Monograph |
|
Corresponding author: Gloria E. Barboza ( gbarboza@imbiv.unc.edu.ar ) Corresponding author: Carolina Carrizo García ( ccarrizo@imbiv.unc.edu.ar ) Academic editor: Sandy Knapp
© 2022 Gloria E. Barboza, Carolina Carrizo García, Luciano de Bem Bianchetti, María V. Romero, Marisel Scaldaferro.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Barboza GE, García CC, Bianchetti LB, Romero MV, Scaldaferro M (2022) Monograph of wild and cultivated chili peppers (Capsicum L., Solanaceae). PhytoKeys 200: 1-423. https://doi.org/10.3897/phytokeys.200.71667
|
Abstract
Capsicum L. (tribe Capsiceae, Solanaceae) is an American genus distributed ranging from the southern United States of America to central Argentina and Brazil. The genus includes chili peppers, bell peppers, ajíes, habaneros, jalapeños, ulupicas and pimientos, well known for their economic importance around the globe. Within the Solanaceae, the genus can be recognised by its shrubby habit, actinomorphic flowers, distinctive truncate calyx with or without appendages, anthers opening by longitudinal slits, nectaries at the base of the ovary and the variously coloured and usually pungent fruits. The highest diversity of this genus is located along the northern and central Andes. Although Capsicum has been extensively studied and great advances have been made in the understanding of its taxonomy and the relationships amongst species, there is no monographic treatment of the genus as a whole. Based on morphological and molecular evidence studied from field and herbarium specimens, we present here a comprehensive taxonomic treatment for the genus, including updated information about morphology, anatomy, karyology, phylogeny and distribution. We recognise 43 species and five varieties, including C. mirum Barboza, sp. nov. from São Paulo State, Brazil and a new combination C. muticum (Sendtn.) Barboza, comb. nov.; five of these taxa are cultivated worldwide (C. annuum var. annuum, C. baccatum var. pendulum (Willd.) Eshbaugh, C. baccatum var. umbilicatum (Vell.) Hunz. & Barboza, C. chinense Jacq. and C. frutescens L.). Nomenclatural revision of the 265 names attributed to chili peppers resulted in 89 new lectotypifications and five new neotypifications. Identification keys and detailed descriptions, maps and illustrations for all taxa are provided.
Keywords
America, Capsicum, chili peppers, cytogenetics, morphology, phylogeny, taxonomy
Introduction
Capsicum L., with 43 species, is placed in the tribe Capsiceae (subfam. Solanoideae, Solanaceae) together with Lycianthes (Dunal) Hassl. It is native to temperate, subtropical and tropical regions of the Americas, growing from the southern United States of America to central Argentina and Brazil, with the primary centre of diversity in the Andes.
Capsicum is an important crop genus, comprising the chili peppers, bell peppers, ajíes, habaneros, jalapeños, ulupicas or pimientos, with five main domesticated species: C. annuum L., C. chinense Jacq. and C. frutescens L., now widely cultivated throughout the world and C. baccatum L. and C. pubescens Ruiz & Pav., cultivated predominantly in South America. The genus comprises a diverse group of sweet and hot chili peppers, which have been used as spices since 6000–6500 BCE (
Chili peppers are known for their high nutritional value, health benefits and medicinal properties (
The genus is characterised by a distinctive truncate calyx, without or with appendages (vein prolongations) borne below the entire margin and by variously coloured and usually pungent berries (
In the molecular phylogenetic reconstructions of Solanaceae (
Although Capsicum has been extensively studied and great advances have been made in the understanding of its taxonomy and the relationships amongst the species, there is no taxonomic monograph of the genus as a whole. As part of ongoing projects to revise the genera Capsicum and Lycianthes, we present here a comprehensive taxonomic treatment of Capsicum, including updated information about morphology, anatomy, karyology, phylogeny and distribution and a revision of the nomenclature and typification of the 265 names in the genus. An identification key and descriptions of wild and domesticated taxa, together with distribution maps and illustrations for each, are provided.
Circumscription and infrageneric classification of Capsicum
Since
There has been little agreement on an infrageneric classification of Capsicum.
The taxonomic division of Capsicum was re-examined in Hunziker’s Capsicum synopsis (
The amount of new evidence produced in recent years has allowed considerable progress in the characterisation of infrageneric groups in Capsicum. Some attempts to group the species were made, based on cytogenetic studies (
Species relationships in Capsicum have been analysed following a phylogenetic approach using a range of molecular data. Several early phylogenetic hypotheses involved and primarily concerned domesticated species, although they also included a small number of wild ones (
Capsicum clades and species composition (after
| Clade | Species |
|---|---|
| Unassigned | C. benoistii Hunz. ex Barboza * |
| Andean | C. dimorphum (Miers) Kuntze |
| C. geminifolium (Dammer) Hunz. | |
| C. hookerianum (Miers) Kuntze | |
| C. lanceolatum (Greenm.) C.V.Morton & Standl. | |
| C. longifolium Barboza & S.Leiva | |
| C. lycianthoides Bitter | |
| C. piuranum Barboza & S.Leiva | |
| C. regale Barboza & Bohs | |
| C. rhomboideum (Dunal) Kuntze | |
| Atlantic Forest | C. campylopodium Sendtn. |
| C. carassense Barboza & Bianch. | |
| C. cornutum (Hiern) Hunz. | |
| C. friburgense Bianch. & Barboza | |
| C. hunzikerianum Barboza & Bianch. ** | |
| C. mirabile Mart. | |
| C. mirum Barboza | |
| C. muticum (Sendtn.) Barboza | |
| C. pereirae Barboza & Bianch. | |
| C. recurvatum Witasek | |
| C. schottianum Sendtn. | |
| C. villosum Sendtn. | |
| Flexuosum | C. flexuosum Sendtn. |
| Caatinga | C. caatingae Barboza & Agra |
| C. parvifolium Sendtn. | |
| Longidentatum | C. longidentatum Agra & Barboza |
| Bolivian | C. caballeroi M.Nee |
| C. ceratocalyx M.Nee | |
| C. coccineum (Rusby) Hunz. | |
| C. minutiflorum (Rusby) Hunz. | |
| C. neei Barboza & X.Reyes | |
| Purple Corolla | C. cardenasii Heiser & P.G.Sm. |
| C. eshbaughii Barboza | |
| C. eximium Hunz. | |
| Pubescens | C. pubescens Ruiz & Pav. |
| Tovarii | C. tovarii Eshbaugh, P.G.Sm. & Nickrent |
| Baccatum | C. baccatum L. |
| C. chacoense Hunz. | |
| C. rabenii Sendtn. | |
| Annuum | C. annuum L. |
| C. chinense Jacq. | |
| C. frutescens L. | |
| C. galapagoense Hunz. |
Capsicum phylogeny. Cladogram summarising findings from
Taxonomic history of Capsicum
The quest of Europeans for the “Indies” (namely the Americas) was accompanied by the discovery of new aromatic plants that extensively enriched cuisines around the world; amongst these were the chili peppers (reviewed in
Fifty years after Columbus’ first voyage to the West Indies, Leonhard Fuchs, a German physician and botanist, published the first three scientific illustrations of chili peppers (
The word ‘capsicum’ was coined in the pre-Linnaean literature for the first time by Matthias de
Subsequently the taxonomy of the genus has been complicated by both generic circumscription (see above) and by differing species concepts. Opinions as to the number of taxa that belong to Capsicum range from as many as 61 species (plus infraspecific taxa) in the genus (e.g.
Taxonomy of the domesticated species
After Linnaeus, the British gardener Philip
The German botanist, Johann Heinrich
The first illustrated monograph of Capsicum was published by the German botanist, Karl Anton
In his monumental treatment of Solanaceae, Michel-Felix
In extreme contrast, other authors tended to reduce the number of accepted species to two (
As a crop genus, Capsicum has inspired researchers to follow a number of in-depth approaches since the mid-1900s, such as classical and molecular cytogenetic analyses, crossing experiments, biochemical and protein electrophoretic studies, molecular characterisation through genotypic markers (restriction fragment length polymorphism, RFLP, amplified fragment length polymorphism, AFLP, random amplified polymorphic DNA, RAPD, microsatellite or simple sequence repeat, SSR, random amplified microsatellite polymorphism, RAMPO and direct amplification of minisatellite DNA, DAMDPCR), phytogeographic and phylogeographic analyses, chloroplast and nuclear DNA and whole-genome sequencing studies (see
Taxonomy of the wild species
Taxonomic work on wild Capsicum species began in the 19th century. Initially, some authors (
During the early 20th century, sporadic descriptions of new Capsicum taxa continued (
In the last two decades, field explorations across South America, mainly in the central Andean countries and Brazil, have enabled us to gain a better understanding of the genus as a whole. Thirteen new wild species have been described and partial keys for the identification of the species for particular areas have been provided (
Morphology
Habit and stems
Members of Capsicum plants are erect (e.g. C. schottianum, C. geminifolium), compact (e.g. C. chacoense, C. annuum var. annuum) or somewhat prostrate (e.g. C. annuum var. glabriusculum). They are subshrubs or shrubs, rarely trees (e.g. C. rhomboideum), short-lived perennials (e.g. C. chinense) or annual herbs (e.g. C. annuum var. annuum). Capsicum coccineum is unusual in being sprawling vines or scrambling shrubs. Stems are woody at the base (1.5–2.5 cm in diameter, rarely more) and some species have fissured bark and lenticels (e.g. C. rhomboideum, C. hookerianum); young stems are angular, herbaceous, usually hollow and weak and, occasionally, somewhat scrambling, range from glabrous to densely pubescent and may have anthocyanin along their length. The nodes are inflated and commonly green or purple.
Capsicum plants have typical solanaceous sympodial growth, giving the stems a “zig-zag” appearance. Initially, the vegetative growth is monopodial and the first stem to emerge has 8–39 leaves (C. annuum cultivars,
Leaves
Species of Capsicum have simple leaves that are generally membranous or less frequently coriaceous (e.g. C. hunzikerianum, C. longifolium, C. pereirae), concolourous to discolourous, ovate to elliptical, rarely lanceolate or narrowly elliptical (C. longifolium, C. carassense) in outline; in taxa with geminate leaves, the minor leaves can be orbicular and sessile (C. dimorphum, C. lycianthoides). Leaf margins are always entire, rarely slightly revolute (C. caballeroi, C. ceratocalyx, C. hunzikerianum) and the leaf base is asymmetric, attenuate or truncate and sometimes decurrent on to the petiole (C. piuranum, C. rabenii). Leaf apices are obtuse or acute to acuminate or long-acuminate in few species (e.g. C. benoistii, C. piuranum, C. hunzikerianum). Petioles are longer in the domesticated species and in the major leaves of geminate leaf pairs in wild species.
Leaf and petiole anatomy of Capsicum members were investigated in domesticated species (
Pubescence
Trichomes in Capsicum are mostly eglandular and simple, although branched trichomes can also occur (e.g. C. longidentatum, C. rhomboideum and C. parvifolium). Simple trichomes are uniseriate and usually 1–11-celled (Fig.
Glandular trichomes are common in Capsicum species. In most species, glandular trichomes are simple, with short uni- or bicellular stalks and globose to ellipsoid multicellular heads (Fig.
Density of pubescence is highly variable both within and between species. Sometimes, this variability has been considered diagnostic at the infraspecific level. For example, the densely pubescent plants of C. chacoense, C. baccatum, C. eshbaughii or C. annuum have been recognised at the infraspecific level (see descriptions and synonymy of those species).
Inflorescences
Capsicum species have axillary flowers; they are solitary only in C. chacoense (Fig.
All Capsicum flowers have distinct, usually pubescent, pedicels that may be terete (Fig.
Flower morphology in Capsicum species A C. rabenii B C. annuum var. glabriusculum C C. lanceolatum D C. schottianum E C. frutescens F C. eximium G C. eshbaughii H C. cornutum I C. galapagoense J C. recurvatum K C. cardenasii L C. lycianthoides M C. chacoense N C. baccatum var. baccatum O C. caballeroi. Abbreviations. im interpetalar membrane sp staminal plaque.
A–G Fruit morphology in Capsicum species H, J epicarp structure A C. chinense B C. baccatum var. baccatum C C. hookerianum D C. lanceolatum E C. coccineum F C. regale G C. schottianum H epicarp with regular epidermal cells I epicarp with some sclereids amongst the regular epidermal cells J epicarp exclusively with sclereids. Abbreviation. sc sclereids. Scale bar: 10 μm (H, I, J).
Calyces
The calyx in Capsicum species is usually 5-merous (4–8-merous in domesticated taxa) and entirely synsepalous (Fig.
Corollas
Capsicum species have 5-merous (6–8-merous in domesticated taxa) sympetalous corollas. Corollas are usually of intermediate size (6–14 mm long), the smallest measuring 4–5 mm long (e.g. C. galapagoense) and the largest ones reaching 17–18 mm long (e.g. C. caballeroi, C. piuranum). Most species have stellate corollas (Fig.
Corolla colour is highly variable in Capsicum species. Corollas can be entirely white (e.g. C. chacoense, C. galapagoense, C. annuum var. annuum), dull white or greenish-white (C. chinense, C. frutescens), light yellow (C. neei), yellow (e.g. C. piuranum, C. caballeroi) or violet or fuchsia (C. friburgense). Other species have corollas with a predominant primary colour (white, yellow or purple), as well as markings (spots) with diverse pigmentation. The abaxial surface (outer surface) of the corollas may have the same colouration as the adaxial surface (inner surface) (e.g. C. geminifolium, C. regale) or it may have a faded or a different colouration (e.g. C. tovarii, C. pereirae). In many species, the co-occurrence of different pigments results in multi-coloured corollas, for example, white corollas with purple (or variations) spots at the base of the lobes and limb and green or greenish-yellow centre (e.g. C. villosum, C. schottianum, C. pereirae). Descriptions in literature or on specimen labels usually refer to the colour of the inner (adaxial) corolla surface. In this monograph, description of the corolla colour of both surfaces is provided for each species; corolla colour can be very difficult to see on herbarium specimens.
Corolla pigmentation is due to anthocyanins which produce violet or purple shades (e.g. C. lycianthoides, C. lanceolatum, Fig.
The adaxial surfaces of the corollas are glabrous (many Andean species) or may be covered by sparse glandular trichomes (e.g. C. ceratocalyx, C. tovarii) or have a continuous ring of glandular trichomes (Fig.
Androecium
Capsicum species have usually five (sometimes 6–8 in domesticated taxa) equal stamens; unequal stamens have only been observed in three species: C. campylopodium (and sometimes also C. lycianthoides), which has two stamens longer than the other three (
Pollen is yellow or white, trizonocolporate, spheroidal, prolate, prolate-spheroidal or oblate-spheroidal, with a triangular to circular outline in polar view. It is usually small, from 15 µm in C. rhomboideum (
Gynoecium
The gynoecium in Capsicum species is usually bicarpellate (2–5-carpellate in domesticated species). The ovary is superior with axile placentation, glabrous and usually subglobose to ovoid, rarely ellipsoid (e.g. C. frutescens). The style is simple, straight or slightly curved, cylindrical (the same width from the proximal to distal end, Fig.
Fruits
The fruit is usually a bicarpellate berry (Fig.
Fruit anatomy in Capsicum species A, D–F C. baccatum var. pendulum B C. baccatum var. umbilicatum C, G, H C. pubescens A fruit, in cross section (note giant cells in the pericarp) B one locule of a fruit, in cross section (note the absence of giant cells in the pericarp) C, D epicarp and some layers of mesocarp (in D, observe cuticular wedges) E sector of pericarp (the arrow indicates the increase of the cell size ending in the giant cells) F detail of two adjacent giant cells G sector of homogeneous endocarp H sclereids of the endocarp. Abbreviations. c cuticle, cw cuticular wedge, epc epidermal cells, gc giant cells, p pericarp. Scale bars: 1 mm (A, B, E); 10 μm (C, D, H); 100 μm (F, G).
Fruiting pedicels are usually green (Fig.
Pericarp structure
The development of the pericarp in Capsicum species is the typical of a true berry (
The epicarp consists of a uniseriate epidermis covered by a smooth (e.g. C. annuum, C. chacoense, C. pubescens, Fig.
The mesocarp consists of (5–) 7–26 layers, with up to 30 layers found in the thick pericarp of a sweet C. annuum var. annuum cultivar known as “calahorra”. The mesocarp can be homogeneous or heterogeneous; a homogeneous mesocarp is formed exclusively by parenchyma (thin-walled cells) or collenchyma (thick-walled cells) (e.g. C. rhomboideum, C. hookerianum), whilst a heterogeneous mesocarp consists of both collenchyma and parenchyma with a variable number of layers for each tissue, depending on the species (see Suppl. material
Hard inclusions of sclereids (stone cells) are developed in the mesocarp of some species (5 spp., see Suppl. material
The endocarp develops from the inner epidermis of the ovary wall; it consists of one layer of polygonal or irregular cells (surface view) with straight or sinuate cell walls. The endocarp can be homogeneous, that is entirely with thin (e.g. C. recurvatum) or pitted thick-walled cells (sclereids) (e.g. C. rhomboideum, C. pubescens, Fig.
Some structural features of the pericarp useful in species-level taxonomy are detailed in Suppl. material
Septum and placenta
The presence of the pungent principles (capsaicinoids) of Capsicum fruits within cells of the interlocular septum and placenta has been demonstrated by histochemical (
An ultrastructural study demonstrated that the major reservoir of capsaicinoids is in the capsisome, a specific capsaicinoid biosynthesising and accumulating vacuole, different from the vacuoles regarded as reservoirs of organic acids (
Capsaicinoids have also been found in the pericarp and seeds in significant or low amounts in some species and cultivars (e.g. C. chinense, C. baccatum) (
Seeds
The gross morphology of the seeds and details of the sculpturing of the seed coat for all Capsicum species are summarised in Suppl. material
Seed morphology and seed coat structure A Capsicum chinense B C. chacoense, longitudinal section C, D C. schottianum, cross section (D detail of the seed coat structure) E C. annuum var. annuum, detail of seed coat structure (the rectangle indicates a cell of the seed coat) F, G C. eximium, cross sections at the seed margin and seed body, respectively H C. dimorphum, cross section at the seed body. Abbreviations. aw anticlinal cell wall, bp beak prominence, em embryo, en endosperm, h hilum, im inferior seed margin, ipw inner periclinal wall, opw outer periclinal wall, sb seed body, sc seed coat, sm superior seed margin, w seed width, l seed length. Scale bars: 200 μm (A–C); 100 μm (D, F, H); 20 μm (E, G).
Seed shape (and size) is influenced by the position in the berry. The seeds are flattened to slightly angled, mostly C- or D-shaped (
The hilum is always marginal (on the inferior margin, Fig.
Seeds and seed coat morphology in species of the Annuum Clade A–D C. annuum var. annuum E–H C. annuum var. glabriusculum I–L C. frutescens M–P C. chinense Q–T C. galapagoense A, I seeds with testa partly digested B seed coat with the external periclinal cell wall partly removed C, D cross section of the seed at the seed margin and seed body, respectively; E, M, N, Q untreated seeds showing subterminal hilum (E, N) and medial hilum (Q) F, J, O, P, S, T detail of a non-digested portion of the seed coat G, K testa pattern with the external periclinal cell wall removed H, L detail of testa cells R hilum. Abbreviation. opw outer periclinal cell wall. Scale bars: 200 μm (A, E, I, M, N, Q); 20 μm (B, F–H, K, L, P, S, T); 50 μm (C, J, O, R); 10 μm (D).
Seeds and seed coat morphology in species of the Baccatum Clade A–D C. baccatum var. baccatum E C. baccatum var. pendulum F–H C. baccatum var. umbilicatum I–L C. chacoense M–O C. rabenii. A, I, M seeds with testa partly digested B, G detail of a non-digested portion of the seed coat C, H, J, K, O testa pattern of treated seeds showing anticlinal cell walls with fibrils (C, K), papillae (H) and ridge (O) D detail of a testa cell E, F untreated seeds L cross section of the seed at the seed body N seed showing the subterminal elliptical hilum. Scale bars: 10 μm (H); 20 μm (B–D, G, K, L, O); 100 μm (J); 200 μm (A, E, F, I, M, N).
Capsicum annuum (and its varieties) has been the most frequently studied species (
Seed ornamentation refers to the appearance of the seed coat (testa) (Figs
Five major types of seed coat sculpture were observed after the outer periclinal wall was removed by enzymatic digestion: (1) reticulate, with straight to wavy cell walls (Fig.
Seeds and seed coat morphology in species of the Atlantic Forest Clade A–D C. campylopodium E–H C. carassense I–L C. cornutum M–P C. friburgense Q–T C. mirabile A untreated seed B, I, Q seeds with testa partly digested C, F, K, O, S marginal testa pattern of treated seeds (note pillar-like outgrowth in K, O, S) D, G, H, L, P, T testa pattern at the seed body of treated seeds showing anticlinal cell walls papillate and punctate E, J, M, R treated seeds (in J showing lateral prominence) N hilar zone with a linear hilum. Abbreviation. lp lateral prominence. Scale bars: 200 μm (A, B, E, I, J, M, Q, R); 100 μm (C, G, K, N, O, S); 20 μm (D, F, H, L, P, T).
Seeds and seed coat morphology in species of the Atlantic Forest Clade A–C C. muticum D–G C. pereirae H–K C. mirum L–P C. schottianum Q–T C. recurvatum A, E, I, N, Q treated seeds B, F, J, O, S marginal testa pattern of treated seeds with pillar-like outgrowths C, G, P, T testa pattern at the seed body of treated seeds showing anticlinal cell walls papillate and punctate D, H, L untreated seeds K detail of papillate anticlinal cell walls M detail of a non-digested portion of the seed coat R hilar zone with a linear hilum. Scale bars: 200 μm (A, D, E, H, I, L, N, Q); 100 μm (B, F, J, O, R); 20 μm (C, G, K, M, P, S, T).
Seeds and seed coat morphology in species of the Atlantic Forest Clade A–D C. hunzikerianum E–I C. villosum A, G seeds with testa digested B seed showing medial and linear hilum C, H marginal testa pattern of treated seeds D, I testa pattern at the seed body of treated seeds showing anticlinal cell walls papillate (D) and punctate (I) E untreated seed F detail of a non-digested portion of the seed coat. Scale bars: 200 μm (A, B, E, G); 20 μm (C, D, F, I); 100 μm (H).
Seeds and seed coat morphology in species of the Andean Clade A–D C. dimorphum E–H C. geminifolium I–L C. lanceolatum M–P C. longifolium Q–T C. lycianthoides A, Q, S seeds untreated B, E, I, J, M seeds with testa digested C, G, K, O testa pattern at the seed body of treated seeds showing anticlinal cell walls punctate (C, J, O) and with fibrils (G, K, O) D detail of ridge H, L, P detail of testa cells F, N hilar zone R, T detail of a non-digested portion of the seed coat. Scale bars: 200 μm (A, B, E, I, J, M, Q, S); 100 μm (C, K, N, O); 20 μm (D, F, G, H, L, P, R, T).
Seeds and seed coat morphology in species of the Andean Clade A–D C. hookerianum E–H C. piuranum I–K C. rhomboideum A untreated seed B, E treated seeds C, D, G, J, K testa pattern of treated seeds showing anticlinal cell walls punctate (C, D, J, K) and papillate (G) F hilar zone H detail of testa cells densely papillate I seed partly digested. Scale bars: 200 μm (A, B, E, I); 20 μm (C, D, F, G, H, J, K).
The anticlinal cell walls observed with SEM are either: (1) papillate, with papillae 2.5–4 µm in diameter on the cell walls (Figs
Seeds and seed coat morphology in species of the Bolivian Clade A–D C. caballeroi E–H C. ceratocalyx I–L C. minutiflorum M–P C. neei Q–T C. coccineum A, Q seeds with testa partly digested B, F, G, J, O, R marginal testa pattern of treated seeds showing anticlinal cell walls punctate and densely papillate (G, O), punctate (J) and with ridge (R) C, H, K, P, S detail of testa cells D, L, T detail of papillae E, I, M treated seeds N linear hilum. Scale bars: 300 μm (A, E, I, M, N, Q); 50 μm (B, F, G, H, O, P); 20 μm (C, J, K, L, R, S); 5 μm (D, T).
Seeds and seed coat morphology in species of the Caatinga, Longidentatum, Flexuosum and Tovarii Clades A–D C. caatingae E–H C. parvifolium I–L C. longidentatum M–O C. flexuosum P–R C. tovarii A, M, P seeds with testa partly digested (in A hilum in terminal position, P hilum subterminal) B detail of a non-digested portion of the seed coat C, F, J, N, Q marginal testa pattern of treated seeds showing anticlinal cell walls papillate (F), with fibrils (J), punctate (N) and with fringe (Q) D, G, K, O, R detail of testa cells E, I treated seeds H, L papillae on anticlinal cell walls Scale bars: 200 μm (A, E, I, M, P); 20 μm (B, D, G, H, J, K, L, N, O, Q, R); 100 μm (C, F).
Seeds and seed coat morphology in species of the Purple corolla and Pubescens Clades A–D C. cardenasii E–H C. eshbaughii I–L C. eximium M–P C. pubescens A, M untreated seeds (in A hilum terminal, M hilum medial) B treated seed C, G, K, O testa pattern of treated seeds showing anticlinal cell walls with fringe (C) and fibrils (G) D, H, L, M detail of testa cells E, I, N seeds with testa partly digested (in I hilum subterminal) F, J detail of a non-digested portion of the seed coat. Scale bars: 200 μm (A, B, E, I, M, N); 20 μm (C, D, G, H, J, K, L, P); 50 μm (F, O).
The distal end of the anticlinal cell walls may have three different types of appendages: (1) a thin ridge (Figs
Embryo
The embryo in Capsicum is usually imbricate, meaning that the cotyledon tips are parallel or almost parallel to the radicle (
Floral biology and pollination
Most work on pollination and floral biology in Capsicum has been done with the domesticated species used for their fruits. Capsicum species are generally reported to be self-compatible, although the studied cases concerned mainly domesticated species and a few wild relatives (e.g. C. annuum and C. galapagoense from the Annuum clade or C. baccatum and C. chacoense from the Baccatum clade) (
Flowering phenology, with particular attention to the timing of gynoecium and androecium maturity, has been studied to improve pollination, fertilisation and, ultimately, the fruit set, as well as to analyse the chances of doing targeted crosses (e.g. through bud pollination). Anther dehiscence has been registered to occur after flower opening, whereas the stigma is receptive before anther dehiscence, even in the buds and receptivity is maintained throughout the lifespan of the flower (
Capsicum species have served as models to examine unilateral self-incompatibility using reciprocal interspecific crosses mainly between the domesticated species and their closest relatives, both within and between clades that include domesticated species (e.g.
Nectar production and its presentation in Capsicum is due to the formation of nectar ducts, structures also found in other Solanaceae genera, such as Jaltomata Schltdl., Physalis L. (
Fruit and seed dispersal
Wild Capsicum species typically produce colourful, juicy, fleshy, conspicuous, many-seeded berries that are attractive to their consumers. The fruits in most Capsicum species contain capsaicinoids (mainly capsaicin), the chemical principles responsible for their pungency, which are highly concentrated in the placental and septum tissues. Some authors (
It is expected that the non-pungent red or orange fruits of the Andean Capsicum species are also dispersed by birds. It would be would be interesting to test in nature if the directed deterrence hypothesis functions in a similar way in the Brazilian Atlantic forest species whose greenish-golden yellow fruits are not as showy as the red-fruited species; they are pendent and are somewhat masked amongst the copious green foliage of the plant, perhaps attractive to fauna moving underneath the plants.
Cytogenetics
Capsicum species are mostly diploid, with two chromosome numbers: 2n = 24 (x = 12) and 2n = 26 (x = 13), the latter appearing only in wild species (Heiser and Smith 1958;
| Taxon and voucher number | n | 2n | Haploid karyotype formula | Chromosomes with active NORs | Hc amount (HKL in µm) | 1C DNA co ntent in pg | References |
|---|---|---|---|---|---|---|---|
| C. annuum var. annuum | |||||||
| No voucher cited | - | 24 | - | - | - | - |
|
| cytotype 1 EAM 193, 251, 203 | - | 24 | 10 m + 1 sm + 1 st | 11 sm | 1.80 (68.51) | 3.41* |
|
| cytotype 2 EAM 204, 252; NMCA 10544, 10272 | - | 24 | 10 m + 1 sm + 1 st | 11 sm, 12 st | 2.88 (70.40) | 3.32* |
|
| Cuneo w.no. Doux Long des Landes w.no. | - | - | - | - | - | 3.83† |
|
| C. annuum var. glabriusculum | |||||||
| No voucher cited | - | 24 | - | - | - | - |
|
| cytotype 1 NMCA 10955 | - | 24 | 10 m + 1 sm + 1 st | 11 sm | 2.26 (59.53) | - |
|
| cytotype 2 NMCA 10983 | - | 24 | 11 m + 1 st | 1 m, 5 m | 3.54 (51.95) | - |
|
| cytotype 3 LQ w. no | - | 24 | 11 m + 1 st | 11 m | 2.33 (55.13) | - |
|
| cytotype 4 YSG w. no | - | 24 | 11 m + 1 st | 5 m, 12 st | 6.33 (53.56) | - |
|
| cytotype 5 Neth 804750009 | - | 24 | 11 m + 1 sm | 12 sm | 3.37 (55.43) | - |
|
| cytotype 6 PI 511885 | - | 24 | 11 m + 1 st | 1 m, 5 m, 6 m, 12 st | 2.97 (80.38) | - |
|
| cytotype 7 PI 511886 | - | 24 | 11 m + 1 st | 1 m, 2 m, 5 m, 8 m | 3.83 (70.05) | - |
|
| C. baccatum var. baccatum | |||||||
| Vouchers not cited | - | 24 | - | - | - | - |
|
| GEB 163 | - | 24 | 11 m + 1 st | 1 m, 3 m, 10 m, 12 st | 7.45 (66.84) | 3.71* |
|
| Tuscia University, Italy w. no | - | - | - | - | - | 4.22† |
|
| C. baccatum var. pendulum | |||||||
| No voucher cited | - | 24 | - | - | - | - |
|
| cytotype 1 EAM 192, 209 | - | 24 | 11 m + 1 st | 1 m, 3 m, 12 st | 7.30 (75.53) | 3.71* |
|
| cytotype 2 EAM 205, 206, 247; ATH 25382; EAM & RN 211 | - | - | 11 m + 1 st | 1 m, 3 m, 10 m, 12 st | 7.56 (74.31) | 3.68* |
|
| Tuscia University, Italy w. no, Sao Paulo University w. no | 74.92 | - | - | - | - | 4.20† |
|
| C. baccatum var. umbilicatum | |||||||
| EAM 197, 253 | - | 24 | 11 m + 1 st | 1 m, 3 m, 10 m, 12 st | 9.06 (74.27) | 3.76* |
|
| C. caatingae | |||||||
| LBB 1560 | 12 | - | - | - | - | - |
|
| LBB 1560 | 12 | 24 | - | - | - | - |
|
| cytotype 1 ATH 25233 | - | 24 | 11 m + 1 st | 12 st | 5.52 (82.40) | - |
|
| cytotype 2 ATH 25233 bis | - | 24 | 12 m | 12 m | 7.47 (77.60) | 5.77* |
(as C. parvifolium) |
| C. caballeroi | |||||||
| GEB et al. 3655 | - | 24 | - | - | - | - | this monograph |
| C. campylopodium | |||||||
| LBB 1566 | 13 | - | - | - | - | - |
|
| LBB 1566 | 13 | 26 | - | - | - | - |
|
| cytotype 1 ATH 25116 | - | 26 | 5 m + 6 sm + 1 st + 1 t | 7 sm | 32.49 (88.30) | 5.74* |
|
| cytotype 2 ATH 25128, 25130, 25136 | - | 26 | 10 m + 2 sm + 1 st | 11 sm | 20.41 (87.95) | 4.53* |
|
| C. cardenasii | |||||||
| Heiser 4196 | 12 | - | - | - | - | - | Heiser and Smith 1958 |
| No voucher cited | - | 24 | - | - | - | - |
|
| CORD 135 | - | 24 | - | - | - | - |
|
| cytotype 1 Neth 904750136 | - | 24 | 11 m + 1 sm | 7 m, 12 sm | 6.91 (62.00) | - |
|
| cytotype 2 AAC w. no; GEB w. no | - | 24 | 11 m + 1 sm | 7 m, 12 sm | 10.41 (76.01) | - |
|
| Budapest, Hungary w. no | - | - | - | - | - | 4.49† |
|
| C. chacoense | |||||||
| Argentina (Córdoba), no voucher cited | 12 | - | - | - | - | - |
|
| No voucher cited | - | 24 | - | - | - | - |
|
| LB et al. 498 | 12 | - | - | 1 m, 12 st | - | - |
|
| cytotype 1 EAM 104, 195, 207, 250; AAC et al. 973 | - | 24 | 11 m + 1 st | 1 m, 12 st | 2.94 (65.02) | 3.34* |
|
| cytotype 2 LB & LG 525 | - | 24 | 11 m + 1 st | - | 2.44 (71.25) | 3.36* | |
| Tuscia University, Italy w. no | - | - | - | - | - | 3.83† |
|
| C. chinense | |||||||
| C 323, C 324 | 12 | - | - | - | - | - |
|
| No voucher cited | - | 24 | - | - | - | - |
|
| cytotype 1 GEB et al. 797; GEB 807 | - | 24 | 11 m + 1 st | 12 st | 3.91 (61.31) | 3.43* |
|
| cytotype 2 EAM 201 | - | 24 | 11 m + 1 st | 12 st | 5.52 (61.36) | 3.41* |
|
| LBB 1720 | 12 | - | - | - | - | - |
|
| MVR 9 | - | 24 | 11 + 1 st | 12 st | - | - |
|
| Sao Paulo University w. no, Reading University U. K. w. no, I.N.R.A. France w. no | - | - | - | - | - | 4.02† |
|
| C. cornutum | |||||||
| LBB 1542, 1546 | 13 | - | - | - | - | - |
|
| LBB 1546 | - | 26 | - | - | - | - |
|
| C. eshbaughii | |||||||
| CCG 91 | - | 24 | - | - | - | - |
|
| C. eximium | |||||||
| No voucher cited | 12 | - | - | - | - | - | Heiser and Smith 1958 |
| No voucher cited | - | 24 | - | - | - | - |
|
| cytotype 1 EAM 254 | - | 24 | 11 m + 1 sm | 7 m, 12 sm | 4.90 (68.89) | 4.06* |
|
| cytotype 2 EAM 255 | - | - | 11 m + 1 sm | 7 m, 12 sm | 2.10 (69.65) | - |
|
| University of Reading, U. K. w. no, Budapest, Hungary w. no | - | - | - | - | - | 4.35† |
|
| C. flexuosum | |||||||
| RSu, EAM 4133 | 12 | - | - | - | - | - |
|
| LBB 1552 | 12 | - | - | - | - | - |
|
| LBB 1552 | - | 24 | - | - | - | - |
|
| GEB et al. 3631 | - | 24 | - | - | - | - | this monograph |
| GEB et al. 1034; JD & AIH 599 | - | 24 | 11 m + 1 st | 2 m, 5 m | 16.82 (103.69) | - |
|
| No voucher cited | - | - | - | - | - | 7.2¤ |
|
| C. friburgense | |||||||
| LBB 1565 | 13 | - | - | - | - | - |
|
| C. frutescens | |||||||
| No voucher cited | - | 24 | - | - | - | - |
|
| GEB et al. 795; EAM 200 | - | 24 | 11 m + 1 st | 1 m, 12 st | 5.55 (66.63) | 3.40* | Moscone et al. 1996; |
| Tuscia University, Italy w. no, Sao Paulo University w. no | - | - | - | - | - | 3.97† |
|
| C. galapagoense | |||||||
| No voucher cited | 12 | - | - | - | - | - | Heiser and Smith 1958 |
| No voucher cited | - | 24 | - | - | - | - |
|
| PI 639682 | - | 24 | 11 m + 1 st | 12 st | 2.24 (48.66) | - |
|
| C. hunzikerianum | |||||||
| GEB et al. 5041 | - | 26 | - | - | - | - | this monograph |
| C. lanceolatum | |||||||
| NMCA 90016 | 13 | - | - | - | - | - |
|
| C. longidentatum | |||||||
| MFA & GEB 7086 | - | 24 | 12 m | 12 m | - | - |
|
| C. longifolium | |||||||
| GEB & SLG 4821 | - | 26 | 9 m + 3 sm + 1 st | 10 sm | 3.77 (23.86) | - |
|
| C. lycianthoides | |||||||
| GDB 85 | - | 26 | 9 m + 3 sm + 1 st | 10 sm | - | - |
|
| C. mirabile | |||||||
| NMCA 50029 | 13 | - | - | - | - | - |
|
| LBB 1559, 1564, 1568 | 13 | - | - | - | - | - |
|
| LBB 1550, 1554 | 13 | - | - | - | - | - |
|
| cytotype 1 ATH 25238, 25251 | - | 26 | 9 m + 2 sm + 1 st + 1 t | 7 m | 29.64 (83.81) | - |
|
| cytotype 2 ATH 25238, 25255 | - | 26 | 8 m + 3 sm + 1 st + 1 t | 7 m | 29.25 (93.72) | - |
|
| cytotype 3 ATH 25238, 25267 | - | 26 | 9 m + 3 sm + 1 t | 9 m | 30.93 (103.4) | - |
|
| C. parvifolium | |||||||
| MFA & GEB 7075 | - | 24 | 11 m + 1 sm | 12 sm | - | - |
|
| C. pereirae | |||||||
| LBB 1558 | 13 | - | - | - | - | - |
|
| LBB 1558 | - | 26 | - | - | - | - |
|
| cytotype 1 ATH 26137 | - | 26 | 9 m + 1 sm + 2 st + 1 t | 4 m, 11 st | 11.42 (74.52) | - |
|
| cytotype 2 ATH 25249 | - | 26 | 10 m + 2 st + 1 t | 6 m, 11 st | 16.04 (75.85) | - |
|
| C. piuranum | |||||||
| GEB & SLG 4841 | - | 26 | 9 m + 3 sm + 1 st | 10 sm | 2.84 (22.97) | - |
|
| C. pubescens | |||||||
| No voucher cited | - | 24 | - | - | - | - |
|
| GEB 79; EAM 198, 202, 208, 256, 257 | - | 24 | 11 m + 1 st | 10 m, 12 st | 18.95 (80.53) | 4.47* |
|
| Budapest, Hungary w. no | - | - | - | - | - | 4.86† |
|
| C. rabenii | |||||||
| No voucher cited | 12 | - | - | - | - | - | Heiser and Smith 1958 |
| No voucher cited | - | 24 | - | - | - | - |
|
| LBB 1553, 1555 | 12 | - | - | - | - | - |
|
| LBB 1524, 1553, 1555 | - | 24 | - | - | - | - |
|
| cytotype 1 PI 441654 | - | 24 | 11 m + 1 st | 7 m, 12 st | 10.96 (72.55) | - |
|
| cytotype 2 EFM 05-17 | - | 24 | 11 m + 1 sm | 6 m, 12 sm | 14.92 (76.20) | - |
|
| Budapest, Hungary w. no, Gatersleben, Germany w. no | - | - | - | - | - | 4.57† |
|
| C. recurvatum | |||||||
| LBB 1523 | 13 | - | - | - | - | - |
|
| LBB 1523 | - | 26 | - | - | - | - |
|
| GEB et al. 915; GEB et al. 1629, 1632 | - | 26 | 10 m + 2 sm + 1 st | 12 sm | 5.73 (68.55) | - |
|
| C. regale | |||||||
| AOR et al. 3034 | - | 26 | - | - | - | - |
|
| C. rhomboideum | |||||||
| No voucher cited | - | 26 | - | - | - | - |
|
| YSG 19, 20 | - | 26 | 10 m + 1 sm + 2 st | 9 m | 4.88 (42.13) | - |
|
| No voucher cited | - | - | - | - | - | 2.08¤ |
|
| C. schottianum | |||||||
| LBB 1535, 1536, 1540, 1544, 1545 | 13 | - | - | - | - | - |
|
| LBB 1535, 1540 | - | 26 | - | - | - | - |
|
| ATH 25160 | - | 26 | 9 m + 2 sm + 1 st + 1 t | 11 sm | 23.28 (93.71) | - |
|
| C. tovarii | |||||||
| No voucher cited | 12 | - | - | - | - | - |
|
| cytotype 1 ATH & GEB 25653 | - | 24 | 11 m + 1 sm | 10 m, 12 sm | 38.91 (70.32) | - |
|
| cytotype 2 NMCA 90008 | - | 24 | 11 m + 1 sm | 6 m, 7 m, 12 sm | 4.89 (67.02) | - |
|
| The Netherlands w. no | - | - | - | - | - | 3.97 † |
|
| C. villosum | |||||||
| LBB 1538, 1539, 1543, 1557 | 13 | - | - | - | - | - |
|
| LBB 1538, 1543, 1557 | - | 26 | - | - | - | - |
|
| ATH 25169; GEB et al. 1653 | - | 26 | 9 m + 3 sm + 1 t | 12 sm | 9.74 (75.89) | - |
|
Cytogenetics provides a valuable and irreplaceable source of information for solving taxonomic, evolutionary and applied questions (
Almost half of the taxa that have been cytogenetically studied exhibit intraspecific karyotype variation, differing in karyotype formulas, number and location of active NORs, heterochromatin content and banding patterns (
Capsicum disploidy (the presence of two basic chromosome numbers) has been examined in relation to genome size evolution and species diversification. The chromosome number 2n = 24 is dominant across the recognised Capsicum clades, whereas the 2n = 26 taxa are restricted to Andean and Atlantic Forest clades only. These last two clades are the more species-rich and include almost one-half of wild species of the genus.
Species with 2n = 24 chromosomes show rather uniform and comparatively the most symmetrical karyotypes, with the 11 m + 1 st karyotype formula, although 11 m + 1 sm is also frequent. In contrast, species with 2n = 26 karyotype formulas have more asymmetric, with nine different karyotypes amongst ten taxa. Out of these, the species of the Atlantic Forest clade are the most asymmetric, with seven different karyotype formulas found amongst them (Table
It has been suggested that species with 13 chromosome pairs are derived from species with 12 pairs, since the latter have more symmetrical karyotypes (
Heterochromatin amount (Hc), indicated as percentage of haploid karyotype length (HKL), is quite variable in the genus (from 1.80 to 38.91) and correlates positively with the HKL in most of the taxa. Capsicum annuum and C. tovarii have the lowest and highest Hc, respectively, but across clades, the Annuum clade has the lowest Hc, whereas the highest Hc is found in the Atlantic Forest clade (Table
DNA content analysis and characterisation of the 5S and 18S-5.8S-26S (45S) rDNA by FISH has been completed for only a few species of Capsicum (
Domestication
Five Capsicum species, C. annuum, C. chinense, C. frutescens, C. baccatum and C. pubescens, were independently domesticated for their fruits in different areas of Central and South America (
Domestication processes typically modify a few genes that affect domestication traits (
Distribution and habitat
Capsicum species are widely distributed across the Americas, from central Argentina and southern Brazil to the southern extreme of the United States of America, although most of the clades recognised here correspond to a particular geographic region (Fig.
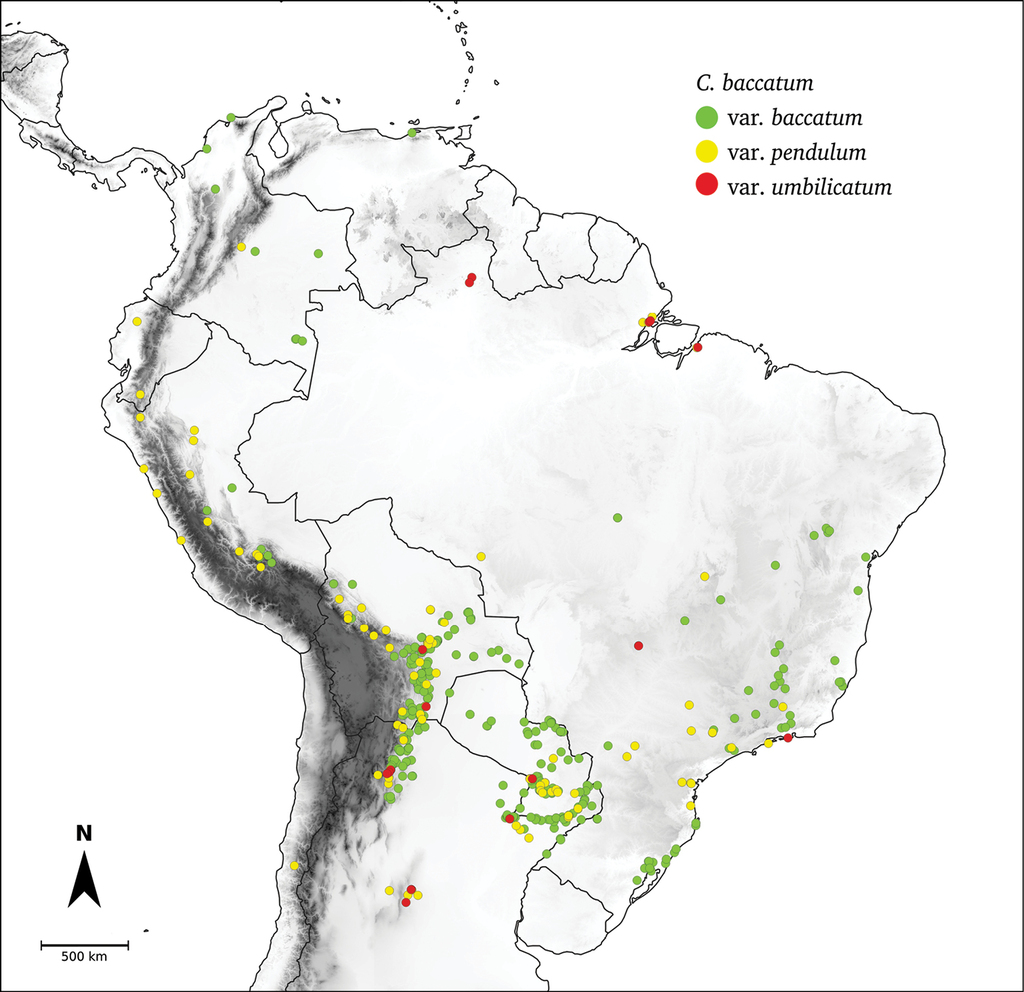
Capsicum geographic distribution. Georeferenced collection points of all wild Capsicum species/varieties. Circles are coloured by clades (Andean: orange; Atlantic Forest: bright green; Flexuosum: bright light blue; Caatinga: lilac; Longidentatum: dark blue; Bolivian: yellow; Purple corolla: red; Tovarii: fuchsia; Baccatum: dark green; Annuum: pale light blue).
Wild Capsicum species are found in a wide variety of habitats, from xeric shrublands to rainforests (Suppl. material
Materials and methods
Trichomes
To analyse the trichomes, temporary preparations of the epidermis of leaves, stems, calyx and corolla were made by making direct peels of the epidermis or cross sections; observations were made under light microscope and drawings were done with the help of a camara lucida.
Fruit anatomy
Mature fruits were used to analyse the anatomy of the pericarp. Fresh fruits were collected in the wild, bought at various markets (domesticated species) or obtained from plants cultivated at the University of Cordoba (Argentina) (see Suppl. material
Seeds
Mature seed samples were taken from herbarium material or collected from wild or cultivated sources (see Suppl. material
Cytogenetics
Chromosome counts, cytogenetic information and DNA content are based on voucher specimens for which we were able to verify their correct identifications (vouchers cited in Table
Taxonomy
The monograph is based on results from many years of herbarium study and field work to collect these taxa across South America. Fresh material was preserved in FAA (formaldehyde–acetic acid–ethanol) or ethanol (70°) to perform measurements of reproductive organs using a Zeiss Stemi 2000-C stereomicroscope at 6.5–50× magnification or trichomes using a Leitz light microscope at 10–40× magnification. Descriptions were based on living plants observed during fieldwork and examination of ca. 6,900 herbarium specimens loaned from or inspected at 213 herbaria (acronyms follow
Measurements of dried material were made from dissections of flowers or fruits rehydrated in hot water, supplemented by measurements from living materials. Information about flower, fruit and seed colour was taken mainly from our own field observations and, in a few cases, flower colour was described from herbarium label data (e.g. C. hookerianum). The terminology used in the mature fruit descriptions of the domesticated species is based on the list of descriptors for Capsicum (IPGRI et al. 1995); pungency of immature and mature fruits was tested by tasting them in the field.
Distribution maps were produced using QGIS 3.16.0-Hannover (
Preliminary conservation status was assessed using
Typification of cultivated taxa has been a particularly difficult task. For many taxa, the authors did not cite specimens or locality of the type. We searched for original material in potential herbaria and when we succeeded or duplicates were found, we designated lectotypes. In other taxa, lectotypifications were based on an illustration cited by the author in the protologue (e.g. Fingerhuth’s illustrations). For taxa recognised only as synonyms, we have cited the taxa in synonymy and indicated that duplicates have not been found rather than neotypifying these taxa. In cases where taxa were described from collections of living material cultivated in botanical gardens from unknown origin or the original material was destroyed in World War II, we designated neotypes when probable original material could be found (e.g. in C. ovatum) or using a modern collection (C. flexuosum). However, for the species described by Philip
In cases where specific herbaria have not been cited in protologues, we designate lectotypes rather than assuming holotypes exist (
Type specimens are cited with their barcodes in square brackets after each herbarium acronym, according to the style used in each herbarium (e.g. GOET003420 or MO-562486); we also indicate the sheet number after the barcode when available (i.e. IND-0153285, acc. # 139721). When barcodes are missing, we indicate only the sheet number (i.e. LIL acc. # 173409). In a few cases, we do not cite barcode or accession number (e.g. some type material from LE).
Identities of all numbered collections seen are presented in the List of Exsiccatae. Numbered and un-numbered collections are presented in Suppl. material
Common names were taken from herbarium label data and reliable literature if we could verify the identity of taxa, but the list of common names for the cultivars of C. annuum, C. frutescens and C. chinense is not complete since we did not comprehensively examine the vast amount of literature where this information appears. Indigenous names are given in a separate paragraph for clarity indicating in brackets the indigenous language (if given). We cite only one specimen by provenance of the common and indigenous names per administrative division of each country. Uses as foods, spices or in ritual practices are cited in the species treatment and folk medicinal uses are summarised in Table
| Taxa | Organ | Use | Country | Voucher/Reference |
|---|---|---|---|---|
| C. annuum var. annuum | Colombia | |||
| Leaf, fruit | Medicinal (no specification) | Amazonas | Alvarado C. 198 | |
| Ecuador | ||||
| Fruit | For snake bite | Morona-Santiago | Evans 4384 | |
| Peru | ||||
| Leaf (juice) | For pregnant women to help birth easily | Loreto | Williams et al. 10922 | |
| C. annuum var. glabriusculum | Brazil | |||
| Leaf | To cure acne | Amapá |
|
|
| Colombia | ||||
| Fruit | To increase body temperature and for the skin fungi | Huila | Buendía 2 | |
| Fruit | To soothe haemorrhoid pains | Valle del Cauca | Duque Jaramillo 4083-A | |
| Ecuador | ||||
| Leaf | To reduce body temperature | Orellana | Carrillo & Reyes 434 | |
| Fruit | Stomach medicine (for sore belly) | Napo | Davis & Yost 994 | |
| Fruit | To kill parasites | Morona-Santiago | Bedoya 2 | |
| Fruit | For skin diseases (measles, pox) | Zamora-Chinchipe | Santín et al. 100 | |
| Fruit | For conjunctivitis | Guatemala | Kufer 99 | |
| Mexico | ||||
| Leaf | To relieve rashes in children (warm bath) | Quintana Roo | Serralta P. 104 | |
| Fruit | For infected wounds | Querétaro | Martínez Torres 82 | |
| Fruit | To treat ulcers | Tabasco | Orozco-Segovia 368 | |
| Fruit | For skin wounds | Yucatán | Ucan 4617 | |
| Fruit | Medicinal | Yucatán | Simá 517 | |
| U.S.A. | ||||
| Fruit | Stimulant | Texas | Chávez Jr. s.n. | |
| C. chacoense | Fruit | Anti-rheumatic | Argentina |
|
| Fruit | Digestive |
|
||
| Fruit | Anti-spasmodic, vermifuge, stomach pain |
|
||
| Fruit extract | Anti-inflammatory activity (mice) |
|
||
| Fruit | Anti-parasitic | Paraguay |
|
|
| C. chinense | Colombia | |||
| Seedling | For haemorrhoids | Meta | Quevedo et al. 1816 | |
| Ecuador | ||||
| Leaf | To treat joint pains | Sucumbíos | Reyes & Moya 234 | |
| - | Anti-parasitic | Napo | Bolotin 21 | |
| Fruit | For stomach ache | Napo | Davis & Yost 993 | |
| Fruit | For eye infections and coughing | Napo | Miller et al. 2404 | |
| Fruit | For dysentery | Orellana | Herrera & Guerrero 186 | |
| Fruit | Medicinal: cardiotonic | Sucumbíos | Moya & Reyes 206 | |
| Mexico | ||||
| Leaf | To treat wounds | Yucatán | Ucan Ek 4652 | |
| C. coccineum | Bolivia | |||
| Entire plant | In baths to relieve stomach pain | La Paz | Vargas 1310 | |
| C. frutescens | Brazil | |||
| Leaf (infusion) | Used for dizziness | Minas Gerais | Pereira 3219 | |
| Immature fruits | For flu | Minas Gerais | Pereira 3219 | |
| C. frutescens | Colombia | |||
| Root (infusion) | To facilitate childbirth | Guaviare | Garzón et al. 3214 | |
| Buds | To cure hand infection | Guaviare | Garzón et al. 3214 | |
| Fruit | Medicinal | Cundinamarca | García Barriga 20315A | |
| Ecuador | ||||
| Leaf | For fungal diseases | Esmerlada | Kvist 40356 | |
| Leaf, fruit | To facilitate the fall of the baby’s umbilical cord | Napo | Siquihua 4 | |
| Fruit | For snake bites | Morona-Santiago | van Asdall 82-59 | |
| Fruit | For snake bites | Zamora-Chinchipe | Ortega 51 | |
| Guatemala | ||||
| Fruit | To treat conjunctivitis | Chiquimula | Kufer 100 | |
| C. pubescens | Ecuador | |||
| Leaf | For bites of dogs | Loja | Ellemann 66689 | |
| Fruit | For headache, weakness and cold | Loja | Ellemann 66689 | |
| Fruit? | Veterinary: to treat “moquillo” (catarrhal disease in dogs and cats) and “tos de nermo” (cough in horses) | Peru | ||
| Oxapampa | Chuck 137 | |||
| C. rhomboideum | Ecuador | |||
| - | To heal skin eruptions | Pichincha | Cerón 6953 |
All species are illustrated with line drawings, colour illustrations or both; photos were taken by the authors of this treatment or were provided by other colleagues (credits are cited in each case). For some species (C. caatingae, C. friburgense, C. hunzikerianum, C. mirum), photos were provided and taken in the field by members of the Associazione PepperFriends (Verona, Italy); these photos have no herbarium voucher, but the identification was verified by the senior author of this treatment (GEB).
Biomes and ecoregions
Ecoregions were determined according to
Taxonomic treatment
Capsicum
Capsicum section Decameris Bitter, Abh. Naturwiss. Vereins Bremen 24(2): 293. 1919. Type: C. dusenii Bitter
Capsicum section Capsicum, Huitième Congr. Int. Bot. Paris. Comptes Rend. Séances Rapp. & Commun. 1954, sect.4: 73. 1956. Type: C. annuum L.
Type
C. annuum L. (lectotype, designated by
Description
Shrubs, subshrubs, rarely trees, vines or short-lived perennials or annuals, occasionally with a thick lignified xylopodium, glabrous or glabrescent or sparsely to densely pubescent with simple, branched, eglandular or glandular, uniseriate trichomes. Stems woody at the base, sometimes with fissured bark and lenticels; young stems angled, herbaceous, usually weak and fragile and occasionally somewhat scrambling. Sympodial units difoliate or unifoliate, the leaves usually geminate, blades simple, entire, concolorous or discolorous, glabrous to densely pubescent with eglandular and/or glandular simple or branched uniseriate trichomes; petioles generally well-developed. Inflorescences axillary, usually unbranched (rarely branched), with few to many (up to 20 or more) flowers clustered or, more rarely, on short rachis or spaced along an elongate rachis, sometimes with flowers solitary or paired. Flowers 5-merous (4–8-merous in domesticated species), actinomorphic, all perfect. Pedicels erect, slightly spreading or pendent, geniculate at their distal end or non-geniculate. Calyx truncate, entire, circular or five-angled in outline, often with 3–10 appendages. Corolla stellate, rotate-stellate, campanulate or campanulate-urceolate, entirely white, yellow, violet or fuchsia or with greenish-yellow and/or maroons or purple spots within, rarely entirely greenish-white or mostly purple, the lobes spreading or reflexed at anthesis, usually with interpetalar membrane. Stamens five (up to eight in domesticated species), usually equal (rarely unequal), the filaments glabrous and broadened at the base to form a staminal plaque fused to the corolla base, each plaque with two short lateral auricles, the anthers dorsifixed, ellipsoid or ovoid, yellow, cream or blue to purple, connivent in pre-anthesis, usually free when mature, dehiscent by longitudinal slits. Gynoecium usually bicarpellate, rarely 3–4-carpellate; ovary superior, glabrous, subglobose to ovoid (rarely ellipsoid), with an annular nectary at the base; styles straight or slightly curved, cylindrical or clavate, glabrous, commonly exserted beyond the anthers, sometimes heteromorphic (long, medium and short styles); stigma globose or discoid, sometimes somewhat bilobed, finely papillate. Fruit glabrous berry, globose, subglobose or somewhat elongate, the mesocarp juicy, the pericarp red, orange-red, greenish-golden yellow or, rarely, dark burgundy or purple-blue at maturity (in domesticated species, fruits of various shapes and colours), pungent or not; fruiting pedicels erect or deflexed; fruiting calyx discoid or campanulate, not accrescent or slightly accrescent. Seeds flattened to slightly angled, mostly C- or D-shaped, subglobose or ellipsoid (rarely reniform or teardrop-shaped), pale yellow to yellow, brownish-yellow to brown or brownish-black to black, seed coat smooth, reticulate or reticulate marginally tuberculate. Stone cells absent or present, if present, not more than six. Embryo usually imbricate (less frequently annular or coiled); endosperm firm, whitish and relatively abundant. Chromosome number: 2n = 24, 26 (see Table
Distribution
(Fig.
Artificial key to Capsicum
| 1 | Stem and mature leaf blades completely glabrous, if trichomes present, sparsely distributed on the veins and margins | 2 |
| – | Stem and mature leaf blades variously pubescent | 10 |
| 2 | Calyx appendages (3–) 5, strongly incurved; flowering pedicels slightly winged and conspicuously winged in fruit; leaves coriaceous; Bolivia | C. ceratocalyx |
| – | Calyx appendages absent or up to 10, straight; flowering and fruiting pedicels not winged; leaves coriaceous or membranous | 3 |
| 3 | Corolla tubular-campanulate to broadly campanulate, lobed less than 1/3 of the way to the base | 4 |
| – | Corolla usually stellate or stellate-campanulate, lobed 1/3 up to nearly halfway to the base | 6 |
| 4 | Calyx appendages 10, unequal (5 long, 5 short); corolla lobes recurved; fruits pungent; seeds 3–4 (–5) mm long, pale yellow to nearly white; Bolivia | C. caballeroi |
| – | Calyx appendages 2–5, equal or subequal; corolla lobes erect; fruits non-pungent; seeds 1.5–2.5 mm long, brown to black | 5 |
| 5 | Leaves coriaceous; calyx appendages (2–) 3–5, spreading or reflexed; filaments 1–2.5 mm long; corolla broadly campanulate; Colombia and Ecuador | C. lycianthoides |
| – | Leaves membranous; calyx appendages 5, erect; filaments 3–5 mm long; corolla tubular-campanulate; Peru | C. piuranum |
| 6 | Inflorescences with 5–13 flowers on an elongate rachis, sometimes rachis forked; fruiting calyx with a conspicuous annular constriction at the junction with the pedicel; fruiting pedicels erect, brilliant dark purple; fruits dark blue to purple; Colombia, Ecuador and Peru | C. regale |
| – | Inflorescences with 3–7 (–9) axillary flowers, rarely on a very short unbranched rachis or flowers solitary; fruiting calyx without an annular constriction at the junction with the pedicel; fruiting pedicels pendent or rarely curved, green or greenish-purple; fruits of other colours | 7 |
| 7 | Calyx appendages absent or 5, minute (< 0.5 mm long); corolla tube and base of the lobes with a sparse but continuous ring of glandular trichomes adaxially; fruits greenish-golden yellow; Brazil | 8 |
| – | Calyx appendages 2–10, longer (1–5 mm long); corolla tube and base of the lobes glabrous adaxially; fruits orange or greenish-golden yellow | 9 |
| 8 | Leaves membranous, elliptic to ovate; flowering pedicels 9–14 mm long, erect, geniculate at anthesis; corolla small, 4.5–6.5 (–8) mm long; stamens unequal (3+2); ovules 2 per locule; fruits 4-seeded; Brazil (Rio de Janeiro, Minas Gerais, Espírito Santo) | C. campylopodium |
| – | Leaves coriaceous, elliptic to narrowly elliptic, flowering pedicels 15–30 mm long, pendent, non-geniculate at anthesis; corolla larger, 9–10 mm long; stamens equal; ovules more than 2 per locule; fruits multi-seeded (up to 20 seeds); Brazil (Bahía, Espírito Santo, Minas Gerais, São Paulo) | C. pereirae |
| 9 | Major leaves narrowly elliptic; calyx appendages 2–3, triangular-compressed wings; flowering pedicels 3–8 mm long, pendent, non-geniculate; corolla 6–8.5 mm long, 8–11 mm in diameter, entirely yellow or with red-brown pigmentation within; fruits orange, non-pungent; seeds 1.7–2.3 mm long; Peru and Ecuador | C. longifolium |
| – | Major leaves ovate to elliptic; calyx appendages 5 (6–10), cylindrical; flowering pedicels (13–) 20–38 (–48) mm long, erect to spreading, geniculate; corolla 10–14 (–16) mm long, (10–) 15–23 mm in diameter, white with diffuse brown-purple spots and a greenish-yellow centre within; fruits greenish-golden yellow, pungent; seeds 2.5–3.2 mm long; Brazil | C. hunzikerianum |
| 10 | Pubescence mostly of branched eglandular or glandular trichomes, few simple trichomes | 11 |
| – | Pubescence mostly of simple eglandular trichomes, rarely furcate eglandular trichomes or simple glandular trichomes | 13 |
| 11 | Dense pubescence of long furcate and simple glandular trichomes; calyx appendages usually 10 (rarely 5 or up to 12); filaments 2.5–3 mm long; flowering and fruiting pedicels erect; fruits pungent; Bolivia | C. eshbaughii |
| – | Dense pubescence of furcate to dendritic eglandular trichomes mixed with few simple eglandular trichomes; calyx appendages usually 5 (rarely 3–4 or 6); filaments 1.2–2.3 mm long; flowering and fruiting pedicels pendent; fruits non-pungent | 12 |
| 12 | Calyx appendages (4.5–) 5–8.5 mm long; corolla stellate, lobed almost halfway to the base, white with dark greenish-yellow spots within, with small glandular trichomes adaxially; style exserted ca. 1 mm beyond the anthers; seeds < 20 per fruit, 3–3.7 mm long, 2.5–2.8 mm; inflorescences usually (1–) 2–5-flowered; Brazil | C. longidentatum |
| – | Calyx appendages 0.9–3 mm long; corolla campanulate or campanulate-rotate, shallowly lobed, entirely yellow or sometimes tinged greenish within, glabrous adaxially; style barely exserted beyond the anthers; seeds > 20 per fruit, 2.4–2.8 mm long, 1.8–2.2 mm wide; inflorescences usually (1–) 3–8 (–13)-flowered; Mexico to Peru | C. rhomboideum |
| 13 | Corolla nearly lobed to the base, the tube 4–4.5 times shorter than the lobes; Ecuador | C. benoistii |
| – | Corolla shallowly lobed or lobed halfway or to 2/3 of the way to the base, the tube as long as the lobes or 1.5 times shorter than the lobes | 14 |
| 14 | Staminal plaques with conspicuous auricles not fused to the corolla at the point of insertion of the filaments; Bolivia, Argentina and Paraguay | C. chacoense |
| – | Staminal plaques with inconspicuous auricles fused to the corolla at the point of insertion of the filaments | 15 |
| 15 | Major leaves narrowly elliptic to lanceolate, the length/width ratio 5–10 (–16) | 16 |
| – | Major leaves ovate or elliptic, if elliptic the length/width ratio (2–) 2.5–4 (–4.5) | 17 |
| 16 | Calyx appendages 5, erect, green; corolla stellate, white with large purple spots and a cream centre within, with glandular trichomes in the throat and lobes adaxially; flowering pedicels erect to spreading, geniculate at anthesis; anthers blue; berry 6–7 mm in diameter, greenish-golden yellow, pungent; seeds 3.5–4 mm long, 2.5–3 mm wide; Brazil | C. carassense |
| – | Calyx appendages (2–) 3–5, spreading or erect, green, greenish-purple or purple; corolla campanulate, entirely yellow or yellow with smaller maroon or purple spots within, glabrous adaxially; flowering pedicels pendent, non-geniculate at anthesis; anthers cream, yellow or rarely white; berry 7–12 mm in diameter, pale orange or orange, non-pungent; seeds 1.8–2.3 mm long, 1.3–1.5 mm wide; Colombia, Ecuador and Peru | C. geminifolium |
| 17 | Flowering pedicels pendent, non-geniculate at anthesis | 18 |
| – | Flowering pedicels erect to slightly spreading, geniculate at anthesis | 29 |
| 18 | Flowers 5–7-merous; calyx thick, strongly 5–10-nerved; style heteromorphic (included at the same level as the stamens or exserted); corolla usually entirely white, dull white or greenish-white, rarely entirely purple or pale yellow; seeds pale yellow or nearly white, the seed coat smooth; fruits of various shape, size and colour; widely cultivated in the Americas | 19 |
| – | Flowers 5-merous (rarely 4-merous); calyx usually thin, comparatively weakly 5–10-nerved; style usually homomorphic, rarely dimorphic (included and exserted); corolla entirely yellow, yellow with maroon or purple spots within, white with greenish-yellow spots within or primarily purple or lilac; seeds usually brown or brownish-black to black, rarely yellow or pale yellow, the seed coat uniformly reticulate or reticulate and tuberculate at margins; fruits usually globose or subglobose, not more than 16 mm in diameter, orange to red or greenish-golden yellow; wild species, mostly from South America | 20 |
| 19 | Flowers solitary, rarely in pairs or more; petioles up to 10 cm long; corolla 8–15 mm long, entirely white, rarely entirely purple or pale yellow; fruiting calyx without a prominent annular constriction at junction with the pedicel | C. annuum var. annuum |
| – | Flowers 2–4 (–5); petioles up to 3.5 cm long; corolla (5–) 6.5–8 mm long, entirely dull white or greenish-white (occasionally with purple spots); fruiting calyx with a prominent annular constriction at junction with the pedicel | C. chinense |
| 20 | Corolla tubular-campanulate, 14.5–17 mm long; calyx appendages 5; stone cells 2; northern Peru | C. piuranum |
| – | Corolla campanulate, campanulate-stellate, rotate-stellate or stellate, 4.5–12 (–15) mm long; calyx appendages absent or up to 10; stone cells absent or up to 6 | 21 |
| 21 | Calyx appendages absent or up to 5, equal and minute, < 1 mm long | 22 |
| – | Calyx appendages 2–10, subequal or unequal, > 1 mm long | 25 |
| 22 | Young stems, leaves and calyx with simple eglandular trichomes mixed with small dark glandular trichomes | 23 |
| – | Young stems, leaves and calyx only with simple eglandular trichomes, glandular trichomes absent | 24 |
| 23 | Corolla campanulate to campanulate-stellate, lobed less than ⅓ of the way to the base; style dimorphic, short style 1–1.6 mm, long style 4.1–4.2 mm long; flowering pedicels 3–10 mm long; inflorescences few-flowered (2–8 flowers); fruiting pedicels erect, green; central Peru | C. tovarii |
| – | Corolla stellate, lobed nearly halfway to the base; style homomorphic, 4.3–4.8 mm long; flowering pedicels longer, 7–21 (–28) mm long; inflorescences multi-flowered (5–13 flowers or up to 20 or more); fruiting pedicels pendent, green or purple; north-eastern Brazil | C. caatingae |
| 24 | Leaf pair strongly dissimilar in shape and size; flower buds ovoid, purple or yellowish; fruits non-pungent; seeds 1.9–2.7 mm long, 1.8–2.1 mm wide; corolla entirely yellow or with purple or maroon spots within; Colombia, Ecuador and Peru | C. dimorphum |
| – | Leaf pair similar or dissimilar in size, similar in shape; flower buds globose, white with green spots; fruits pungent; seeds 2.8–3.4 mm long, 2.2–3 mm wide; corolla white with greenish-yellow spots, rarely also with purple spots; Argentina, Paraguay and Brazil | C. flexuosum |
| 25 | Calyx appendages 8–10, unequal | 26 |
| – | Calyx appendages 2–5 (–7), subequal | 27 |
| 26 | Leaves coriaceous; inflorescences (1–) 2-flowered; flowering pedicels 20–40 (–50) mm long; corolla ≥ 10 mm long, 4–6 mm in diameter; filaments 4–6 mm long; style 7–9 mm long; fruits pungent; fruiting calyx appendages appressed to the berry; Bolivia | C. caballeroi |
| – | Leaves membranous; inflorescences with (1–) 2–7 flowers; flowering pedicels shorter, 8–15 mm long; corolla 7.5–9 mm long, 8–10 mm in diameter, filaments (1.5–) 1.8–2 mm long; style ca. 4 mm long; fruits non-pungent; fruiting calyx appendages spreading or reflexed; Ecuador and Peru | C. hookerianum |
| 27 | Leaf pair dissimilar in size, similar in shape; major leaves ovate; corolla stellate, lobed nearly halfway to the base; fruits greenish-golden yellow, pungent; seeds 3–3.8 mm long, 2.7–3 mm wide; Colombia, Venezuela and Brazil | C. parvifolium |
| – | Leaf pair markedly dissimilar in size and shape, rarely similar in shape; major leaves elliptic to lanceolate; corolla campanulate, lobed ⅓ of the way to the base; fruits orange to orange-red, non-pungent; seeds 1.8–2.8 mm long, 0.8–1.8 mm wide | 28 |
| 28 | Mature leaves glabrous adaxially; flowers solitary, rarely 2 per node; calyx appendages (4–) 5, spreading or strongly reflexed; corolla purple with white interpetalar membrane; North America (Mexico) and Central America (Guatemala and Honduras) | C. lanceolatum |
| – | Mature leaves sparse to densely pubescent adaxially; flowers 2–5 (–6) per node; calyx appendages 2–3 (–5), erect or spreading; corolla entirely yellow or with purple or maroon pigmentation within and yellow interpetalar membrane; South America: Colombia, Ecuador and Peru | C. geminifolium |
| 29 | Corolla broadly campanulate, campanulate-urceolate, rotate or rotate-stellate, lobed 1/3 or less of the way to the base | 30 |
| – | Corolla stellate, lobed more than 1/3 up to 2/3 of the way to the base | 36 |
| 30 | Stems and mature leaves with minute dark simple glandular trichomes mixed with sparse eglandular trichomes; corolla broadly campanulate or campanulate-urceolate | 31 |
| – | Stems and mature leaves lacking minute dark glandular trichomes mixed with abundant or sparse eglandular trichomes; corolla rotate or rotate-stellate | 32 |
| 31 | Major leaves (5.5–) 8.5–13 (–21) cm long; corolla campanulate-urceolate, entirely fuchsia or violet, glabrous adaxially, the lobes spreading to strongly recurved; fruits greenish-golden yellow, slightly pungent; seeds brownish-black to black; Brazil | C. friburgense |
| – | Major leaves 3–5 (–6) cm long; corolla campanulate, almost completely violet or lilac and a greenish-yellow to white centre within, with short glandular trichomes adaxially, the lobes erect or spreading; fruits red, pungent; seeds pale yellow to brownish-yellow; Bolivia | C. cardenasii |
| 32 | Flowers 4–8-merous; corolla 8.5–15 mm long; fruiting pedicels pendent; fruits large, > 10 mm in diameter, persistent, variously coloured; cultivated in the Americas | 33 |
| – | Flowers 5-merous (rarely 4-merous); corolla 4–7 mm long; fruiting pedicels erect; fruits small, < 10 mm in diameter, deciduous, orange or red; wild or semi-domesticated in South America | 35 |
| 33 | Leaves densely pubescent, rarely glabrescent; flower buds dark purple on pendent pedicels; style clavate; seeds 5.5–7 mm long, 4.8–6 mm wide, brownish-black to black, the seed coat reticulate; corolla dark purple or violet with a white or yellowish-green centre within | C. pubescens |
| – | Leaves glabrous to sparsely pubescent; flower buds greenish-white or purple on geniculate pedicels; style cylindrical; seeds 3–5.2 mm long, 3–4 mm wide, pale yellow to yellow, the seed coat smooth to slightly reticulate; corolla white with large greenish-yellow spots and white centre within | 34 |
| 34 | Fruits pungent, rarely non-pungent, usually elongate, endocarp alveolate, pericarp with giant cells | C. baccatum var. pendulum |
| – | Fruits non-pungent, campanulate-umbilicate, endocarp smooth, pericarp without giant cells | C. baccatum var. umbilicatum |
| 35 | Leaves with dense pubescence, especially abaxially; corolla marginally purple with greenish-yellow centre; Brazil and Paraguay | C. rabenii |
| – | Leaves mostly glabrescent, more rarely moderately pubescent; corolla white with greenish-yellow spots within, purple pigmentation absent; widely distributed across South America | C. baccatum var. baccatum |
| 36 | Flowers 4–8-merous; style usually heteromorphic (three different lengths), when homomorphic carpels 2; fruits of various size, shape and colours; mostly cultivated or semi-domesticated plants across the Americas | 37 |
| – | Flowers always 5-merous; style homomorphic; fruits small, not more than 15 mm in diameter, globose or subglobose, most rarely ellipsoid or ovoid, usually red or red-orange or greenish-golden yellow; wild plants from South America | 41 |
| 37 | Calyx appendages absent or if present, minute, ≤ 0.5 mm long | 38 |
| – | Calyx appendages > 0.5 mm long | 40 |
| 38 | Flowers solitary, rarely in pairs or more; petioles up to 10 cm long; corolla 8–15 mm long, entirely white, rarely entirely purple or pale yellow; fruits usually large, up to 300 mm long, pungent or non-pungent | C. annuum var. annuum |
| – | Flowers (1–) 2–5; petioles up to 3.5 cm long; corolla 3.75–8 mm long, entirely dull white or greenish-white (occasionally with purple spots); fruits small to medium-sized, up to 100 mm long, pungent, rarely non-pungent | 39 |
| 39 | Corolla glabrous adaxially; style heteromorphic; fruits highly variable in shape (domesticated), with the base obtuse or truncate (fruits subglobose in wild populations); fruiting calyx discoid or shallowly cup-shaped, with a prominent annular constriction at junction with the pedicel; ovary subglobose, 2–2.5 mm long, 2.5–3.5 mm in diameter | C. chinense |
| – | Corolla with small glandular trichomes adaxially; style homomorphic; fruits usually elongate and narrowly triangular, with the base abruptly narrowed; fruiting calyx deeply cup-shaped lacking a constriction at junction with the pedicel; ovary oblong-ovoid, 2.5–4 mm long, 1.3–1.8 mm in diameter | C. frutescens |
| 40 | Young leaves rugose; flowers 4–8-merous; corolla 10–15 mm long, 15–22 (–25) mm in diameter; style heteromorphic; fruiting pedicels pendent; fruits > 10 mm in diameter, persistent; seeds 5.5–7 mm long, 4.8–6 mm wide, brownish-black to black, the seed coat reticulate; cultivated from Mexico to Bolivia | C. pubescens |
| – | Young leaves plane; flowers 5-merous, rarely 4-merous; corolla 6–7 mm long, (9–) 12–15 mm in diameter; style homomorphic; fruiting pedicels erect; fruits < 10 mm in diameter, deciduous; seeds 3–4.2 mm long, 2.5–2.8 mm wide, yellow, the seed coat smooth. Wild or semi-domesticated; Brazil and Paraguay | C. rabenii |
| 41 | Androecium heterodynamous (three long and two short filaments); ovules two per locule; fruits 4-seeded; corolla white or cream with golden yellow or ochraceous spots within; Brazil | C. campylopodium |
| – | Androecium homodynamous (filaments equal or slightly subequal); ovules more than two per locule; fruits many-seeded; corolla variously coloured | 42 |
| 42 | Calyx appendages absent or five, minute, < 0.5 mm long | 43 |
| – | Calyx appendages 2–10, the main appendages ≥ 0.5 mm long | 47 |
| 43 | Filaments < 2 mm long; fruiting pedicels erect; seeds pale yellow, yellow or brownish-yellow; fruits yellow, red-orange or red | 44 |
| – | Filaments ≥ 2 mm long; fruiting pedicels pendent; seed brownish-black to black; fruits greenish-golden yellow | 46 |
| 44 | Flowers 4–13 (–18) on a short rachis; style clavate; seeds 4–4.6 mm long, (–2.8) 3.2–3.75 mm wide, yellow to brownish-yellow; sprawling vines or scrambling shrubs, with stems to 7 m long; Peru, Bolivia and Brazil | C. coccineum |
| – | Flowers 1–2 per axil, rarely up to 3; style cylindrical; seeds 3–4 mm long, 2.5–3.2 mm wide, pale yellow to yellow; perennial herbs or low shrubs or subshrubs up to 2 m, rarely larger | 45 |
| 45 | Calyx circular in outline; corolla 4–5 mm long, ca. 6 mm in diameter, with glandular trichomes adaxially; style 2.25–2.5 mm long, exserted 0.5–0.8 mm beyond the anthers; seed coat smooth; plants densely pubescent, the trichomes spreading; Ecuador: Galapagos Islands | C. galapagoense |
| – | Calyx pentagonal in outline; corolla (5–) 6–8 mm long, 8–10 (–12) mm in diameter, glabrous adaxially; style 4–4.8 mm long, exserted 1.5–2 mm beyond the anthers; seed coat reticulate to obscurely reticulate; plants glabrescent to densely pubescent, the trichomes appressed-antrorse, sometimes spreading; widespread in the Americas | C. annuum var. glabriusculum |
| 46 | Stems, leaves, pedicels and calyx densely pubescent with long spreading eglandular trichomes 0.5–2 mm long; major leaves elliptic to narrowly elliptic; corolla white with large greenish-yellow spots and sparse diffuse purple or brown spots within, the lobes widely triangular; Brazil (Rio de Janeiro) | C. muticum |
| – | Stems, leaves, pedicels and calyx glabrescent to moderately pubescent, with short antrorse eglandular trichomes 0.25–0.5 mm long; major leaves elliptic to ovate; corolla white with large or small purple or brownish spots and large greenish-yellow spots within (in some populations purple or brownish pigmentation completely absent); the lobes triangular or ovate; Brazil (Minas Gerais, Rio de Janeiro and São Paulo) | C. schottianum |
| 47 | Leaves coriaceous; calyx appendages strongly incurved, flattened laterally; Bolivia | C. ceratocalyx |
| – | Leaves membranous; calyx appendages straight or recurved, filiform or cylindrical | 48 |
| 48 | Fruiting pedicels usually erect, rarely pendent; fruits red; seeds nearly white or yellow to brown | 49 |
| – | Fruiting pedicels pendent; fruits greenish-golden yellow; seeds brownish-black to black | 52 |
| 49 | Calyx strongly 10-nerved with prominent venation; seeds pale yellow to nearly white, the seed coat smooth and reticulate at margins; Bolivia | C. neei |
| – | Calyx slightly 5-nerved with inconspicuous venation; seeds yellow to brown, the seed coat faintly reticulate and slightly tuberculate at margins | 50 |
| 50 | Inflorescences many-flowered (4–18 flowers); pedicel scars prominent, corky; filaments < 2 mm long; sprawling vines or scrambling shrubs; Peru, Bolivia and Brazil | C. coccineum |
| – | Inflorescences few-flowered (up to 5 flowers); pedicels scars inconspicuous; filaments ≥ 2 mm long; erect shrubs or subshrubs | 51 |
| 51 | Calyx appendages 5, 1–1.5 mm long; filaments 2–2.5 mm long; corolla yellow with small and faint greenish-yellow spots within; seeds 4–4.5 mm long, 3–3.5 mm wide, dark brown; Bolivia | C. minutiflorum |
| – | Calyx appendages (4–) 5, 1.2–2.7 (–3) mm long; filaments 2.7–3.8 mm long; corolla lilac, purple or magenta with a continuous greenish-yellow or ochre tube within, sometimes the corolla white with greenish-yellow spots; seeds 2.5–4 (–4.2) mm long, 2.1–3 mm wide, brownish-yellow; Bolivia and Argentina | C. eximium |
| 52 | Calyx appendages five | 53 |
| – | Calyx appendages 10 or 6–10, rarely five | 54 |
| 53 | Plants densely pubescent, the stem trichomes spreading; Brazil: Rio de Janeiro, Minas Gerais and São Paulo | C. villosum |
| – | Plants glabrescent to moderately pubescent, the stem trichomes antrorse; Brazil: Bahia, Espírito Santo, Minas Gerais, Rio de Janeiro and São Paulo | C. mirabile |
| 54 | Calyx appendages 10, subequal; filaments 3–3.2 mm long; style barely exserted beyond the anthers; anthers lilac or pale blue; corolla almost entirely purple; Brazil (São Paulo) | C. mirum |
| – | Calyx appendages ranging from 5 to 10, unequal; filaments 1.4–2.5 mm long; style exserted 0.8–1.3 mm beyond the anthers; anthers yellow, light green or grey; corolla white with greenish-yellow or purple spots | 55 |
| 55 | Flowering calyx appendages cylindrical or triangular-compressed, glabrous to moderately pubescent with antrorse trichomes, the longest appendages 1–2.5 mm; corolla 6–7 mm long, ca. 11 mm in diameter, white with greenish-yellow spots within; style 3.2–3.5 mm long; fruiting calyx appendages strongly recurved; fruiting pedicels 18–25 mm long; Brazil (Minas Gerais, Paraná, Rio de Janeiro, Santa Catarina, and São Paulo) | C. recurvatum |
| – | Flowering calyx appendages linear or subulate, densely pubescent with spreading trichomes, the longest appendages 2.5–5 (–6) mm long; corolla (8–) 9–14 mm long, 18–22 mm in diameter, white with purple or reddish-brown spots within; style 4–6.8 mm; fruiting calyx appendages spreading; fruiting pedicels (25–) 30–38 mm long; Brazil (São Paulo and Rio de Janeiro) | C. cornutum |
Key to the wild Capsicum species from North America, Central America and the Caribbean
| 1 | Leaf pair subequal in size and shape; calyx appendages absent or five, minute, < 0.5 mm long; corolla (5–) 6–8 mm long, stellate, lobed nearly halfway or up to 2/3 of the way to the base, entirely white or almost pale yellow, rarely greenish-white; style cylindrical; flowering and fruiting pedicels erect; fruits pungent; seeds pale yellow to yellow; perennial herbs or prostrate subshurb; southern United States of America, Mexico, Central America and the Caribbean islands | C. annuum var. glabriusculum |
| – | Leaf pairs markedly dissimilar in size and shape; calyx appendages (3–4) 5, > 0.5 mm long; corolla 5–14 mm long; campanulate or campanulate-rotate, lobed not more than 1/3 of the way to the base, yellow or purple marginally white; style clavate; flowering and fruiting pedicels pendent; fruits non-pungent; seeds brown or brownish-black to black; erect shrubs or shrubs, rarely trees | 2 |
| 2 | Plants glabrescent to densely pubescent with simple, furcate or dendritic trichomes; major leaves ovate, elliptic or rhomboid-ovate; inflorescences of 3–8 (–13) flowers, rarely flowers solitary; calyx appendages erect or spreading, 0.9–3 mm long; corolla (5–) 6–10 mm long, entirely yellow or with diffuse greenish spots within; Mexico, Guatemala, Honduras, El Salvador, Nicaragua and Costa Rica (also South America) | C. rhomboideum |
| – | Plants glabrous or glabrescent only with simple eglandular trichomes; major leaves elliptic to lanceolate; inflorescence of a solitary flower, rarely two; calyx appendages spreading or strongly reflexed, (2–) 3–5 mm long; corolla 9.8–14 mm long, purple with white margin within; Mexico, Guatemala and Honduras | C. lanceolatum |
Key to the wild Andean and adjacent Andean (Venezuela, Colombia, Ecuador, Peru, Bolivia and Argentina) Capsicum species
| 1 | Stem and mature leaf blades completely glabrous, if trichomes present sparsely distributed on the veins and margins | 2 |
| – | Stem and mature leaf blades variously pubescent | 7 |
| 2 | Calyx appendages (3–) 5, strongly incurved; flowering pedicels slightly winged and conspicuously winged in fruit; leaves coriaceous; Bolivia | C. ceratocalyx |
| – | Calyx appendages absent or up to 10, straight; flowering and fruiting pedicels not winged; leaves coriaceous or membranous | 3 |
| 3 | Corolla tubular-campanulate to broadly campanulate, lobed less than 1/3 of the way to the base | 4 |
| – | Corolla stellate or stellate-campanulate, lobed between 1/3–2/3 of the way to the base | 6 |
| 4 | Calyx appendages 10, unequal (five long, five short); corolla lobes recurved; fruits pungent; seeds 3–4 (–5) mm long, pale yellow to nearly white; Bolivia | C. caballeroi |
| – | Calyx appendages 2–5, equal or subequal; corolla lobes erect; fruits non-pungent; seeds 1.5–2.5 mm long, brown to black | 5 |
| 5 | Leaves coriaceous; calyx appendages (2–) 3–5, spreading or reflexed; filaments 1–2.5 mm long; corolla broadly campanulate; Colombia and Ecuador | C. lycianthoides |
| – | Leaves membranous; calyx appendages five, erect; filaments 3–5 mm long; corolla tubular-campanulate; Peru | C. piuranum |
| 6 | Leaves coriaceous; major leaves narrowly elliptic (length/width ratio 6–10.8); calyx tube membranaceous; stamens equal, 2–2.6 mm long; fruits 8–13 mm in diameter, orange at maturity; fruiting pedicels 10–16 mm long, green, pendent; fruiting calyx green-purple or green; seeds 1.7–2.3 mm long, 1.7–2.2 mm wide, seeds D or teardrop-shaped, the surface reticulate; Peru and Ecuador | C. longifolium |
| – | Leaves membranaceous; major leaves elliptic (length/width ratio 2.5–4); calyx tube fleshy; stamens subequal (one longer), (2–) 3–4.3 mm long; fruits 6–9 mm in diameter, dark blue to purple at maturity; fruiting pedicels ca. 18 mm long, brilliant dark purple, erect; fruiting calyx entirely brilliant purple; seeds 2.75–3.40 mm long, 2.25–2.70 mm wide, C-shaped, the surface smooth and tuberculate at margins; Colombia, Ecuador, and Peru | C. regale |
| 7 | Pubescence mostly of branched eglandular or long forked glandular trichomes, few simple trichomes | 8 |
| – | Pubescence mostly of simple eglandular trichomes, rarely furcate eglandular trichomes or simple long glandular trichomes | 9 |
| 8 | Dense glandular pubescence of long furcate and simple trichomes; calyx appendages usually 10 (rarely 5 or up to 12); corolla stellate, lobed nearly halfway to the base, white with greenish-yellow spots within (sometimes nearly white or with purple spots in the lobes); flowering and fruiting pedicels erect; fruits pungent; inflorescences usually 2–3 (–4)-flowered; Bolivia | C. eshbaughii |
| – | Dense eglandular pubescence of simple, furcate and dendritic trichomes; calyx appendages usually five (rarely 3–4); corolla campanulate or campanulate-rotate, shallowly lobed, entirely yellow or sometimes tinged greenish within; flowering and fruiting pedicels pendent; fruits non-pungent; inflorescences usually 3–8 (–13)-flowered; Venezuela to Peru (also in Mexico and Central America) | C. rhomboideum |
| 9 | Corolla nearly lobed up to the base, tube 4–4.5 times shorter than the lobes; Ecuador | C. benoistii |
| – | Corolla shallowly lobed or lobed halfway or up to 2/3 of the way to the base, tube as long as the lobes or 1.5 times shorter than the lobes | 10 |
| 10 | Calyx appendages (3–) 5, strongly incurved; flowering pedicels nearly winged and conspicuously winged in fruit; leaves coriaceous; Bolivia | C. ceratocalyx |
| – | Calyx appendages absent or up to 10, straight; flowering and fruiting pedicels not winged; leaves coriaceous or membranous | 11 |
| 11 | Young stems, lower surface of the leaves and calyx with small dark glandular trichomes mixed with simple eglandular trichomes; corolla primarily purple, violet or lilac; fruits pungent | 12 |
| – | Young stems, leaves and calyx only with simple eglandular trichomes, glandular trichomes absent; corolla entirely yellow or nearly white or primarily purple and usually with maroon, purple or greenish-yellow pigmentation within; fruits pungent or non-pungent | 13 |
| 12 | Calyx appendages absent or five, minute ≤ 0.5 mm long; leaves moderately to densely pubescent; flowering pedicels usually pendent, non-geniculate at anthesis, 3–10 mm long; style dimorphic; fruiting pedicels erect; Peru | C. tovarii |
| – | Calyx appendages five, 1–2 mm long; leaves glabrescent; flowering pedicels usually erect, geniculate at anthesis, 8–18 (–22) mm long; style homomorphic; fruiting pedicels pendent; Bolivia | C. cardenasii |
| 13 | Corolla tubular-campanulate or campanulate, lobed less than 1/3 of the way to the base | 14 |
| – | Corolla stellate or rotate-stellate, lobed between 1/3–2/3 of the way to the base | 17 |
| 14 | Calyx appendages 8–10, unequal | 15 |
| – | Calyx appendages 2–5, equal or subequal | 16 |
| 15 | Leaves coriaceous; inflorescences 2-flowered or flowers solitary; flowering pedicels 20–40 (–50) mm long; corolla ≥ 10 mm long, 4–6 mm in diameter; filaments 4–6 mm long; style 7–9 mm long; fruits pungent; fruiting calyx appendages appressed to the berry; seeds pale yellow to nearly white; Bolivia | C. caballeroi |
| – | Leaves membranous; inflorescences with 2–7 flowers, rarely flowers solitary; flowering pedicels shorter, 8–15 mm long; corolla 7.5–9 mm long, 8–10 mm in diameter, filaments (1.5–) 1.8–2 mm long; style ca. 4 mm long; fruits non-pungent; fruiting calyx appendages spreading or reflexed; seeds yellow or brown; Ecuador and Peru | C. hookerianum |
| 16 | Calyx appendages five, erect; corolla tube narrow, ca. 6 mm in diameter; filaments 3–5 mm long; mature leaves glabrescent adaxially; stone cells two; northern Peru | C. piuranum |
| – | Calyx appendages 2–3 (–5), erect or spreading; corolla tube broad, 8–10 mm in diameter; filaments 2–3 mm; mature leaves sparse to densely pubescent adaxially; stone cells 1–5 or absent; Colombia, Ecuador and Peru | C. geminifolium |
| 17 | Flowers 4–13 (–18) on a short rachis; pedicel scars prominent, corky; fruiting calyx usually recurved; sprawling vines or scrambling shrubs; Peru, Bolivia (also Brazil) | C. coccineum |
| – | Flowers 2–6 (–8) per axil, rarely flowers solitary; pedicel scars usually inconspicuous, rarely prominent; fruiting calyx not recurved; usually scandent or erect shrubs or subshrubs, rarely perennial herbs | 18 |
| 18 | Calyx appendages absent or minute, ≤ 1 mm long | 19 |
| – | Calyx appendages (4–) 5–10, > 1 mm long | 21 |
| 19 | Leaf pair strongly dissimilar in shape and size; flower buds ovoid, purple or yellowish; fruits non-pungent; seeds 1.9–2.7 mm long, 1.8–2.1 mm wide, brownish-black to black; corolla entirely yellow or with purple or maroon spots within; Colombia, Ecuador and Peru | C. dimorphum |
| – | Leaf pair similar or dissimilar in size, similar in shape; flower buds globose or ovoid, white cream or greenish-white; fruits pungent; seeds 3.2–4 mm long, 2.5–4 mm wide, pale yellow to yellow; corolla entirely white or pale yellow, rarely greenish-white | 20 |
| 20 | Calyx circular in outline; corolla 4–5 mm long, ca. 6 mm in diameter, with glandular trichomes adaxially; style 2.25–2.5 mm long, exserted 0.5–0.8 mm beyond the anthers; seed coat smooth; plants densely pubescent, the trichomes spreading; endemic; Ecuador, Galapagos Islands | C. galapagoense |
| – | Calyx pentagonal in outline; corolla (5–) 6–8 mm long, 8–10 (–12) mm in diameter, glabrous adaxially; style 4–4.8 mm long, exserted 1.5–2 mm beyond the anthers; seed coat reticulate to obscurely reticulate; plants glabrescent to densely pubescent, the trichomes appressed-antrorse, sometimes spreading; widespread; Colombia, Venezuela, Ecuador, Peru, Bolivia (also in North and Central America, the Caribbean Islands and Brazil | C. annuum var. glabriusculum |
| 21 | Flowers solitary; corolla entirely white; filaments with conspicuous auricles not fused to the corolla at the point of insertion; Bolivia and Argentina (also Paraguay) | C. chacoense |
| – | Flowers 2–8 per axil, rarely solitary; corolla white, purple or yellow with greenish-yellow spots or greenish-yellow centre within; filaments with inconspicuous auricles fused to the corolla at the point of the insertion | 22 |
| 22 | Flowering pedicels pendent, non-geniculate at anthesis; calyx appendages 5–10 | 23 |
| – | Flowering pedicels erect, geniculate at anthesis; calyx appendages (4–) 5 | 24 |
| 23 | Calyx appendages always 10; calyx tube strongly 10-nerved with prominent venation; pedicels scars inconspicuous; corolla entirely yellow or with small greenish-yellow spots within; fruits red; seeds 4–5 mm long, 3–4.25 mm wide, pale yellow to white; Bolivia | C. neei |
| – | Calyx appendages 5 (–7); calyx tube strongly 5-nerved with prominent venation; pedicels scars prominent; corolla purple with a narrow white margin and yellowish-green centre; fruits greenish-golden yellow; seeds 3–3.8 mm long, 2.7–3 mm wide, brownish-black; Colombia and Venezuela (also Brazil) | C. parvifolium |
| 24 | Corolla rotate-stellate; white with greenish-yellow spots within; seeds pale yellow to yellow; fruits globose, subglobose or ellipsoid; Colombia to Argentina (also Paraguay and Brazil) | C. baccatum var. baccatum |
| – | Corolla stellate; primarily yellow or lilac, purple or magenta; seeds brownish-yellow or dark brown; fruits globose | 25 |
| 25 | Calyx appendages five, 1–1.5 mm long; filaments 2–2.5 mm long; corolla yellow with small and faint greenish-yellow spots within; seeds 4–4.5 mm long, 3–3.5 mm wide, dark brown; Bolivia | C. minutiflorum |
| – | Calyx appendages (4–) 5, 1.2–2.7 (–3) mm long; filaments 2.7–3.8 mm long; corolla lilac, purple or magenta with a continuous greenish-yellow or ochre tube within, sometimes the corolla white with greenish-yellow spots; seeds 2.5–4 (–4.2) mm long, 2.1–3 mm wide, brownish-yellow; Bolivia and Argentina | C. eximium |
Key to the wild Capsicum species occurring in Brazil, Paraguay and Argentina
| 1 | Stem and mature leaf blades completely glabrous, if trichomes present, sparsely distributed on the veins and margins | 2 |
| – | Stem and mature leaf blades variously pubescent | 4 |
| 2 | Calyx appendages five (6–10), unequal, 1–5 mm long; corolla 10–14 (–16) mm long; Brazil (São Paulo) | C. hunzikerianum |
| – | Calyx appendages absent or five, minute, < 0.5 mm long; corolla 4.5–10 mm long | 3 |
| 3 | Leaves membranous, elliptic to ovate; flowering pedicels 9–14 mm long, erect, geniculate at anthesis; corolla small, 4.5–6.5 (–8) mm long; stamens unequal (3+2); ovules 2 per locule; fruits 4-seeded; Brazil (Rio de Janeiro, Minas Gerais, Espírito Santo) | C. campylopodium |
| – | Leaves coriaceous, elliptic to narrowly elliptic, flowering pedicels 15–30 mm long, pendent, non-geniculate at anthesis; corolla larger, 9–10 mm long; stamens equal; ovules more than 2 per locule; fruits multi-seeded (up to 20 seeds); Brazil (Bahía, Espírito Santo, Minas Gerais, São Paulo) | C. pereirae |
| 4 | Pubescence of furcate to dendritic eglandular trichomes mixed with few simple eglandular trichomes; fruits non-pungent; calyx appendages long, (4.5–) 5–8.5 mm long; Brazil (Bahía, Minas Gerais, Pernambuco) | C. longidentatum |
| – | Pubescence of simple eglandular trichomes, rarely furcate trichomes; fruits usually pungent; calyx appendages absent or, if present, up to 6 mm long | 5 |
| 5 | Corolla campanulate-urceolate, rotate or rotate-stellate, lobed less than 1/3 of the way to the base | 6 |
| – | Corolla stellate, lobed more than 1/3 up to 2/3 of the way to the base | 8 |
| 6 | Corolla campanulate-urceolate, entirely fuchsia or violet, glabrous adaxially, the lobes spreading to strongly recurved; fruiting pedicels pendent; fruits greenish-golden yellow, slightly pungent; seeds brownish-black to black; Brazil (Rio de Janeiro) | C. friburgense |
| – | Corolla rotate or rotate-stellate, white or primarily purple or lilac with greenish-yellow pigmentation within, with glandular trichomes adaxially; the lobes spreading; fruiting pedicels erect; fruits usually red, pungent; seeds pale yellow to yellow | 7 |
| 7 | Leaves with dense pubescence, especially underneath; corolla marginally purple or lilac with greenish-yellow centre; Brazil and Paraguay | C. rabenii |
| – | Leaves mostly glabrescent, more rarely moderately pubescent; corolla white with greenish-yellow spots within, purple pigmentation absent; widely distributed across South America | C. baccatum var. baccatum |
| 8 | Calyx appendages absent or up to five, minute, < 0.5 mm long | 9 |
| – | Calyx appendages 2–10, > 0.5 mm long | 16 |
| 9 | Flowering pedicels pendent, non-geniculate at anthesis | 10 |
| – | Flowering pedicels erect, geniculate at anthesis | 11 |
| 10 | Young stems, leaves and calyx with simple eglandular trichomes mixed with small dark glandular trichomes; inflorescences multi-flowered (5–13 flowers or up to 20 or more); corolla purple with a white margin within; fruiting pedicels green or purple, with a constriction at the junction with the calyx; seeds pale yellow; Brazil | C. caatingae |
| – | Young stems, leaves and calyx only with simple eglandular trichomes, glandular trichomes absent; inflorescences few-flowered (2–6 flowers), rarely solitary flowers; corolla white with greenish-yellow spots, rarely also with purple spots; fruiting pedicels green, without a constriction at the junction with the calyx; seeds brownish-black; Argentina, Paraguay and Brazil | C. flexuosum |
| 11 | Flowers 4–13 (–18) on a short rachis; pedicels scars prominent, corky; fruiting calyx usually recurved; seeds 4–4.6 mm long, (–2.8) 3.2–3.75 mm wide, yellow to brownish-yellow; sprawling vines or scrambling shrubs, with stems to 7 m long; Brazil (also Peru and Bolivia) | C. coccineum |
| – | Flowers 2–5 (–7) per axil, rarely solitary; pedicels scars inconspicuous; fruiting calyx not recurved; seeds 2–4 mm long, 2.5–3.5 mm wide, pale yellow or brownish-black to black; erect shrubs or subshrubs up to 2.5 m tall, rarely low perennial herbs or small trees | 12 |
| 12 | Stamens unequal (3+2); ovules two per locule; fruits 4-seeded; Brazil (Rio de Janeiro, Minas Gerais, Espírito Santo) | C. campylopodium |
| – | Stamens equal; ovules more than two per locule; fruits multi-seeded (up to 20 seeds) | 13 |
| 13 | Filaments short, < 2 mm long; fruiting pedicels erect; seeds pale yellow to yellow; fruits yellow, red-orange or red | 14 |
| – | Filaments longer, 2.4–4 mm long; fruiting pedicels pendent; seeds brownish-black to black; fruits greenish-golden yellow | 15 |
| 14 | Fruiting calyx without a prominent annular constriction at junction with the pedicel | C. annuum var. glabriusculum |
| – | Fruiting calyx with a prominent annular constriction at junction with the pedicel | C. chinense |
| 15 | Stems, leaves, pedicels and calyx densely pubescent with long spreading eglandular trichomes (0.5–2 mm long); major leaves elliptic to narrowly elliptic; corolla white with large greenish-yellow spots and sparse diffuse purple or brown spots within, the lobes widely triangular; Brazil (Rio de Janeiro) | C. muticum |
| – | Stems, leaves pedicels, and calyx glabrescent to moderately pubescent, with short antrorse eglandular trichomes (0.25–0.5 mm long); major leaves elliptic to ovate; corolla white with large or small purple or brownish spots and large greenish-yellow spots within (in some populations, purple or brownish pigmentation absent entirely); the lobes triangular or ovate; Brazil (Minas Gerais, Rio de Janeiro and São Paulo) | C. schottianum |
| 16 | Flowers solitary; corolla entirely white; staminal plaques with auricles not fused to the corolla at the point of insertion; Argentina and Paraguay (also Bolivia) | C. chacoense |
| – | Flowers 2–18, rarely flowers solitary; corolla yellow, purple or white tinged of different colours within; staminal plaques with auricles fused to the corolla at the point of insertion | 17 |
| 17 | Corolla usually yellow with yellowish-green or purple-brown spots within; fruiting calyx usually recurved; seeds 4–4.6 mm long, (–2.8) 3.2–3.75 mm wide, yellow to brownish yellow; sprawling vines or scrambling shrubs, with stems to 7 m long; western Brazil (also Peru and Bolivia) | C. coccineum |
| – | Corolla primarily purple or white; fruiting calyx not recurved; seeds (2–) 2.2–3.5 (–4) mm long, (–1.8) 2–3 (–3.5) mm wide, yellow or brownish-black to black; erect shrubs or subshrubs, up to 5 m tall | 18 |
| 18 | Calyx appendages 10 or 6–10, rarely five | 19 |
| – | Calyx appendages five (very rarely up to seven) | 21 |
| 19 | Calyx appendages 10, subequal; filaments 3–3.2 mm long; style barely exserted beyond the anthers; anthers lilac or pale blue; corolla almost entirely purple; Brazil (São Paulo) | C. mirum |
| – | Calyx appendages ranging from 6 to 10, rarely five, unequal; filaments 1.4–2.5 mm long; style exserted 0.8–1.3 mm beyond the anthers; anthers yellow, light green or grey; corolla white with greenish-yellow or purple spots within | 20 |
| 20 | Flowering calyx appendages cylindrical or triangular-compressed, glabrous to moderately pubescent with antrorse trichomes, the longest appendages 1–2.5 mm; corolla 6–7 mm long, ca. 11 mm in diameter, white with greenish-yellow spots within; fruiting calyx appendages strongly recurved; fruiting pedicels 18–25 mm long; Brazil (Minas Gerais, Paraná, Rio de Janeiro, Santa Catarina and São Paulo) | C. recurvatum |
| – | Flowering calyx appendages linear or subulate, densely pubescent with spreading trichomes, the longest appendages 2.5–5 (–6) mm long; corolla (8–) 9–14 mm long, 18–22 mm in diameter, white with purple or reddish-brown spots within; fruiting calyx appendages spreading; fruiting pedicels (25–) 30–38 mm long; Brazil (São Paulo and Rio de Janeiro) | C. cornutum |
| 21 | Major leaves ovate; corolla primarily purple with greenish-yellow or cream centre within | 22 |
| – | Major leaves elliptic or narrowly elliptic, more rarely ovate | 23 |
| 22 | Pedicels scars prominent; flowering and fruiting pedicels pendent; fruits greenish-golden yellow; seeds brownish-black, the seed coat reticulate and tuberculate at margins; Brazil (also Colombia and Venezuela) | C. parvifolium |
| – | Pedicels scars inconspicuous; flowering and fruiting pedicels erect; fruits orange or red; seeds pale yellow or yellow, the seed coat smooth; Paraguay and Brazil | C. rabenii |
| 23 | Plants densely pubescent on stems, petioles, pedicels and sometimes also on the leaf nerves beneath, the trichomes spreading; Brazil (Rio de Janeiro, Minas Gerais, São Paulo and Espírito Santo) | C. villosum |
| – | Plants glabrescent to densely pubescent on stems, leaves and pedicels, the trichomes appressed-antrorse; calyx appendages up to 5 mm long | 24 |
| 24 | Plants glabrous to sparsely pubescent; major leaves elliptic to ovate, rarely narrowly elliptic (length/width ratio: (2–) 2.5–4 (–4.9), apex acuminate to long acuminate; calyx appendages (0.4–) 0.5–1.5 (–3) mm; flower buds purple; corolla (6–) 7.5–12 mm long, (9–) 10–13 mm in diameter; Brazil (Bahia, Espírito Santo, Minas Gerais, Rio de Janeiro and São Paulo) | C. mirabile |
| – | Plants moderately to densely pubescent; major leaves narrowly elliptic to lanceolate (length/width ratio: (4–) 5–10 (–16), apex acute to obtuse; calyx appendages (2.8–) 3–4 (–5) mm; flower buds cream with greenish and purple spots; corolla (8–) 10–12 mm long, 13–20 mm in diameter; Brazil (Minas Gerais) | C. carassense |
Key to the domesticated Capsicum species
| 1 | Calyx appendages absent or minute, ≤ 0.5 mm long | 2 |
| – | Calyx appendages > 0.5 mm long | 4 |
| 2 | Flowers solitary, rarely in pairs or more; petioles up to 10 cm long; corolla 8–15 mm long, entirely white, rarely entirely purple or pale yellow; fruits usually large, up to 300 mm long, pungent or non-pungent | C. annuum var. annuum |
| – | Flowers 2–5, rarely solitary; petioles up to 3.5 cm long; corolla 3.75–8 mm long, entirely dull white or greenish-white (occasionally with purple spots); fruits small to medium-sized, up to 100 mm long, pungent, rarely non-pungent | 3 |
| 3 | Corolla glabrous adaxially; style heteromorphic; fruits subglobose to highly variable in shape, with the base obtuse or truncate; fruiting calyx discoid or shallowly cup-shaped, with a prominent annular constriction at junction with the pedicel; ovary subglobose, 2–2.5 mm long, 2.5–3.5 mm in diameter | C. chinense |
| – | Corolla with small glandular trichomes adaxially; style homomorphic; fruits usually elongate, narrowly triangular, with the base abruptly narrowed; fruiting calyx deeply cup-shaped lacking a constriction at junction with the pedicel; ovary oblong-ovoid, 2.5–4 mm long, 1.3–1.8 mm in diameter | C. frutescens |
| 4 | Leaves densely pubescent, rarely glabrescent, the youngest leaves rugose; flower buds dark purple on pendent pedicels; corolla dark purple or violet with a white or yellowish-green centre within; style clavate; seeds 5.5–7 mm long, 4.8–6 mm wide, brownish-black to black, the seed coat reticulate | C. pubescens |
| – | Leaves glabrous to sparsely pubescent; the youngest leaves even; flower buds greenish-white (rarely purple) on geniculate pedicels; corolla white with large greenish-yellow spots and white centre within, style cylindrical; seeds 3–5.2 mm long, 3–4 mm wide, pale yellow to yellow, the seed coat smooth to slightly reticulate | 5 |
| 5 | Fruits pungent, rarely non-pungent, usually elongate, endocarp alveolate, pericarp with giant cells | C. baccatum var. pendulum |
| – | Fruits non-pungent, campanulate-umbilicate, endocarp smooth, pericarp without giant cells | C. baccatum var. umbilicatum |
Species descriptions
Capsicum annuum
Type
“Habitat in America meridionali” Herb. Clifford: 59, Capsicum 1 (lectotype, designated by
Capsicum annuum var. annuum
Capsicum grossum L., Mant. Pl.: 47. 1767. Type. “Habitat in India … H.U.” HU [Horto Upsaliensis]: Fructu vario crasso. Caulis biennis, Herb. Linn. N° 249.5 (lectotype, designated here: LINN [LINN-HL249-5]).
Capsicum cordiforme Mill., Gard. Dict. ed. 8, no. 2. 1768. Type. Cultivated at the Chelsea Physic Garden (no specimens cited; no original material located).
Capsicum tetragonum Mill., Gard. Dict. ed. 8, no. 3. 1768. Type. Cultivated at the Chelsea Physic Garden (no specimens cited; no original material located).
Capsicum angulosum Mill., Gard. Dict. ed. 8, no. 4. 1768. Type. Cultivated at the Chelsea Physic Garden (no specimens cited; no original material located).
Capsicum olivaeforme Mill., Gard. Dict. ed. 8, no. 6. 1768. Type. Cultivated at the Chelsea Physic Garden, seeds from “Barbadoes” (no specimens cited; no original material located).
Capsicum pyramidale Mill., Gard. Dict. ed. 8, no. 7. 1768. Type. Cultivated at the Chelsea Physic Garden, seeds from Egypt (no specimens cited; no original material located).
Capsicum conicum Lam., Tabl. Encycl. 2: 26. 1794. Type. “Ex Indiis” Herb. Lamarck s.n. (lectotype, designated here: P-LAM [P00357734]).
Capsicum bicolor Jacq., Fragm. Bot. 66, tab 99, fig. 1. 1809. Type. “Patriam ignoro” (no specimens cited; lectotype, designated here: Jacquin, Fragm. Bot.: 66, tab 99, fig. 1. 1809).
Capsicum grossum Willd., Enum. Pl. [Willdenow] 1: 241. 1809, nom. illeg., not Capsicum grossum L. (1767). Type. “Habitat in India orientali” Capsicum grossum [sheet] 2, Herb. Willdenow (lectotype, designated here: B [B-W04425-02-0]).
Capsicum sphaericum Willd., Enum. Pl. [Willdenow] 1: 241. 1809. Type. “Habitat…. ” (lectotype, designated here: B [B-W04426-01-0, F neg. 2886]).
Capsicum nigrum Willd., Enum. Pl. [Willdenow] 1: 242. 1809, nom. illeg. superfl. Type. Based on Capsicum bicolor Jacq. (cited in synonymy).
Capsicum purpureum Vahl ex Hornem., Hort. Bot. Hafn. 1: 224. 1813. Type. [Denmark]. Hort. Haf., 1802, Herb. Vahl s.n. (lectotype, designated here: C [C10019148]).
Capsicum ovatum DC., Cat. Pl. Horti Monsp.: 86. 1813. Type. “Habitat….” (no specimens cited; no original material located; Capsicum ovatum, Anonymous s.n. (neotype, designated here: G-DC [G00200072]).
Capsicum longum DC., Cat. Pl. Horti Monsp.: 86. 1813. Type. “Hab... in hortis frequens” (no specimens cited; lectotype, designated here [illustration]: “Piper Calecuticum sive Capsicum oblongius, Bauhin et al., Hist. pl. 2: 943, f. I. 1651).
Capsicum globiferum G.Mey., Prim. Fl. Esseq.: 113. 1818. Type. “In plantationibus”, no specimens cited; [Guyana]. Río Essequibo, E.K. Rodschied 29 (lectotype, designated here: GOET [GOET003420]).
Capsicum purpureum Roxb., Fl. Ind., ed. Carey & Wall. 2: 259. 1824, nom. illeg., not Capsicum purpureum Vahl ex Hornem. (1813). Type. “Most likely from the Molucca Islands” (no specimens cited; neotype, designated here: “C. purpureum, H.B.C.” [Horto Botanici Calcutta]: K [K001132446]).
Capsicum indicum var. vulgatum Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 22. 1829, nom. illeg. superfl. Type. Based on Capsicum annuum L. (cited in synonymy).
Capsicum indicum var. longum (DC.) Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 23. 1829. Type. Based on Capsicum longum DC.
Capsicum indicum var. tetragonum (Mill.) Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 23. 1829. Type. Based on Capsicum tetragonum Mill.
Capsicum indicum var. angulosum (Mill.) Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 25. 1829. Type. Based on Capsicum angulosum Mill.
Capsicum indicum var. cordiforme (Mill.) Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 25. 1829. Type. Based on Capsicum cordiforme Mill.
Capsicum indicum var. grossum (L.) Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 26. 1829. Type. Based on Capsicum grossum L.
Capsicum indicum var. sphaericum (Willd.) Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 27. 1829. Type. Based on Capsicum sphaericum Willd.
Capsicum indicum var. ovatum (DC.) Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 27. 1829. Type. Based on Capsicum ovatum DC.
Capsicum indicum var. pyramidale (Mill.) Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 28. 1829. Type. Based on Capsicum pyramidale Mill.
Capsicum indicum var. olivaeforme (Mill.) Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 28. 1829. Type. Based on Capsicum olivaeforme Mill.
Capsicum indicum var. nigrum (Willd.) Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 29. 1829. Type. Based on Capsicum nigrum Willd.
Capsicum axi
Vell., Fl. Flumin.: 61. 1829 (“1825”); Fl. Flumin. Icon. 2: t. 6. 1831 (“1827”). Type. Brazil. [Rio de Janeiro]: “Colitur hortis” (lectotype, designated by
Capsicum silvestre
Vell., Fl. Flumin. 60. 1829 (“1825”); Fl. Flumin. Icon. 2: t. 1. 1831 (“1827”). Type. Brazil. [Rio de Janeiro]: “Ad declivium Alpium Fluminensium” (lectotype, designated by
Capsicum annuum var. rugosulum Fingerh., Monogr. Capsic.: 13. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. II b. 1832).
Capsicum annuum var. acuminatum Fingerh., Monogr. Capsic.: 13. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. II c. 1832).
Capsicum annuum var. subangulosum Fingerh., Monogr. Capsic. 13. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. II d. 1832).
Capsicum annuum var. ovoideum Fingerh., Monogr. Capsic.: 14. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. II e. 1832).
Capsicum annuum var. abbreviatum Fingerh., Monogr. Capsic.: 14. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. II f. 1832).
Capsicum annuum var. olivaeforme Fingerh., Monogr. Capsic.: 14. 1832. Type. “Crecit in America meridionali et India oriental” (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. II g. 1832).
Capsicum bicolor var. purpureum (Vahl ex Hornem.) Fingerh., Monogr. Capsic.: 16. 1832. Type. Based on Capsicum purpureum Vahl ex Hornem.
Capsicum strictum Fingerh., Monogr. Capsic.: 21. 1832. Type. “Patria…..” (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. V a. 1832).
Capsicum grossum Willd. var. pomiforme Fingerh., Monogr. Capsic.: 22. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. V c. 1832).
Capsicum grossum var. ovatum Fingerh., Monogr. Capsic.: 22. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. V d. 1832).
Capsicum grossum Willd. var. cordatum Fingerh., Monogr. Capsic.: 22. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. VI a. 1832).
Capsicum grossum Willd. var. angulosum Fingerh., Monogr. Capsic.: 22. 1832. Type: “Patria India orientalis (Herb. Wight et Herb. Hamilt.)” (no specimens found; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. VI d. 1832).
Capsicum ceratocarpum Fingerh., Monogr. Capsic.: 22. 1832. Type. “Patria….” (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. VI c. 1832).
Capsicum longum var. incrassatum Fingerh., Monogr. Capsic.: 24. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. VII a. 1832).
Capsicum longum var. latum Fingerh., Monogr. Capsic.: 25. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. VII b (as - - [Capsicum longum] luteum). 1832).
Capsicum longum var. rectum Fingerh., Monogr. Capsic.: 25. 1832. Type. “Cresit in Indiis et America meridionali” (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. VII c. 1832).
Capsicum pendulum var. torulosum Fingerh., Monogr. Capsic.: 26. 1832. Type. [Indonesia] “in Amboina” (no specimens cited; lectotype, designated here [illustration]: Capsicum rubrum minus Rumphius, Herbarium Amboinense 5, Tab. LXXXVIII, fig. 1, 1747, cited in synonymy).
Capsicum angulosum var. conicum Fingerh., Monogr. Capsic.: 28. 1832. Type. No locality cited (no specimens cited, no original material located).
Capsicum angulosum var. ovale Fingerh., Monogr. Capsic.: 28. 1832. Type. “Patria….?” (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. VIII b. 1832).
Capsicum hamiltonii G.Don, Gen. Hist. 4: 447. 1838. Type. [Caribbean Islands] “Native of the Island of Nevis, in gardens” (no specimens cited, no original material located).
Capsicum annuum var. longum (DC.) Sendtn., Fl. Bras. (Martius) 10(6): 144. 1846. Type. Based on Capsicum longum DC.
Capsicum annuum var. grossum (Willd.) Sendtn., Fl. Bras. (Martius) 10(6): 147. 1846. Type. Based on Capsicum grossum Willd.
Capsicum annuum var. cordiforme (Mill.) Sendtn., Fl. Bras. (Martius) 10(6): 148. 1846. Type. Based on Capsicum cordiforme Mill.
Capsicum abyssinicum A.Rich., Tent. Fl. Abyss 2: 96. 1850. Type. [Ethiopia] “Abyssinia, Ouedjerate”, R. Quartin Dillon s.n. (lectotype, designated here: P [P00329903]; isolectotypes: P [P00329904, P00329905]).
Capsicum annuum var. oblongum Dunal, Prodr. [A. P. de Candolle] 13(1): 412. 1852. Type. “Capsicum annuum α oblongum fructibus rubris”, 1844, Herb. Dunal (lectotype, designated here: G-DC [G00131768]).
Capsicum pyramidale var. longicorne Dunal, Prodr. [A. P. de Candolle] 13(1): 414. 1852. Type. [Indonesia] Java, 1843, H. Zollinger 489 (lectotype, designated here: G-DC [G00131841]; isolectotypes: G [G00390281], LE).
Capsicum bicolor var. purpureum (Vahl ex Hornem.) Dunal, Prodr. [A. P. de Candolle] 13(1): 414. 1852. Type. Based on Capsicum purpureum Vahl ex Hornem.
Capsicum testiculatum Vis. ex Dunal, Prodr. [A. P. de Candolle] 13(1): 424. 1852. Type. In Hort. Montpellier [seeds sent by R. de Visiani], 1837, Anonymous s.n. (lectotype, designated here: G-DC [G00200067]; isolectotype: MPU [MPU023039]).
Capsicum angulosum var. macrocarpum Dunal, Prodr. [A. P. de Candolle] 13(1): 426. 1852. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. VIII a (as Capsicum angulosum M.). 1832).
Capsicum leucocarpon
Dunal, Prodr. [A. P. de Candolle] 13(1): 429. 1852. Type. Cultivated in England “Capsicum americanum latifolium, fructu oblongo erecto candido” (
Capsicum dulce Dunal, Prodr. [A. P. de Candolle] 13(1): 428. 1852. Type. Cultivated in Montpellier, France, “In hortis botanicis cultum” (no specimens cited; no original material located).
Capsicum annuum var. cordiforme (Mill.) Alef., Landw. Fl.: 132. 1866. Type. Based on Capsicum cordiforme Mill.
Capsicum annuum var. angulosum (Mill.) Alef., Landw. Fl.: 132. 1866, as ‘angulatum’. Type. Based on Capsicum angulosum Mill.
Capsicum annuum var. pyramidale (Mill.) Alef., Landw. Fl.: 132. 1866. Type. Based on Capsicum pyramidale Mill.
Capsicum annuum var. globiferum (G.Mey.) Alef., Landw. Fl.: 132. 1866. Type. Based on Capsicum globiferum G.Mey.
Capsicum annuum var. longum (DC.) Alef., Landw. Fl.: 132. 1866. Type. Based on Capsicum longum DC.
Capsicum annuum var. tetragonum (Mill.) Alef., Landw. Fl.: 133. 1866. Type. Based on Capsicum tetragonum Mill.
Capsicum annuum var. tetragonum (Mill.) Alef., Landw. Fl.: 133. 1866. Type. Based on Capsicum tetragonum Mill.
Capsicum annuum var. purpureum (Roxb.) Alef., Landw. Fl.: 134. 1866. Type. Based on Capsicum purpureum Roxb.
Capsicum annuum var. ceratocarpum (Fingerh.) Alef., Landw. Fl.: 134. 1866. Type. Capsicum ceratocarpum Fingerh.
Capsicum annuum var. bicolor (Jacq.) Alef., Landw. Fl.: 134. 1866. Type. Capsicum bicolor Jacq.
Capsicum fasciculatum Sturtev., Bull. Torrey Bot. Club 15(5): 133. 1888. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: “Tenjikumamori, Capsicum annuum L. (Solaneae)”, Tanaka & Motoyoshi, Sô-Mokou-Zoussets, vol. 3, Tab. 38. 1874).
Capsicum annuum var. longum (DC.) Kuntze, Revis. Gen. Pl. 2: 449. 1891. Type. Based on Capsicum longum DC.
Capsicum annuum var. erectum Kuntze, Revis. Gen. Pl. 2: 449. 1891. Type. “Java, cult.” (no specimens cited, no original material located).
Capsicum annuum var. grossum (L.) Kuntze, Revis. Gen. Pl. 2: 449. 1891. Type. Based on Capsicum grossum L.
Capsicum annuum var. fasciculatum (Sturtev.) Irish, Rep. (Annual) Missouri Bot. Gard. 9: 68, pl. 9, f. 4. 1898. Type. Based on Capsicum fasciculatum Sturtev.
Capsicum frutescens var. lanicaule Greenm., Proc. Amer. Acad. Arts 39: 88. 1903. Type. Mexico. Jalisco: along Ave. Vallarta in Ciudad Granja, on the western outskirts of Guadalajara, 31 Dec 1886, E. Palmer 639 (lectotype, designated here: US [00816554, acc. # 92534], isolectotype: BM [BM000775827]).
Capsicum velutinum De Wild., Pl. Bequaert. 1: 413. 1922. Type. [Democratic Republic of the Congo]. Basankusu, Mar 1913, O. Lamboray 22 (lectotype, designated here: BR [BR000000649909]).
Capsicum frutescens var. fasciculatum (Sturtev.) L.H.Bailey, Gentes Herbarum 1: 129. 1923. Type. Based on Capsicum fasciculatum Sturtev.
Capsicum frutescens var. grossum (Willd.) L.H.Bailey, Gentes Herbarum 1: 129. 1923. Type. Based on Capsicum grossum Willd.
Capsicum annuum forma erectum Makino, J. Jap. Bot. 3(8): 29. 1926, as “Capsicum annuum var. fasciculatum f. erectum”. Type. “Hab. JAPAN, cultivated” (no specimens cited, no original material located).
Capsicum annuum forma pendulum Makino, J. Jap. Bot. 3(8): 29. 1926, as “Capsicum annuum var. fasciculatum f. pendulum”. Type. “Hab. JAPAN, cultivated, rare” (no specimens cited, no original material located).
Capsicum petenense Standl., Publ. Carnegie Inst. Wash. 461(4): 84. 1935. Type. Guatemala. Distr. Peten, La Libertad, Jun 1933, C. L. Lundell 3754 (holotype: F [v0072800F, acc. # 685329]; isotypes: CORD [CORD00101764 fragment ex MICH], MICH [1109873]).
Capsicum sonitpurense J.Sarma & G.Dutta, Bangladesh J. Pl. Taxon. 24(2): 215. 2017. Type. India. Assam, Sonitpur, Tezpur, 49 m, 22 Oct 2016, J. Sarma & G. Dutta 394 (holotype: ASSAM [acc. # 95893, sheet 394A]; isotypes: TUH [Tezpur University Herbarium, 3 sheets 394 B, C, D]).
Description
Annual herbs or short-lived, compact, low subshrubs, 1–1.5 m tall, the main stem 0.5–1 cm in diameter at base, branched from near the base. Young stems 3–4-angled, fragile, green to brownish-green, sometimes with purple lines, glabrous, glabrescent to moderately pubescent, rarely densely pubescent, with appressed-antrorse, simple, uniseriate, (5–) 8–13)-celled, eglandular trichomes 0.5–1 (–2) mm long; nodes green or with purple spots; bark of older stems light brown or brown, glabrous to sparsely pubescent; lenticels absent or few. Sympodial units difoliate, the leaves geminate; leaf pair similar in size and shape. Leaves membranous, concolorous, pale to dark green, glabrous to moderately pubescent on both sides, especially on the main veins abaxially, the trichomes similar to those of the stems; blades of all leaves 3–7 (–15.5) cm long, 2.5–5 (–8) cm wide, ovate to elliptic, the major veins (3–) 5–8 on each side of mid-vein, the base truncate to cordate or cuneate to attenuate, the margins entire, the apex acuminate or long-acuminate; petioles (0.5–) 4–7 (–10) cm, with the same pubescence as the stems. Inflorescences axillary, 1 (– 2) flowers per axil, rarely more; flowering pedicels (6–) 10–40 mm long, angled, erect and geniculate at anthesis or pendent and non-geniculate, green or purple, glabrous to moderately pubescent, the eglandular trichomes usually short, antrorse; pedicels scars inconspicuous. Buds globose, white or purple. Flowers 5–7-merous. Calyx 1–4 mm long, 3–5 mm wide, cup-shaped, green, strongly 5–10-nerved, glabrous to moderately pubescent with similar short or long eglandular trichomes as the stems, the calyx appendages usually 5 (–7), minute, 0.3–0.5 mm long. Corolla 8–15 mm long, (8–) 10–22 mm in diameter, entirely white, rarely entirely pale yellow or purple, stellate with narrow interpetalar membrane, lobed ca. halfway or 2/3 of the way to the base, glabrous adaxially and abaxially, the tube 3–8 mm long, the lobes 5–7 mm long, 3.5–5.5 mm wide, ovate, spreading, the margins finely ciliate, the tips acute, papillate. Stamens 5–7, equal; filaments 1–3 mm long, white or cream, sometimes purple, inserted on the corolla 1–1.5 mm from the base, with auricles fused to the corolla tube at the point of insertion; anthers 2–3 mm, ellipsoid or ovoid, pale blue to purplish, very rarely yellow, connivent or not connivent at anthesis. Gynoecium with ovary 1.5–3 mm long, 1.2–2.5 mm in diameter, ovoid or globose, green; nectary ca. 0.5 mm tall, pale green; style heteromorphic, short style 2.2–2.5 mm, not exceeding the anthers, medium style nearly the same height as the anthers, long style 3–5.1 mm, exserted 1.3–2.3 mm beyond the anthers, cylindrical, white or purple; stigma 0.1–0.2 mm long, ca. 0.4 mm wide, discoid or capitate, pale green or yellow. Berry highly variable in shape, size and colour, usually blocky or elongate, less commonly globose, up to 300 mm long, 6–65 mm in diameter, green, yellow or purple when immature, yellow, red, brown, purple or purple-black at maturity, persistent, pungent or non-pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 25–50 (–70) mm, erect or pendent, rigid, angled, uniformly widened, green; fruiting calyx 15–25 mm in diameter, slightly accrescent, discoid or rather cup-shaped, green. Seeds more than 50 per fruit, 3.8–4.4 mm long, 3.2–3.6 mm wide, C-shaped, pale yellow, the seed coat smooth to slightly reticulate (SM), cerebelloid (SEM), the cells irregular in shape, the lateral walls sinuate; embryo imbricate.
Capsicum annuum var. annuum A flower bud on pendent pedicel B flower with connivent anthers C flower with hexamerous corolla (note nectar droplets on the limb) and style near the same length as the anthers D flower with heptamerous purple corolla E flower with pentamerous corolla and style exceeding the anthers F, H mature fruits on pendent pedicels G mature fruits on upright pedicels A–H no specimen vouchers, photos by G.E. Barboza and C. Carrizo García taken at different greenhouses.
Distribution
Capsicum annuum var. annuum is the most extensively cultivated pepper worldwide.
Ecology
Capsicum annuum var. annuum is found in diverse habitats throughout its wide distribution and is well adapted to the highlands environments (0–2,600 m elevation).
Phenology
Flowering and fruiting all year.
Chromosome number
2n = 2x = 24 (
Common names
Argentina: Ají balita (Jujuy, Moscone 204), Pimiento (Corrientes, Anzótegui & Benitez 237; Salta, Hunziker 25498), Serrano (Salta, Hunziker 25492), Pimiento Calahorra (Córdoba, Hunziker 29428); Bolivia: Ají (Beni, Balderrama 10; Santa Cruz, Saldías P. 759), Urubibi (Beni, Ticona & Saravia May 10), Pimentón colorado (Santa Cruz,
Indigenous names
Bolivia: Ta (Beni, Ticona & Saravia May 10); Colombia: Aati (Curripaco, Guainía, Espina et al. 189), Aii (Cauca, Plowman & Vaughan 5370), Asi (Piapoco, Vichada, Rodríguez 177), Azi (Piapocos, Vichada, Rodríguez 165), Biaá (Tanimuka, Amazonas, Cárdenas et al. 9406), Coc (Puinabe, Vichada, Rodríguez 169), Curripaati (Tucano, Guainía, Marín & Rodríguez 502), Fecogɨ (Bora, Amazonas, Torres et al. 4020), Fekorai (Huitoto-Mɨnɨka, Amazonas, Henao 167), Jipujou (Caquetá, Cárdenas et al. 9330), Jumerien (Sukuare, Vichada, Rodríguez 1), Mèe (Colona, Amazonas, Torres & Rodríguez 2021), Munɨ (Huitoto, Amazonas, Posada 2577), Nubata (Andoque, Amazonas, Torres et al. 4047), Pidá (Emberá, Chocó, La Rotta & Martínez 737), Rɨairai (Huitoto-Mɨnɨka, Amazonas, Henao & Kuiru 172), Arera rɨairai (Huitoto-Mɨnɨka, Amazonas, Henao 316), Yicane (Miraña, Caquetá, Cárdenas et al. 9375), Jeba gayebá (Mui, Castro et al. 238), Masan via (Amazonas, Cárdenas et al. 9424), Pipita deé (Mui, Amazonas, Castro 305), Viahoracá carunoje (Tanimuka, Amazonas, Cárdenas et al. 9432); Ecuador: Aatyu (Chapalaachi, Yañez et al. 1485), Uchu (Quichua, Napo, Kohn 1225), Ahí bia (Siona & Secoya Indians, Napo, Vickers 211), Suara pia (Siona & Secoya Indians, Napo, Vickers 227), Soa horo bia (Siona & Secoya Indians, Napo, Vickers 200); Mexico: Cants (Huave, Oaxaca, Zizumbo & Colunga 145), Chaunik (Yucatán, Vargas 66), Guiin-cànár (Zapateco, Oaxaca, Hunn OAX-1345), Guiin-ló-yág (Zapateco, Oaxaca, Hunn OAX-1341), Guiin-ló-ngÚbidz (Zapateco, Oaxaca, Hunn OAX-1343), Guiin-nàl-zhàb (Zapateco, Oaxaca, Hunn OAX-1342), Guiin-txxtlé (Zapoteco, Oaxaca, Hunn OAX-1344), Moo-o-re (Oaxaca, Hernández Ortega 482), Moo-o-qui (Oaxaca, Hernández Ortega 481), Niiy (Oaxaca, Antonio B. GUI 201), Xcatic (Maya, Quintana Roo, Villanueva 591), X-mash ik (Quintana Roo, Gutiérrez 85-26), X-mehen (Quintana Roo, Gutiérrez 85-27), Xkat-ik (Maya, Quintana Roo, Gutiérrez 26), Ya Jimia (Morona-Santiago, Evans 4384), Ya’axik (Maya, Quintana Roo, Gutiérrez 109), Chaua ik (Yucatán, May 39), Ixa nadun (Guerrero, Wagenbreth 130), Kat ik (Yucatán, Ucan et al. 3529), Nadam kanc (Huave, Oaxaca, Bamonte 77), Namis kanc (Huave, Oaxaca, Bamonte 79), Yaá dia (Mixteco, Guerrero, Díaz Rico 221), Yak ik (Quintana Roo, Gutiérrez 85-71), Ixe dun xkuiya smidi (Guerrero, Wagenbreth 687); Peru: Iwiá (Mayna Jívaro, Loreto, Lewis et al. 10922), Kistian jima (Amazonas, Ancuash 297), Mun hima (Amazonas, Berlín 1572), Tsitikana ogat-santsakarioni (Machiguenga, Cuzco, Johnson 70).
Uses
Capsicum annuum var. annuum is the economically most important member of the genus. The fruits are widely used in international cuisine in a broad spectrum of meals and preparations, because of their aroma, flavour, texture and level of pungency. Some cultivars have good acceptance as ornamental plants due to the colour of the leaves and the brightness of the colourful and usually erect fruits (e.g. Christmas peppers, Bolivian rainbow, Fig.
Preliminary conservation assessment
Capsicum annuum var. annuum is not under threat.
Discussion
The domesticated taxon C. annuum var. annuum belongs to the Annuum clade, together with C. chinense, C. frutescens and C. galapagoense (
The vast majority of the modern landraces, varietals and hybrids of chili peppers belong to this variety (
Due to the selective pressure for domestication and diversification, defining a characteristic group of traits for var. annuum is difficult; however, the most distinctive features are its herbaceous to shrubby, annual or perennial habit, the solitary axillary flowers (rarely two or more), the strongly 5–10-nerved calyx, the large white (or purple) corollas (up to nearly 22 mm in diameter) and the usually persistent and pendent fruits, which are highly variable in size, form, colour and pungency. Some of these traits contrast with those of var. glabriusculum which has a shrubby habit, 5-nerved calyx, smaller corollas (≤ 12 mm in diameter) and small (< 10 mm in diameter), globose, ellipsoid or ovoid, erect, red or red-orange, deciduous fruits.
Philip Miller was the curator of the Chelsea Physic Garden in London in the late 18th century. Many of the plants he grew there were new taxa in his “Gardener’s Dictionary” (1768). He described several Capsicum species (C. cordiforme, C. tetragonum, C. angulosum, C. olivaeforme and C. pyramidale), based on cultivated specimens obtained from seeds of different provenance. As was the practice at the time, he did not cite specimens and is likely to have based his descriptions on living plants. Most of these plants were described as annuals with white flowers and a variety of fruit sizes, shapes (heart-shaped, angular-obtuse, oval-shaped, pyramidal), colours (yellow, scarlet, red), textures and positions (pendent or upright), characters that are highly variable due to human selection in these domesticated species. Specimens made from plants grown by Miller are found in several different places, mostly at BM and its associated historical herbaria. As these names are almost certainly described from living plants and, thus, will need neotypification, we do not typify them here, but leave that for a separate study when these materials, including any non-digitised specimens, can be studied in detail.
We found a collection in the Lamarck Herbarium with a label indicating that it belongs to C. conicum (P00357734) which we designate here as the lectotype.
Capsicum bicolor was probably described only from living material cultivated in the gardens of Schönbrunn Palace near Vienna (Austria).
There are two sheets of original material labelled C. grossum in Willdenow’s Herbarium held at Berlin. Both contain reproductive branches; one of these (B-W 04425 -02 0) consists of two fruiting branches that exactly match Willdenow’s description (
In the protologue of C. purpureum,
When coining the name C. longum,
Capsicum purpureum is based on a single plant found in the Botanic Garden of Calcutta (India), whose exact origin is unknown, but
In the protologue of C. abyssinicum,
In his description of C. pyramidale var. longicorne,
In the protologue of Capsicum testiculatum,
In the protologue of C. angulosum var. macrocarpum,
Dunal based C. leucocarpum on
Capsicum velutinum (
Capsicum annuum var. glabriusculum
Capsicum hispidum var. glabriusculum
Dunal, Prodr. [A. P. de Candolle] 13(1): 420. 1852. Type. [United States of America. Texas: Bexar Co., San Antonio]: “Mexico, circa Bejar”, Sep 1828, J.L. Berlandier 1863 (lectotype, designated by
Capsicum minimum Mill., Gard. Dict. ed. 8, no. 10. 1768. Type. “Cultivated in England” (no specimens cited; no original material located).
Capsicum havanense Kunth, Nov. Gen. Sp. [H.B.K.] 3: 38. 1818. Type. [Cuba]. “in arenosis maritimis, prope Havanam (Insulae Cubae)” [Havana] s.d., F.W.H.A. von Humboldt & A.J.A. Bonpland 4518 (lectotype, designated here: P [P00670653]).
Capsicum indicum var. aviculare Dierb., Arch. Apotheker-Vereins Nördl. Teutschl. 30(1): 30. 1829. Type. Based on Capsicum minimum Mill. and C. microcarpon DC. (cited in synonymy), PANAMA: Coclé, 10 mi. E of Nata at Rio Grande, 4 Jan 1969, E.L. Tyson 5222 (neotype, designated here: MO [MO-562584, acc. # 1980106]; isoneotype, FSU [000064909, acc. # 119808]).
Capsicum frutescens var. minus Fingerh., Monogr. Capsic.: 17. 1832. Type. “Crecit in India orientali et America meridionali” (no specimens cited; lectotype, designated here [illustration]: “Capsicum rubrum minimum” Rumphius, Herbarium Amboinense 5, Tab. 88, fig. 2, 1747]).
Capsicum pendulum var. minus Fingerh., Monogr. Capsic.: 25. 1832. Type. Based on Capsicum havanense Kunth.
Capsicum chlorocladum Dunal, Prodr. [A. P. de Candolle] 13(1): 415. 1852. Type. Mexico. Tamaulipas: “Tampico da Tamaulipas”, 1827, J.L. Berlandier 97 (lectotype, designated here: G-DC [G00131884]; isolectotypes: BM [BM000775807, BM000775821], G [G00342805], F [v0072794F, acc. # 680277], LE [LE01072484], MPU [MPU023049], P [P00410031, P00410147, P00409849]).
Capsicum laurifolium Dunal, Prodr. [A. P. de Candolle] 13(1): 418. 1852. Type. Brazil. Bahia: “partie mérid. de la prov. de Bahia”, 1840, J.S. Blanchet 3098 A (lectotype, designated here: G-DC [G00131882]; isolectotypes: MPU [MPU023046, MPU023047).
Capsicum hispidum Dunal, Prodr. [A. P. de Candolle] 13(1): 419. 1852. Type. Mexico. Tamaulipas: circa Tupan et Tampico de Tamaulipas, 1827, J.L. Berlandier 152 (lectotype, designated here: G-DC [G00131880]; isolectotypes: BM [BM000775838], G [G00390276], G [G00390277], MO [MO-562486, acc. # 1690380], MPU [MPU013437], P [P00409850, P00410137]).
Capsicum angustifolium Dunal, Prodr. [A. P. de Candolle] 13(1): 420. 1852. Type. “In Indiâ utrâque colitur”. Capsicum baccatum hort. Geneve, 1836, Anonymous 1414/6 (lectotype, designated here: G-DC [G00131878]).
Capsicum microphyllum
Dunal, Prodr. [A. P. de Candolle] 13(1): 421. 1852. Type. [Cuba]: La Havanna, 1828, R. de la Sagra 3 (lectotype, designated by
Capsicum pendulum var. minus Dunal, Prodr. [A. P. de Candolle] 13(1): 425. 1852, nom. illeg., not C. pendulum var. minus Fingerh. (1832). Type. Based on Capsicum havanense Kunth.
Capsicum annuum var. minus (Fingerh.) Shinners, Baileya 4: 82. 1956. Type. Based on Capsicum frutescens var. minus Fingerh.
Capsicum annuum var. minimum (Mill.) Heiser, Ci. & Nat. 7: 52. 1964. Type. Based on Capsicum minimum Mill.
Capsicum annuum var. aviculare (Dierb.) D’Arcy & Eshbaugh, Phytologia 25(6): 350. 1973. Type. Based on Capsicum indicum var. aviculare Dierb.
Capsicum frutescens var. glabriusculum (Dunal) M.R.Almeida, Fl. Maharashtra 3B: 356. 2001. Type. Based on Capsicum hispidum var. glabriusculum Dunal.
Type
Based on Capsicum hispidum var. glabriusculum Dunal.
Description
Perennial low herbs or somewhat prostrate subshrubs, (1–) 1.5–2 (–3) tall, the main stem woody, 0.5–1 cm in diameter at base, much branched from near the base, the branches dichotomously spreading in a typical “zig-zag” appearance above. Young stems angled, fragile, green to greenish-grey or purple-striped, glabrescent to densely pubescent, with appressed-antrorse to spreading, simple, uniseriate, (2–) 3–8 (–12)-celled, eglandular trichomes 0.3–0.9 (–2) mm long, rarely furcate trichomes; nodes solid, green or purple; bark of older stems light brown or brown, glabrous to sparsely pubescent; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair subequal in size and shape. Leaves membranous, discolorous, dark green above, light green beneath, glabrescent to densely pubescent on both sides, if glabrescent with an evident tuft of trichomes in the vein axils beneath, the trichomes similar to those of the stems; blades of all leaves 2.5–6 (–8.5) cm long, 1.15–2.5 (–3.4) cm wide, ovate to elliptic, the major veins 4–5 on each side of mid-vein, the base attenuate or truncate and rather unequal, the margins entire, the apex acuminate; petioles (0.5–) 1.5–2.5 (–3) cm, glabrous to moderately pubescent. Inflorescences axillary, 1–2 flowers per axil, more rarely 3 flowers; flowering pedicels 7.5–27.8 mm long, angled, erect, geniculate at anthesis, green, glabrous to moderately pubescent, the eglandular trichomes short, antrorse; pedicels scars inconspicuous. Buds globose, white, cream or greenish-white. Flowers 5-merous. Calyx 1.5–2.5 (–3) mm long, 2–3.8 mm wide, cup-shaped, green, pentagonal in outline, glabrous to moderately pubescent with similar short or long eglandular trichomes as the stems, without appendages or with five minute appendages less than 0.5 mm long. Corolla (5–) 6–8 mm long, 8–10 (–12) mm in diameter, entirely white or almost pale yellow, rarely greenish-white, stellate with narrow interpetalar membrane, lobed ca. halfway or 2/3 of the way to the base, glabrous adaxially and abaxially, the tube (2–) 3–3.5 mm long, the lobes 3–4.5 mm long, 2–2.5 mm wide, triangular, spreading, the margins slightly involute and finely ciliate, the tips acute to long-cucullate, densely papillate. Stamens five, equal; filaments 1–1.25 mm long, white, cream or purple, sometimes lilac at the apex, inserted on the corolla 1–1.3 mm from the base, with auricles fused to the corolla tube at the point of insertion; anthers 0.95–2.55 mm, broadly ellipsoid or ellipsoid, blue, bluish-grey or purple, very rarely yellow, connivent at anthesis. Gynoecium with ovary 1.2–1.5 (–2.5) mm long, 1–2 mm in diameter, green or cream, ovoid or globose; nectary ca. 0.3 mm tall, pale yellow; style homomorphic, 4–4.8 mm, exserted 1.5–2 mm beyond the anthers, cylindrical, white or pale lilac; stigma 0.1–0.2 mm long, ca. 0.3 mm wide, discoid or bilobed, pale bright green or white. Berry 6–8.5 mm in diameter, globose (larger in semi-domesticated specimens, 9–11 mm in diameter) or ellipsoid or ovoid with acute to slightly obtuse apex, 9–13 mm long, 5–6.5 mm in diameter (larger in semi-domesticated specimens, 15–25 mm long, 7–12 mm in diameter), green or green and partly dark purple or purple when immature, bright lemon-yellow, bright red-orange or red at maturity, deciduous, very pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 16–28 (–35) mm, erect, rigid, angled, widened distally, green; fruiting calyx 4–4.5 mm in diameter, persistent, not accrescent, discoid or rather cup-shaped, green. Seeds (6–) 8–26 per fruit, 3.2–4 mm long, 2.5–3.2 mm wide, C- or D-shaped, pale yellow to yellow, the seed coat reticulate to obscurely reticulate (SM), cerebelloid (SEM), the cells irregular in shape, the lateral walls strongly sinuate; embryo imbricate.
Capsicum annuum var. glabriusculum A reproductive branch B eglandular trichome of the leaf C flower D section of the calyx showing the venation E opened corolla F gynoecium G fruit H anatomical detail of the pericarp (note the giant cell in the mesocarp) I seed J seed, in cross section K structure of seed coat at the seed margin L structure of seed coat at the seed body M embryo. A–H from Singleton 195 I–M from Scolnik 19An329. Drawn by L. Ochoa. Published in
Capsicum annuum var. glabriusculum A apex of a reproductive branch B flower bud on geniculate pedicel C–F flowers in anthesis showing variations in corolla, stamens and style colouration G immature fruit H mature and immature fruits A, B, D, F from Barboza et al. 5049, photos by G.E. Barboza C, E, H Carrizo García 102, photos by C. Carrizo García G Leiva González et al. 2105, photo by S. Leiva González.
Distribution
Capsicum annuum var. glabriusculum is the most widely distributed member of the genus, from southern United States of America to northern Bolivia and northern Brazil (Fig.
Ecology
Capsicum annuum var. glabriusculum occupies a wide variety of habitats throughout its wide distribution, including tropical deciduous, semi-deciduous and evergreen forests, less frequently in dry tropical or subtropical forests or in thorny scrub, from sea level to ca. 2,500 m elevation. It is found in shade along roadsides, stream banks, meadows near shores or as a weed in pastures or on the edges of cultivated lands. Indigenous communities and rural people cultivate C. annuum var. glabriusculum for self-consumption and it is often found escaped from cultivation.
Phenology
Flowering and fruiting all year.
Chromosome number
2n = 2x = 24 (
Common names
Bahamas: Bird pepper (Long Island, Richey 98-355), Pepper bush (Bimini, Howard & Howard 10055); Boliva: Ají (Pando, Beck et al. 19150); Brazil: Pimenta-de-mesa, Pimenta-peito-de-moça, Pimenta-ova-de-tamuata (
Indigenous names
Colombia: Aati (Curripaco, Guainía, Espina et al. 191), Aiyo borarede jairai (Huitoto-Nɨpode, Amazonas, Henao & Zɨueche 247), Beeakxtú (And, Amazonas, Castro & Andoke 607), Jɨgɨngo uijɨ (Huitoto-Mɨnɨka, Amazonas, Henao 170), Jimorai (Huitoto-Mɨnɨka, Amazonas, Henao & Padd 168), Kupirapa’ajiné (Amazonas, Castro & Matapí 523), Meniño uijɨ (Huitoto-Mɨnɨka, Amazonas, Henao 173), Wainpiraicha (Guajira, Betancur et al. 11258), Ziorai (Huitoto-Mɨnɨka, Amazonas, Henao 169); Ecuador: Bula uchu (Napo, Irvine 773), Giimo (Oncaye, Napo, Davis & Yost 994), Jimiea (Achuar Jívaro, Pastaza, Lewis et al. 14010), Jimia (Shuar, Zamora-Chinchipe, Van den Eynden et al. 700), Sampíajimia (Shuar, Zamora-Chinchipe, Santín et al. 100), Uchu (Quichua, Napo, Alarcón 102), Uchumuyu (Quichua/Spanish, Pastaza, Lewis et al. 14010); Guatemala: Chi-ik (Alto Verapaz, Standley 90936), Tamut ich (Ch’orti’, Chuiquimula, Kufer 99); Mexico: Guiiña (Zapateco, Oaxaca, Sánchez L. et al. 1190), Skapin (Totonaco, Veracruz, Cortés-Vásquez 552 & 143), Guiiña dxuladi (Zapateco, Oaxaca, Sánchez L. & Trujillo V. 874), Guien guiix (Oaxaca, Ruiz Núñez 7), Guiinya xigundu (Zapateco, Oaxaca, Sánchez L. 317), Kulum its (Huastec, S. Luis Potosí, Alcorn 2369), Max ik (Campeche, Álvarez 89; Yucatán, Ucan et al. 3527 & 3893), Lak’su pin (Tot, Puebla, Villalobos C. & Guerrero 205), Tsakam its (Huastec, S. Luis Potosí, Alcorn 1406; Veracruz, Alcorn 1903), Xmax ik (Yucatán, Ucan 4617), Aj max iik (Yucatán, Ucan 5058); Peru: Ají (Quichua, Loreto, Lewis et al. 12906), Cusharu’ nu’ca” (Chayahuita, Loreto, Odonne 561), Imiá (Achual Jívaro, Loreto, Lewis et al. 11196), Nuca (Loreto, Odonne 25), Nu’ca (Loreto, Odonne 626), Uchu (Loreto, Lewis et al. 12552), Yaa Jimia (Amazonas, Salaün 185), Yampit jima (Amazonas, Berlin 2016), Yanco nu’ca” (Chayahuita, Loreto, Odonne 563); Surinam: Lombo riwit (Ja, Commewijne, Heilbron & Sanredjo 6), Lombo kusti ‘pepper of god’ (Commewijne, Heilbron & Sanredjo 6).
Uses
This taxon is used as an ornamental, for food and for medicine. The fruits are harvested by local people and are widely used and much prized throughout its distribution as a hot seasoning; they are also eaten fresh, dry or in vinegar, raw or toasted. Some medicinal properties have been attributed to the leaves and fruits in different countries (see Table
Preliminary conservation assessment
EOO (37,301,728.615 km2); AOO (4,496 km2). Capsicum annuum var. glabriusculum is not under threat for the time being.
Discussion
Capsicum annuum var. glabriusculum, better known as ‘chiltepin’ or ‘chilipequin’ (with some variations of these names) in Mexico and Central America (see common names) or ‘bird pepper’ in the United States and the Caribbean, belongs to the Annuum clade (
In herbaria or in literature, many names have been misapplied to the specimens of this variety, such as C. baccatum, C. frutescens, C. conoides, C. annuum var. conoides, C. annuum var. baccatum, C. frutescens var. baccatum and so on. Based only on the morphology of the fruits, this variety is sometimes confused with wild C. baccatum var. baccatum. While the fruiting calyx of C. annuum var. glabriusculum has 0–5 inconspicuous appendages and the fruits are generally more ovoid with an acute to slightly obtuse apex (rarely truncate), in C. baccatum var. baccatum, the calyx has five appendages up to 2 mm in length and the fruits are generally more globose or subglobose to ellipsoid with a truncate or flattened apex (very rarely acute to slightly obtuse). In addition to the differences in the fruits, C. annuum var. glabriusculum has solitary or paired flowers (rarely three flowers), stellate corollas that are entirely white to greenish-white without spots within and connivent blue, bluish-grey or purple anthers at anthesis (Fig.
In describing C. chlorocarpum,
Capsicum laurifolium was described, based on two different specimens in G-DC, both mounted on the same sheet. The right hand specimen (Anonymous 67, G00131902) comes from the Island of Guadeloupe in the Leeward Islands, part of the Lesser Antilles in the Caribbean. The left hand specimen is that of Blanchet from Bahia (Brazil). Of these two collections, we have selected the most complete specimen that most closely matches the data in the protologue (Blanchet 3098 A) as the lectotype (G00131882).
Specimens examined
See Suppl. material
Capsicum baccatum
Type
“Habitat in Indiis” Herb. Linn. N° 249.3 (lectotype, designated by
Capsicum baccatum var. baccatum
Capsicum pulchellum Salisb., Prodr. Stirp. Chap. Allerton: 134. 1796, nom. illeg. superfl. Type. Based on Capsicum baccatum L. (cited in synonymy).
Capsicum microcarpum Cav., Descr. Pl. (Cavanilles): 371. 1802. Type. Cultivated in the Royal Botanical Garden in Madrid, Spain “H.R.M. [Hortus Regis Matritensis]. Se cría en la Havana... y se cultiva en el Jardín botánico” (lectotype, designated here: MA [MA-307276]).
Capsicum ciliare Willd., Enum. Pl. [Willdenow] 1: 243. 1809. Type. Cultivated in Berlin, Germany, of unknown origin “Cult. in Hort. Bot. Berol.”, C.L. Willdenow s.n. (lectotype, designated here: B [B-W04430-01-0]).
Capsicum indicum var. ribesium Dierb., Arch. Apotheker-Vereins Nördl. Teutschl. 30 (1): 29. 1829. Type. Based on C. baccatum L.
Capsicum comarim
Vell., Fl. Flumin.: 60. 1829 (“1825”); Fl. Flumin. Icon. 2: t. 2. 1831 (“1827”). Type. Brazil. [Rio de Janeiro]: “Colitur hortis, et sponte undequaque crescit” (lectotype, designated by
Capsicum cumanense Fingerh., Monogr. Capsic.: 17. 1832, nom. illeg. superfl. Type. Based on (renaming of) “Capsicum baccatum Kunth” [= C. baccatum L.] (cited in synonymy).
Capsicum microcarpum forma fruticosum Sendtn., Fl. Bras. (Martius) 10(6): 146. 1846. Type. Brazil “In Brasilia”, Pohl s.n. (lectotype, designated here: M [M-0171544]).
Capsicum microcarpum forma herbaceum Sendtn., Fl. Bras. (Martius) 10(6): 146. 1846. Type. Brazil. “Martius Mss. in Itinerario n. 132”, Prope Polafoco, Sept., C.F.P. Martius 132 (lectotype, designated here: M [M-0171543]; isolectotype, CORD [CORD00101765]).
Capsicum annuum var. microcarpum (DC.) Alef., Landw. Fl.: 133. 1866. Type. Based on Capsicum microcarpum DC.
Capsicum annuum var. baccatum (L.) Kuntze, Revis. Gen. Pl. 2: 449. 1891. Type. Based on Capsicum baccatum L.
Capsicum annuum var. microcarpum (Cav.) Voss, in Vilm. Blumengärtn., ed. 3. 1: 723. 1894. Type. Based on Capsicum microcarpum Cav.
Capsicum frutescens var. baccatum (L.) Irish, Rep. (Annual) Missouri Bot. Gard. 9: 99. 1898. Type. Based on Capsicum baccatum L.
Capsicum microcarpum var. glabrescens
Hassl., Repert. Spec. Nov. Regni Veg. 15: 244. 1918. Type. Paraguay. Canindeyú: “Iter ad Yerbales montium Sierra de Maracayu, in regione cursus superioris fluminis Jejui guazú”, Dec. 1898-99, É Hassler 5703 (lectotype, designated by
Capsicum annuum subsp. baccatum (L.) Terpó, Feddes Repert. 72: 173. 1966. Type. Based on Capsicum baccatum L.
Description
Erect shrubs or perennial herbs 0.50–3 (–3.5) m tall, rarely small trees, the main stem 2-2.5 cm in diameter at base, much branched from near the base and above, the branches spreading in a typical “zig-zag” appearance. Young stems 3–4-angled, fragile, green, sometimes the ridges purple, mostly glabrous to sparsely or moderately pubescent with appressed-antrorse, simple, uniseriate, 4–7-celled, eglandular trichomes 0.2–1.2 mm long; nodes usually purple; bark of older stems fissured, dark brown, glabrous; lenticels abundant. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous, slightly discolorous, dark green above, light green beneath, glabrescent to moderately pubescent with appressed-antrorse trichomes like those of the stems on both surfaces and margins; blades of major leaves 4.5–10 cm long, 2.5–6 cm wide, ovate, the major veins 5–8 on each side of mid-vein, the base somewhat asymmetric and attenuate, the margins entire, the apex acute; petioles (1.5–) 2–4 cm long, moderately to densely pubescent; blades of minor leaves 3.5–4.5 cm long, 2–3 cm wide, ovate, the major veins 4–5 on each side of mid-vein, the base rounded or truncate, the apex acute; petioles 0.5–1 cm long, moderately to densely pubescent. Inflorescences axillary, 2–3 flowers per axil, rarely flowers solitary; flowering pedicels (17–) 20–35 mm long, angled, erect or slightly spreading, geniculate at anthesis, glabrescent to moderately pubescent, the eglandular trichomes short, spreading or antrorse; pedicel scars inconspicuous. Buds globose, white with greenish-yellow spots, occasionally purple. Flowers 5-merous. Calyx 1.5–2 (–2.5) mm long, ca. 2–2.5 mm wide, cup-shaped, thick, green, pubescent with the same trichomes as pedicels and some glandular trichomes, the calyx appendages 5, (0.3–) 0.5–2 mm long, 0.2 mm wide, subequal, thick, erect, cylindrical, inserted close to the margin, pubescent with the same trichomes as calyx tube. Corolla 4.5–7.5 mm long, 10–13 mm in diameter, thick, white with greenish-yellow spots and white centre outside and within, rotate or rotate-stellate, with interpetalar membrane, lobed 1/3 or less of the way to the base, pubescent adaxially with short glandular trichomes (stalk 1–3-celled; head globose, peltate, unicellular) in the throat and base of the lobes, glabrous abaxially, the tube 4–5 mm long, the lobes 2.5–2.7 mm long, 3.4–3.5 mm wide, broadly triangular, spreading, the margins with very short eglandular trichomes, the tips acute, papillate. Stamens five, equal; filaments 2.5–3.5 mm long, white, inserted on the corolla 1–1.1 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.5–1.8 mm long, ellipsoid, white or pale yellow, more rarely greyish, not connivent at anthesis. Gynoecium with ovary 2.5–2.7 mm long, ca. 2 mm in diameter, ovoid, green; nectary 0.3–0.5 mm tall; styles dimorphic, short style 2–2.5 mm long, not exceeding the anthers length, long style ca. 3.5 mm long, exserted 1.4–1.7 mm beyond the anthers, cylindrical, white; stigma 0.3 mm in diameter, globose or discoid, pale green. Berry 6–8 (–10) mm in diameter, globose or subglobose, less frequently ellipsoid with truncate or flattened apex, 10–20 mm long, 4–7 mm in diameter, green when immature turning to greenish-black and bright red at maturity, deciduous, pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 20–35 mm long, erect, strongly angled, widened distally, green; fruiting calyx 3–4.5 mm in diameter, persistent, not accrescent, cup-shaped or discoid, green, the appendages 1.5–2.3 mm long, appressed to the berry or spreading. Seeds 12–15 per fruit, 2.5–4 mm long, 2.3–3 mm wide, ovoid, subglobose or C-shaped, pale yellow to yellow, the seed coat smooth or slightly reticulate (SM), cerebelloid (SEM), the cells irregular in shape, the lateral walls sinuate strongly sinuate; embryo imbricate.
Capsicum baccatum var. baccatum A flowering branch B calyx C glandular trichome of the calyx D eglandular trichome of the calyx E flower, upper view F sector of opened corolla G gynoecium H fruit I seed J seed, in cross section K embryo A–G from Hunziker 7350 H–K from Hunziker 1579. Drawn by N. de Flury. Published in
Capsicum baccatum var. baccatum A plant B flower bud C purple flower bud D, E, F flowers at anthesis, different views G young fruiting branch showing ovary with long and short styles H immature fruits I mature fruit A, C from Barboza 4913 B, D–F, H from Barboza 5038 G from Barboza 2431 bis, I from Barboza et al. 3419. Photos by G.E. Barboza.
Distribution
Capsicum baccatum var. baccatum is widely distributed in South America from northern Venezuela and Colombia through Peru, Bolivia, Paraguay, northern and north-eastern Argentina to south and eastern Brazil (Fig.
Ecology
Capsicum baccatum var. baccatum occurs in dry or humid subtropical or tropical forests with semi-deciduous or deciduous vegetation, between 150 and 1,900 m elevation; it is quite common in Chaco scrub forests, in gallery forests and in the margins or interior of secondary forests. It is frequently a ruderal in disturbed areas.
Phenology
Flowering from October to May. Fruiting from late November to September.
Chromosome number
2n = 2x = 24 (
Common names
Argentina: Coincho (Jujuy, Fabris 3454), Cumbarí (Misiones, Montes 15164), Quitucho (Salta, Hunziker 1985), Puta-parió (Corrientes, Martínez Crovetto 11125), Ají quitucho (Salta, Hunziker 1579), Ají del campo, Ajitucho (Salta, West 8389), Ají del monte (Salta, Rial Alberti s.n.), Pimenta del monte (Misiones, Montes 15164), Pimentón del monte, ají cumbarí (Misiones, Montes 15202); Bolivia: Arabibi (Santa Cruz, Zenteno-R 12798), Aribibe (Santa Cruz, Hurtado 296), Aribibi (Chuquisaca, Debouck 3019; Santa Cruz, de Michel 159), Arivivi (Chuquisaca, Serrano 1903; Santa Cruz, Cárdenas 4702), Cobincho (Tarija, Krapovickas & Schinini 39010), Ají aribibi (Cochabamba, Thomas 705), Aribibi silvestre (Beni, Rivero 218), Ají del campo (Tarija, Krapovickas & Schinini 39010), Arivivi grande o cumbarito (
Indigenous names
Bolivia: Pochetii (Trinitario, Cochabamba, Thomas 705), Winno, Sachimi (Yuracare, Cochabamba, Thomas 705); Colombia: Azi (Piapocos, Vichada, Rodríguez 164), Gugsobia (Amazonas, Cárdenas 9423), Kulana (And, Amazonas, Castro & Matapí 564), Kulana (Yucuna, Amazonas, Cárdenas 9403), Kuraraka (Letuama, Amazonas, Cárdenas 9401); Paraguay: Hõmpita (Ayoreo, Boquerón, Gragson 124), Nuuhá (Alto Paraguay, Schmeda 1584).
Uses
As fruits are generally extremely pungent, they are collected and stored for use as a food condiment by native populations. Some accessions of this wild pepper (“arivivi”) in Bolivia have been considered promising for their interesting agro-morphological and biochemical characteristics with potential for the development of high value products for different markets (
Preliminary conservation assessment
EOO (11,809,545.422 km2); AOO (1,212 km2). Capsicum baccatum var. baccatum is considered Least Concern (LC) for the time being.
Discussion
Capsicum baccatum var. baccatum is a member of the Baccatum clade and is related to C. rabenii and C. chacoense (
In an effort to clarify the taxonomy of C. baccatum, Eshbaugh and collaborators (
Capsicum baccatum var. baccatum typically exhibits 2–3 flowers per node, rarely solitary flowers, geniculate pedicels that are erect or declining at anthesis, 5-merous flowers with white rotate or rotate-stellate corollas with greenish-yellow spots, dimorphic styles and small, globose, subglobose or ellipsoid, erect, deciduous, red fruits (Fig.
Fruiting specimens of C. baccatum var. baccatum are very similar to C. rabenii and it is sometimes impossible to distinguish the two, especially if there are no annotations about the corolla colour (in C. rabenii, corolla lobe margins are purple). However, C. baccatum var. baccatum usually has glabrescent to moderately pubescent leaves in contrast to the densely lanose pubescence found abaxially along the main veins in C. rabenii (Fig.
Capsicum baccatum var. baccatum differs from C. chacoense, with which it is sympatric in some localities of Argentina, Bolivia and Paraguay, by usually having five calyx appendages, a larger and rotate or rotate-stellate corolla with greenish-yellow pigmentation within, staminal plaques with auricles fused to the corolla and long and short styles. In contrast, C. chacoense has a calyx with 5–10 unequal appendages, entirely white and smaller corollas (4–6 mm long), staminal plaques with auricles not fused to the corolla and homomorphic styles (Fig.
Some earlier botanists submerged the epithet baccatum under C. annuum (
Although the pungency of C. baccatum is regarded as low-mild (
For C. microcarpum forma herbaceum,
When
When
Specimens examined
See Suppl. material
Capscium baccatum var. pendulum
Capsicum pendulum Willd., Enum. Pl. [Willdenow]: 242. 1809. Type. Cultivated in the Berlin Botanic Garden, Germany “Habitat ... [Country unknown]. Cult. in Hort. Bot. Berol”., C.L. Willdenow s.n. (lectotype, designated here: B [B-W04431-01-0]).
Capsicum frutescens var. pendulum (Willd.) Besser, Cat. Jard. Bot. Krzemieniec: 29. 1816. Type. Based on Capsicum pendulum Willd.
Capsicum indicum var. pendulum (Willd.) Dierb., Arch. Apotheker-Vereins Nördl. Teutschl. 30: 28. 1829. Type. Based on Capsicum pendulum Willd.
Capsicum pendulum var. majus Dunal, Prodr. [A. P. de Candolle] 13(1): 425. 1852. Type. No locality cited (no specimens cited; no original material located; Dunal may have considered this the typical variety).
Type
Based on Capsicum pendulum Willd.
Description
Erect shrubs or perennial herbs (0.60–) 1–2.5 m tall, with the main stem 1.5–2.5 cm in diameter at base, much branched from near the base and above, the branches spreading in a typical “zig-zag” appearance. Young stems 3–4-angled, fragile, dark green or green, mostly glabrous to sparsely or moderately pubescent with appressed-antrorse, short to long, simple, uniseriate, 4–9-celled, eglandular trichomes 0.5–1.3 mm long; nodes green; bark of older stems green with light brown fissures, glabrous; lenticels absent or few. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, equal or subequal in shape. Leaves membranous, discolorous, dark green above, light green beneath, glabrous to glabrescent with short eglandular trichomes in margins and long, spreading, 5–9-celled, eglandular trichomes along the primary veins and in the vein axils beneath; blades of major leaves 5–12 (–14.5) cm long, 3–5 (–7) cm wide, ovate, the major veins 5–7 on each side of mid-vein, the base asymmetric and attenuate or symmetric and rounded, the margins entire, the apex acute or acuminate; petioles 2.5–7.5 cm long, sparsely pubescent; blades of minor leaves 3–5.8 cm long, 1.3–2.5 cm wide, ovate or elliptic, the major veins 3–4 on each side of mid-vein, the base rounded, the margins entire, the apex acute; petioles 1.7–2 cm long, sparsely to moderately pubescent. Inflorescences axillary, 2–3 flowers per axil or flowers solitary; flowering pedicels 20–50 mm long, terete or angled, erect, sometimes curved, geniculate at anthesis, glabrous, glabrescent to moderately pubescent, the trichomes short, antrorse; pedicels scars inconspicuous. Buds globose, white with greenish-yellow spots, occasionally purple. Flowers 5–8-merous. Calyx 2–3 mm long, 3–4.2 mm wide, cup-shaped, thick, strongly 10-nerved, green, glabrous or glabrescent, the calyx appendages 5–6, rarely up to 8, 0.9–2.5 mm long, 0.2 mm wide, subequal, thick, erect, cylindrical, inserted close to the margin, with the same pubescence as calyx tube. Corolla 8.5–15 mm long, 12–16 (–20) mm in diameter, thick, white with greenish-yellow to tan spots and a white centre outside and within, rotate to rotate-stellate, with interpetalar membrane, lobed less than 1/3 of the way to the base, pubescent adaxially with short glandular trichomes (stalk 1–3-celled; head globose, peltate, unicellular) in the throat and base of the lobes, glabrous abaxially, the tube 4–5 mm long, the lobes 3–3.3 (–5) mm long, 3.5–6 mm wide, triangular to broadly triangular, spreading, the margins finely ciliate, the tips acute, papillate. Stamens 5–8, equal; filaments (2.5–) 3–4 mm long, white, inserted on the corolla 1.2–1.5 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 2–2.6 mm long, ellipsoid or ovoid, yellow, brownish post-dehiscent, not connivent (rarely connivent) at anthesis. Gynoecium with ovary 2.5–3 mm long, 1.6–2.5 mm in diameter, 2–5-carpelar, light green, ovoid; ovules more than two per locule; nectary ca. 1.2 mm tall, yellowish-green; styles dimorphic, short style 1.3–2 mm long, not exceeding the anthers length, long style 2.5–3.5 mm long, at the same level or slightly exserted beyond the anthers, yellowish-white, cylindrical; stigma ca. 0.2 mm long, 0.7–0.8 mm wide, discoid or bilobed, pale green. Berry 20–180 mm long, (10–) 20–40 (–50) mm in diameter, usually elongate or elongate-curved, triangular or campanulate, rarely subglobose, the base truncate or obtuse, the apex rounded, blunt or pointed, dark green or green when immature, green, bright yellow, orange, brown or red at maturity, persistent, pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels (35–) 50–95 mm long, pendent, sometimes strongly curved, terete or slightly angled, widened distally, green; fruiting calyx 9–18 (–20) mm in diameter, persistent, slightly accrescent, campanulate, thick, somewhat corrugated or not, green, the appendages 0.5–2 mm long, appressed to the berry. Seeds 30–80 per fruit, (3–) 4–5.2 mm long, 3–3.8 (–4) mm wide, reniform or C-shaped, pale yellow to yellow, the seed coat smooth or slightly reticulate (SM), minutely reticulate (SEM), the cells polygonal to irregular in shape, the lateral walls straight to wavy; embryo imbricate.
Capsicum baccatum var. pendulum A plant B flower bud C flower, in pre-anthesis D flower short-styled, longitudinal section E flower long-styled, lateral view F–H flowers hexamerous showing connivent anthers and not connivent anthers I immature fruit J–M mature fruit A, B, E from Barboza 4886 C, D, I, K, L, M no specimen vouchers (cult. Pairumani, Cochabamba-Bolivia) F, G, H from Palombo 3 J from Barboza et al. 4824. Photos by G.E. Barboza.
Distribution
Capsicum baccatum var. pendulum is found from low to mid-Andean elevations, mainly in Bolivia and Peru, extending to Ecuador and Colombia in the north and reaching Argentina, Paraguay and south-western Brazil in the south (Fig.
Ecology
Capsicum baccatum var. pendulum is a cultivated plant adapted to many different ecological conditions between 150 and 3,400 m elevation.
Phenology
Flowering and fruiting all year.
Chromosome number
2n = 2x = 24 (
Common names
Argentina: Ají (San Juan, Ariza Espinar 3214; Corrientes, Benítez 76); Varita (Salta, Krapovickas & Schinini 28134), Ají picante (Salta, Hunziker 25496); Ají vainilla (Salta, Hilgert 1363), Puta parió (Corrientes, Martínez Crovetto 11125), Varita larga (Salta, Krapovickas & Schinini 28132), Ají huevo de gallo (Salta, Hilgert 1374); Bolivia: Ají (Santa Cruz, Williams 696; Tarija, Krapovickas & Schinini 39321), Aribibe (Santa Cruz, Hurtado 296), Aribibi (Santa Cruz, Heiser C281a, La Paz, Debouck et al. 3016), Ulupica (Tarija, Krapovickas & Schinini 31056), Aji acabeche (La Paz, Hinojosa & Wásra 1133), Ají amarillo (Cochabamba, Moscone 205), Aji Picante (Santa Cruz, Krapovickas & Schinini 32133), Locato largo (Santa Cruz, Heiser C290), Ají churcu, Ají rojo, Ají redondo, Pimentón colorado, Ají colorado gigantón (Santa Cruz,
Indigenous names
Argentina: Keuí (= picante) (Corrientes, Hunziker 7339); Bolivia: Kîî (Guaraní, Santa Cruz, Roca 0689); Paraguay: Ky y’ (Guaraní, Cordillera, Williams et al. 135), Pimenta í (Guaraní, Guairá, Williams et al. 121).
Uses
This domesticated variety, mostly known as ‘Ají’, ‘Ají amarillo’ or ‘Ají escabeche’, is an important component of the diet of the Bolivian and Peruvian native population, less so in Argentina, Brazil, Ecuador and Colombia. In Bolivia and Peru, many different pod types occur that differ both in morphological (shape, colour, size) and biochemical attributes (e.g. capsaicinoids, vitamin E, flavonoids and quercetin content and antioxidant capacity). These forms are consumed in regional cuisines as spices and vegetables, fresh or dehydrated and ground (
Preliminary conservation assessment
EOO (11,296,813 km2); AOO (356 km2). Capsicum baccatum var. pendulum is a very widespread cultivated plant and can be assigned a category of Least Concern (LC).
Discussion
Capsicum baccatum var. pendulum is a member of the Baccatum clade (
Many studies have been carried out that demonstrate the potential of this domesticated form in crop improvement. Capsicum baccatum var. pendulum encompasses a wide range of fruit morphology (e.g. fruit weight, size, shape and ripe colour), health-related compounds (flavonoids, polyphenols, quercetins, vitamin E, ascorbic acid, fat, amongst others) and capsaicinoids content (low to mild) (
Specimens examined
See Suppl. material
Capsicum baccatum var. umbilicatum
Capsicum umbilicatum
Vell., Fl. Flumin.: 61. 1829. Type. Brazil. [Rio de Janeiro]: “Colitur hortis” (lectotype, designated by
Type
Based on Capsicum umbilicatum Vell.
Description
Erect shrubs 1.50–2 m tall, with the main stem 1–1.5 cm in diameter at base, much branched from near the base, the branches spreading in a typical “zig-zag” appearance. Young stems 3–4-angled, fragile, green, mostly glabrous to sparsely pubescent with appressed-antrorse, short, simple, uniseriate, 4–5-celled, eglandular trichomes 0.3–0.6 mm long; nodes green or purple; bark of older stems green with light brown fissures, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous, concolorous or slightly discolorous, dark green above, light green beneath, glabrous to glabrescent adaxially and in margins with 5–6-celled eglandular trichomes and with long, spreading, 8–12-celled, eglandular trichomes along the primary veins and in the vein axils abaxially; blades of major leaves 5–14 cm long, 2.5–5 cm wide, ovate, the major veins 6–7 on each side of mid-vein, the base asymmetric and attenuate, the margins entire, the apex acute; petioles 2.5–7.5 (–9.5) cm long, sparsely pubescent; blades of minor leaves 4–4.5 cm long, 1.5–2.5 cm wide, ovate, the major veins 4–5 on each side of mid-vein, the base rounded, the apex acute; petioles 0.8–1.5 cm long, sparsely pubescent. Inflorescences axillary, 1–2 flowers per axil, rarely 3-flowered; flowering pedicels 25–35 mm long, angled, erect, geniculate at anthesis, rarely slightly curved and pendent, glabrescent, the trichomes short, antrorse; pedicels scars inconspicuous. Buds globose, white with greenish-yellow spots. Flowers 5–6-merous. Calyx 2–2.5 mm long, 3–3.8 mm wide, subequal, cup-shaped, thick, strongly 5–10-nerved, green, glabrescent to moderately pubescent with simple and some forked eglandular trichomes, the calyx appendages 5 or up to 8, 0.8–1.2 mm long, 0.2 mm wide, subequal, thick, erect, cylindrical, inserted close to the margin, with the same pubescence as calyx tube. Corolla 10–13 mm long, 12–14 mm in diameter, thick, white with greenish-yellow spots and a white centre outside and within, rotate-stellate with interpetalar membrane, lobed ⅓ or less than of the way to the base, pubescent adaxially with short glandular trichomes (stalk 1–3-celled; head globose, peltate, unicellular) in the throat and base of the lobes, glabrous abaxially, the tube 4 mm long, the lobes 5–6 mm long, ca. 3 mm wide, triangular or broadly triangular, spreading, sometimes with sparse eglandular trichomes abaxially, the margins finely ciliate, the tips acute, papillate. Stamens five, equal; filaments 2.5–2.7 long, white, inserted on the corolla 1–1.2 mm from the base, with auricles fused to the corolla at point of insertion; anthers 2.5–2.7 mm long, ellipsoid, yellow, not connivent at anthesis. Gynoecium with ovary 2–2.2 mm long, ca. 1.5 mm in diameter, 3 (–4)-carpelar, light green, ovoid; ovules more than two per locule; nectary ca. 1.2 mm tall, yellowish-green; styles dimorphic, short style 1.25–1.5 mm long, not exceeding the anthers length, long style 2.5–2.8 mm long, at the same level of the anthers or slightly exserted, white, cylindrical; stigma ca. 0.2 mm long, 0.6–0.7 mm wide, discoid, yellowish-green. Berry 25–40 mm long, 30–55 mm in diameter, campanulate-umbilicate, the base truncate or obtuse, the apex rounded, blunt or pointed, light green when immature, orange-red or bright red at maturity, persistent, pungent, the pericarp thick, opaque, without giant cells (endocarp smooth); stone cells absent; fruiting pedicels 25–35 mm long, pendent, sometimes strongly curved, strongly angled, widened distally, green; fruiting calyx 10–16 mm in diameter, persistent, slightly accrescent, slightly campanulate, thick, strongly nerved, green, the appendages 1–2 mm long, spreading or slightly recurved. Seeds 30–68 per fruit, 3.5–4.2 mm long, 3–4 mm wide, C-shaped, pale yellow to yellow, the seed coat slightly reticulate (SM), minutely reticulate (SEM), the cells polygonal to irregular in shape, the lateral walls straight to wavy; embryo imbricate.
Distribution
Capsicum baccatum var. umbilicatum is a cultigen and has been reported from Brazil, Colombia, Peru, Paraguay, Bolivia, Argentina and the Caribbean Islands (Fig.
Ecology
Capsicum baccatum var. umbilicatum is a cultivated plant adapted to wet and semi-shaded places.
Phenology
Flowering from October to June; fruiting from December to July.
Chromosome number
2n = 2x = 24 (
Common names
Argentina: Campanita (Salta, Barboza 164), Farolito, mitra (Distrito Federal, Melchiore s.n.); Brazil: Pimiento pitonga (Rio de Janeiro, Scaldaferro 57), Pimenta-de-cheiro-amarela (Amapá, Pereira et al. 1830), Chapéu-de-frade, Cabeça-de-frade, Chapéu-de-bispo, Pimenta-chapéu, Pimenta-de flor, Pimenta-de-cheiro (Roraima,
Uses
The mildly hot fruits of this cultivated variety are valued for their use in dishes with tropical fruits, sauces, Caribbean fish stews, curries and chutneys (
Preliminary conservation assessment
EOO (6,070,048 km2); AOO (64 km2). Capsicum baccatum var. umbilicatum is a cultigen in the Least Concern (LC) category.
Discussion
Capsicum baccatum var. umbilicatum belongs to the Baccatum clade (
Capsicum baccatum var. umbilicatum A reproductive branch B flower C section of the calyx showing the venation D, E eglandular trichomes of the abaxial surface of the calyx F glandular trichome of the adaxial surface of the calyx G sector of opened corolla H eglandular trichome of the corolla lobes I, J anthers, in dorsal and ventral views, respectively K gynoecium with long style L gynoecium with short style M ovary trilocular, in cross section N seed O seed, in cross section P structure of seed coat at the seed margin Q structure of seed coat at the seed body R embryo. From Rodríguez s.n. (CORD 241). Drawn by N. de Flury. Published in
Capsicum baccatum var. umbilicatum A plant B flower bud C flower, lateral view D flower short-styled E flower long-styled F flower hexamerous G flower, seen from behind H immature fruit I, J mature fruits A–I from Barboza 5163, photos by G.E. Barboza J from Carrizo García 101, photo by C. Carrizo García.
Specimens examined
See Suppl. material
Capsicum benoistii
Type
Ecuador. Tungurahua: Baños, 3 Apr 1931, M.R. Benoist 4204 (holotype: P [P04023406]).
Description
Erect shrubs with few branches. Young stems 3-angled, light brown, glabrous or sparsely pubescent with appressed-antrorse, simple, uniseriate, 3–5-celled, eglandular trichomes 0.3–1.1 mm long; bark of older stems dark brown, angled, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair markedly unequal in size, subequal in shape. Leaves membranous, discolorous, dark green above, light green beneath, glabrous or with sparse trichomes adaxially and abaxially, similar to those of the stems, more abundant on main veins; blades of major leaves 8.5–12 cm long, 2.8–6 cm wide, ovate or elliptic, the major veins 4–5 (–6) on each side of mid-vein, the base asymmetric and attenuate, the margins entire, the apex long-acuminate; petioles 0.5–1 cm long, glabrous or glabrescent; blades of minor leaves 2.4–6 cm long, 1.7–4 cm wide, ovate or elliptic, the major veins 3–4 on each side of mid-vein, the base asymmetric and rounded, the margins entire, the apex acute or rounded; petioles 0.1–1 cm long, glabrescent or sparsely pubescent. Inflorescences axillary, 3–6 flowers per axil; flowering pedicels 13–20 mm long, angled, filiform, pendent, non-geniculate at anthesis, moderately to densely pubescent, the eglandular trichomes long, spreading to antrorse; pedicels scars inconspicuous. Buds ovoid, colour unknown. Flowers 5-merous. Calyx 2–2.5 mm long, ca. 5 mm wide, cup-shaped, thick, colour unknown, moderately pubescent with the same trichomes as pedicels, the calyx appendages 5, 2.5–3.5 mm long, ca. 0.5 mm wide, equal or subequal, thick, erect, subulate, inserted close to the margin, with the same pubscence as calyx tube. Corolla ca. 12–13 mm long, thick, deeply stellate without interpetalar membrane, lobed nearly to the base, glabrous adaxially and abaxially, the tube ca. 3 mm long, the lobes ca. 9 mm long, ca. 2 mm wide, narrowly triangular, erect, the margins and the tips pubescent. Stamens five, equal; filaments 3–3.2 mm long, inserted on the corolla 1.5 mm from the base, with auricles fused to the corolla at the point of insertion; anthers ca. 3 mm long, ellipsoid, not connivent at anthesis. Gynoecium with ovary 1.3–1.7 mm long, 1.2–1.5 mm in diameter, subglobose; ovules more than two per locule; nectary ca. 0.3 mm tall; styles dimorphic, long style ca. 6.5 mm, short style ca. 3.6 mm, clavate; stigma 0.3 mm long, 0.5 mm wide, globose. Berry and seeds unknown.
Capsicum benoistii A flowering branch B flower C calyx D section of the calyx showing the venation E eglandular trichome of the calyx F opened corolla G gynoecium with long style H gynoecium with short style. From Benoist 4204. Drawn by N. de Flury. Published in
Distribution
Ecology
Capsicum benoistii grows in thickets in montane forests, between 1,500 and 2,600 m elevation.
Phenology
Flowering from March to May. Fruiting time unknown.
Chromosome number
Not known.
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (2,627.651 km2); AOO (12 km2). Considering the extent of occurrence, the area of occupancy, the few localities (3) where it was collected and the decline observed in its geographic range, we assign C. benoistii the Endangered (EN; B1+2ab(i,ii) category. The species has not been collected since 1978 despite recent intensive field explorations in the same locations (
Discussion
The affinities of C. benoistii have not yet been explored and, due to the lack of data on some morphological characters, it is not assigned to any of the recognised clades (
The presence of heterostylous flowers in C. benoistii is unusual amongst Capsicum species. It has been reported in C. chinense (
Specimens examined
See Suppl. material
Capsicum caatingae
Type
Brazil. Bahía: Cachoeira, Estação da EMPASA, Vale dos Rios Paraguaçu e Jacuipe, 12°32'39"S, 39°05'00"W, 40–120 m elev., Jun 1980, P. do Cavalo et al. 162 (holotype: HUEFS [HUESF000001216, acc. # 00920]; isotypes: ALCB [ALCB000131, acc. # 07938], RB [RB00461411, acc. # 263323]).
Description
Small trees or erect shrubs 2–4 (–6) m tall, the main stem thick, 2.5–5 cm in diameter at base and with indefinite growth up to 5 m high, few branched, the branches slender or scandent. Young stems 3–4-angled, rigid, grey, glabrescent or, more rarely, sparsely pubescent with antrorse, simple, uniseriate, 3–7-celled, eglandular trichomes 0.2–0.9 mm long, sometimes furcate trichomes 0.7–0.9 mm long or minute, simple, glandular trichomes, ca. 0.1 mm long; nodes solid, green; bark of older stems grey or brown, glabrous; lenticels light brown. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size and similar or dissimilar in shape. Leaves membranous or papery, slightly discolorous, glabrescent to moderately pubescent on both sides, with antrorse, 5–8-celled, eglandular trichomes 0.4–1.2 mm long, sometimes with branched trichomes 0.7–0.9 mm long and small glandular hairs (stalk short, 2–3-celled; head multicellular or unicellular), especially on veins abaxially; blades of major leaves 4–11.5 (–20) cm long, 1.5–2.4 (–8.5) cm wide, ovate to elliptic, the major veins 4–5 on each side of mid-vein, the base unequal and short-attenuate, the margins entire, the apex acute or somewhat acuminate; petioles (0.5–) 0.7–2.5 cm long, moderately pubescent adaxially; blades of minor leaves 1.5–2 cm long, 0.7–1.3 cm wide, ovate, the major veins 3–4 on each side of mid-vein, the base short-attenuate or truncate, the margins entire, the apex acute; petioles 0.2–0.5 cm long, moderately pubescent adaxially. Inflorescences axillary, congested, 5–13 (–20 or more) flowers per axil; flowering pedicels 7–21 (–28) mm long, terete, pendent, non-geniculate at anthesis, green with violet tones, glabrescent to moderately pubescent, the eglandular trichomes short, antrorse; pedicels scars conspicuous, somewhat corky. Buds globose to ellipsoid, cream, greenish-white or purple at the apex. Flowers 5-merous. Calyx 1.2–1.7 (–2) mm long, 2–2.5 mm wide, cup-shaped, circular in outline, thin, weakly 5-nerved, green, greenish-purple or purple, sparsely pubescent with the same antrorse eglandular and glandular trichomes of the leaves, without appendages. Corolla 4.5–6 (–8) mm long, lilac or purple, greenish-yellow at the base outside, with different tones of purple and a narrow white marginal band in the lobes and greenish-yellow to yellowish-white centre within, stellate with narrow interpetalar membrane, lobed nearly halfway to the base, pubescent adaxially with a continuous ring of small glandular trichomes (stalk short, 1–2-celled; head globose, unicellular) in the throat and base of the lobes, glabrous abaxially, the tube 2.8–3.2 mm long, the lobes 2.9–3.5 mm long, 1.7–2.4 mm wide, broadly triangular, spreading, the margins involute and finely ciliate, the tip cucullate, papillate. Stamens five, equal; filaments (0.8–)1.1–1.75 mm long, greenish-white or white, inserted on the corolla ca. 2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.4–2.1 mm long, ellipsoid, light green or yellow, not connivent at anthesis. Gynoecium with ovary 1.1–1.4 in diameter, pale green, subglobose; ovules more than two per locule; nectary ca. 0.3–0.4 mm tall; styles homomorphic, (4.3–) 4.6–4.8 mm, exserted ca. 1 mm beyond the anthers, pale yellow or cream, clavate; stigma ca. 0.6 mm in diameter, globose or 0.15 mm long, 0.6 mm wide, discoid, light green. Berry (5–) 7–11 mm in diameter, globose, slightly flattened at the apex, green or yellowish-green when immature, red at maturity, deciduous, pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels (15–) 20–25 mm long, pendent, terete, inflated, strongly widened distally and with a constriction at the junction with the calyx, green or purple; fruiting calyx 3–4 mm in diameter, persistent, not accrescent, discoid, 5 (–10)-nerved, the margin entire or sometimes easily torn, green or purple. Seeds (9–) 11–17 per fruit, 3.2–3.7 mm long, 2.2–2.8 mm wide, C-shaped, pale yellow, the seed coat reticulate and tuberculate at margins (SM), cerebelloid (SEM), the cells irregular in shape, the lateral walls wavy at margins and strongly sinuate in the central zone; embryo imbricate.
Distribution
Capsicum caatingae is endemic to the north-eastern States of Brazil (Alagoas, Bahia, Pernambuco Sergipe and northern Minas Gerais States, Fig.
Ecology
Capsicum caatingae is a xerophytic species usually found in the margins of arid open Caatinga forests (Seasonal Deciduous and Seasonal Residual Forests) and in the anthropogenic Caatinga, more rarely in degraded humid forests (Floresta Estacional Decidual or Semidecidual). It is quite common in savannahs or amongst granitic and gneissic outcrops (‘inselbergs’), growing with thorny, deciduous arboreal and shrubby vegetation, between 100 and 950 m elevation.
Phenology
Flowering from December to August; fruiting from February to October.
Chromosome number
n = 12 (
Common names
Brazil. Caraibera (Alagoas, Silva & Moura 1586), Murta (Sergipe, Silva 287), Pimenta brava (Bahía, Pinto & Bautista 104), Semente-de-macaco (Alagoas, Oliveira 7).
Uses
None recorded.
Preliminary conservation assessment
EOO (270,442.695 km2); AOO (276 km2). The large extent of occurrence and the number of localities where C. caatingae was collected indicate Least Concern (LC) category. Given its highly specialised habitat limited to the Caatinga Biome, some subpopulations may be adversely affected because deforestation has intensified rapidly in recent years due to the consumption of native firewood for domestic and industrial purposes, over-grazing and changes in the ecosystem due to pasture and agricultural expansion (MMA-Brazil 2020).
Discussion
Capsicum caatingae is a member of the Caatinga Clade (
Capsicum caatingae A flowering branch B branched trichome from leaf C flower D calyx E, F, G glandular trichomes of the calyx and leaf venation H eglandular trichome of the calyx I sector of opened corolla J eglandular trichome of the corolla lobes K gynoecium L ovary, in cross section M node of a fruiting branch N fruit, in cross section O seed P seed, in cross section Q embryo. From Hunziker 25233. Drawn by N. de Flury. Published in
Capsicum caatingae is an unusual species with a combination of uncommon features rarely found amongst its congeners: arborescent habit, with indefinite growth of the main stem reaching up to 6 m high (15 m fide Bautista & Pinto 1023), very congested inflorescences with up to 20 or more flowers per node, corolla with varied colours (lobes white edged, then deep purple or variations of purple colour, tube with greenish-yellow to yellowish-white centre) and terete and inflated fruiting pedicels with a strong annular constriction at the junction with the calyx base (Fig.
Capsicum caatingae A flowering branch B young fruiting branch C immature fruits (note the purple pedicels) D node of a fruiting branch, some fruits already fallen down. No specimen voucher. Photos taken in Federal University of Viçosa (Minas Gerais) by C. dal Zovo (Associazione PepperFriends).
Capsicum caatingae differs from C. parvifolium in the absence of calyx appendages (five appendages in C. parvifolium), the number of flowers per node (up to 20 or more flowers vs. not more than seven flowers in C. parvifolium) and the fruit and seed colour (red berry with pale yellow seeds vs. greenish-golden yellow translucent berry with brownish-black seeds in C. parvifolium). Capsicum caatingae is sometimes sympatric with C. longidentatum from which it differs by its arborescent growth (vs. shrubby growth), the indumentum mostly of simple trichomes (vs. indumentum of branched and dendritic trichomes), the lack of calyx appendages (vs. five, rarely six, calyx appendages), the mostly purple corolla (vs. corolla white with greenish-yellow spots) and the pungent red fruit with pale yellow seeds (vs. non-pungent probably yellowish-green fruit with brown to brownish-black seeds).
Specimens examined
See Suppl. material
Capsicum caballeroi
Type
Bolivia. Santa Cruz. Prov. Caballero: Parque Nacional Amboró, Cerro Bravo, 10 km al N de Comarapa, 17°49.5'S, 64°32.5'W, 2400–2500 m elev., 7–10 Apr 1994, I. Vargas C. & J.M. Camacho 3118 (holotype: USZ; isotypes: CORD [CORD00003917], MO [MO-1921597, acc. # 5959888], NY [00745836], US [00902045, acc. # 3520370]).
Description
Erect shrubs or more rarely small trees 0.70–5 (–7) m tall, with the main stem thick, 2.5–3 cm in diameter at base, few to much branched above. Young stems angled, rigid, green, glabrous to sparsely pubescent, with appressed-antrorse, simple, uniseriate, 3–4-celled, eglandular trichomes 0.07–0.2 mm long; nodes solid, green; bark of older stems brown or brownish-grey, glabrous; lenticels few, light brown. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar or dissimilar in shape. Leaves coriaceous, slightly discolorous, glabrous on both sides or sparsely pubescent along the mid-vein abaxially, with simple, eglandular trichomes 0.2–0.4 mm long; blades of major leaves 4–13.5 cm long, 1.5–4 cm wide, elliptic, the major veins 4–5 on each side of mid-vein, the base acute to short-attenuate, the margins entire slightly revolute, the apex acuminate; petioles 0.2–0.8 cm long, glabrous; blades of minor leaves 2–4.5 cm long, 1–2 cm wide, elliptic or ovate, major veins 2–3 on each side of mid-vein, the base short-attenuate, the margin entire slightly revolute, the apex acute; petioles 0.3–0.5 cm long, glabrous. Inflorescences axillary, 1–2 flowers per axil; flowering pedicels 20–40 (–50) mm long, terete, slightly pendent to pendent, non-geniculate at anthesis, green, glabrous; pedicels scars inconspicuous. Buds ellipsoid, yellow. Flowers 5-merous. Calyx 1.5–3 mm long, ca. 3 mm wide, cup-shaped, thick, pale green, sparsely pubescent with the same antrorse eglandular trichomes of the young stems, the calyx appendages 10, unequal, the five main appendages 1–3.2 mm long, the five secondary appendages 0.8–2 mm long, erect, subulate, inserted close to the margin. Corolla (10–) 11–14 (–18) mm long, 4–6 mm in diameter, thick, entirely pure yellow or lemon-yellow, campanulate without interpetalar membrane, shallowly 5-lobed; glabrous adaxially and abaxially, the tube 7–10 mm long, the lobes 3–4 mm long, ca. 2 mm wide, narrowly triangular, recurved, the margins involute and finely ciliate, the tips deeply acute, papillate. Stamens five, equal; filaments 4–6 mm long, cream, inserted on the corolla 1–2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 2–3 mm long, ellipsoid, yellow, not connivent at anthesis. Gynoecium with ovary ca. 2 mm long, 1.5 mm in diameter, pale green, ovoid; ovules more than two per locule; nectary ca. 0.4–0.5 mm tall; styles homomorphic, 7–9 mm, scarcely exserted beyond the anthers, cream, clavate; stigma ca. 1 mm in diameter, whitish, capitate. Berry (9–) 10–16 mm in diameter, globose, slightly flattened at the apex, pale green or white when immature, bright red at maturity, persistent, pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 25–50 mm long, pendent, curved, terete, strongly widened distally and with a constriction at the junction with the calyx, green; fruiting calyx 3–5 mm in diameter, persistent, not accrescent, discoid, green, the appendages (1–) 3–5 mm long, appressed to the berry. Seeds 5–21 per fruit, 3–4 (–5) mm long, 3.8–5 mm wide, C-shaped or teardrop-shaped, pale yellow or nearly white, the seed coat smooth and reticulate at margins (SM), cerebelloid-reticulate (SEM), the cells irregular and polygonal in shape, the lateral walls strongly sinuate to nearly straight at the seed margin; embryo imbricate or coiled.
Distribution
Capsicum caballeroi is an endemic species from central Bolivia (Santa Cruz, Cochabamba and Chuquisaca Departments, Fig.
Ecology
Capsicum caballeroi is a rare element of montane cloud forests (Yungas and Bosque Tucumano-Boliviano Montano), found in very moist shaded wooded quebradas or at the margin of the forest between 1,000 and 2,600 m elevation.
Phenology
Flowering and fruiting probably all year long; a peak of flowering was observed in November to early January and fruiting from January to June.
Chromosome number
2n = 2x = 24 (Barboza et al. 3655, see Table
Common names
Bolivia. Aribibi (Cochabamba, Fernández T. et al. 2007), Ají de monte (Santa Cruz, Vargas C. & Prado 1282), Ulupica de yunga (Santa Cruz, Vargas C. et al. 1343).
Uses
None recorded.
Preliminary conservation assessment
EOO (14,005.388 km2); AOO (84 km2). Capsicum caballeroi grows mostly in the cloud forest of the Amboró National Park and peripheral areas where the population consists of few individuals; although found in a relatively large geographical area, we observed a severe decline of both the EOO and the AOO of this species due to the continuing indiscriminate deforestation occurring in the last years; in this way, we consider C. caballeroi under threat and assign the Vulnerable category (VU; B1ab(ii,iii)).
Discussion
Capsicum caballeroi belongs to the Bolivian clade (
Capsicum caballeroi A plant B leaf pairs C major leaf D flower bud E flower F flower, in front view G immature fruit H mature fruit I mature fruit, in cross section, showing the seeds A from Barboza et al. 4907 B, C, E, F, H Barboza et al. 3655 D, G, I from Barboza et al. 4908. Photos by G.E. Barboza and S. Leiva González.
Capsicum caballeroi is morphologically most similar to C. piuranum, an endemic species from northern Peru and can be distinguished from that species in its calyx (green calyx with 10 unequal linear appendages vs. purple or greenish-purple calyx with five equal subulate appendages), its corolla lobes (recurved vs. spreading) and its fruits and seeds (red pungent fruits with pale yellow or nearly white seeds vs. orange to red non-pungent fruits with dark brown to black seeds). The fruits and the seeds of C. caballeroi are larger than those of C. piuranum. Capsicum caballeroi is sympatric with C. minutiflorum; they share yellow corollas and red fruits, but are distinguished by the coriaceous leaves, calyx with 10 unequal appendages, campanulate and larger corollas (10–14 mm long) and fruits (9–16 mm in diameter) of C. caballeroi vs. the membranous leaves, calyx with five equal or subequal appendages, stellate, smaller corollas (6.5–8.5 mm long) and fruits (7–10 mm in diameter) of C. minutiflorum (Fig.
Specimens examined
See Suppl. material
Capsicum campylopodium
Capsicum gracilipes Dunal, Prodr. [A. P. de Candolle] 13(1): 418. 1852. Type. [Brazil]. Rio de Janeiro, 1834, C. Gaudichaud 513 (lectotype, designated here: G-DC [G00131901]; isolectotypes: F [F neg. F0BN002869], MPU [MPU013436 fragment], P [P00410015, P00410016]).
Capsicum salicifolium Dunal, Prodr. [A. P. de Candolle] 13(1): 418. 1852. Type. Brazil. Rio de Janeiro: “In provinciã Rio de Janeiro, Serra dos Órgãos”, Oct. 1833, A.-C. Vauthier 528 (lectotype, designated here: G-DC [G00131881]; isolectotypes: CORD [CORD00006953], F [F neg. 6846 ex G-DC + F0093724F fragment, acc. # 644821], GH [GH00077007], MPU [MPU023040 fragment], P [P00410013, P00410014]).
Type
Brazil. “Brasilia”, [no date], F. Sellow 6 (lectotype, designated by
Description
Erect subshrubs or shrubs, 0.5–2 m tall, with the main stem thick, up to 2.5 cm in diameter at base, much branched above, the branches dichotomously spreading in a typical “zig-zag” appearance. Young stems striate, fragile, green, glabrous to glabrescent, with antrorse, curved, simple, uniseriate, 3–4-celled eglandular trichomes 0.3–0.5 mm long; nodes solid, green; bark of older stems dark brown, glabrous; lenticels few, light brown. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous, slightly discolorous, green or dark green above, light green beneath, glabrous or sparsely pubescent with appressed-antrorse, 3–5-celled, eglandular trichomes 0.2–0.4 mm long on both surfaces; blades of major leaves 4–11.5 (–20) cm long, 1.5–2.4 (–8.5) cm wide, elliptic to ovate, the major veins 5–7 on each side of mid-vein, the base attenuate and unequal, the margins entire, the apex acuminate to long-acuminate; petioles 0.5–1.7 cm, glabrescent or glabrous; blades of minor leaves 1.7–3.5 (–4.5) cm long, 0.5–2 cm wide, elliptic to ovate, the major veins 3–4 on each side of mid-vein, the base attenuate, the margins entire, the apex acute; petioles 0–0.5 cm, glabrous. Inflorescences axillary, 2–5 (–7) flowers per axil, rarely flowers solitary; flowering pedicels 9–14 mm, very thin, delicate, terete to slightly striate, erect to slightly spreading, geniculate at anthesis, entirely green or reddish basally, glabrescent, the eglandular trichomes short, antrorse; pedicels scars inconspicuous. Buds ovoid, cream or with greenish-yellow spots. Flowers 5-merous. Calyx (1–) 1.2–1.6 mm long, 1.4–1.5 mm wide, hemispherical, circular in outline, very thin, green, glabrous or rarely glabrescent, without appendages. Corolla 4.5–6.5 (–8) mm long, (6–) 6.4–7.5 (–11) mm in diameter, mostly cream outside, white or cream with two large golden yellow or ochraceous spots on each lobe and part of the limb and cream or white centre within, stellate with narrow interpetalar membrane, lobed more than 1/3 to nearly halfway to the base, pubescent adaxially with a continuous ring of glandular trichomes (stalk long, 2–3-celled; head globose, peltate, unicellular) in the throat and base of the lobes, glabrous abaxially, the tube 2–4 mm long, the lobes 1.8–3.5 (–4.3) mm long, 2–4 mm wide, triangular or broadly triangular, spreading, the margins finely ciliate, the tips acute, cucullate, papillate. Stamens five, unequal in length; three filaments short 1.5–2.3 mm long, the two longer 1.9–3 mm long, white or cream, inserted on the corolla 0.6–1 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 0.8–1.7 mm, ellipsoid, yellow, not connivent at anthesis. Gynoecium with ovary 0.7–1 mm long, 0.9–1.3 mm in diameter, light green, ovoid; ovules two per locule; nectary ca. 2.2 mm tall; styles homomorphic, 2.3–4 mm, somewhat exserted beyond the anthers, cream, clavate, slightly curved; stigma 0.1–0.2 mm long, 0.4 mm wide, discoid, pale green. Berry 3–5 mm long, 5–7 mm in diameter, globose-depressed, green when immature, greenish-golden yellow at maturity, deciduous, pungent, the pericarp thin, translucent, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 14–25 mm, pendent, angled, slightly widened distally, green; fruiting calyx 3–4.5 mm in diameter, persistent, not accrescent, discoid, green. Seeds 4 (–6) per fruit, 3.7–3.9 (–4) mm long, 3–3.3 mm wide, C-shaped or reniform, brownish-black, the seed coat smooth or faintly reticulate and tuberculate at margins (SM), reticulate-cerebelloid with pillar-like outgrowths at margins (SEM), the cells rectangular or polygonal at margin and irregular in seed body, the lateral walls straight to wavy at margins and sinuate in the central zone; embryo imbricate.
Distribution
Capsicum campylopodium is an endemic species from south-eastern Brazil (Rio de Janeiro, Minas Gerais and Espírito Santo States, Fig.
Ecology
Capsicum campylopodium is a typical component of the coastal Atlantic Forest (Mata Atlântica) and of some remnants of interior forests of the same biome. It is found in small colonies of a few individuals in shady or semi-shady places, sometimes also in sun, along roadsides or trails of the Ombrophilous Forest (Floresta Ombrófila Densa Submontana and Montana), between 100 and 1,200 m elevation.
Phenology
Flowering from late September to March; fruiting from December to April.
Chromosome number
n = 13 (
Common names
Brazil. Pimenta da Serra (Rio de Janeiro, Kuhlmann 6288).
Uses
None recorded.
Preliminary conservation assessment
EOO (58,586.312 km2); AOO (212 km2). Capsicum campylopodium is a relatively widespread species that occurs in many formally protected areas, such as Parque Nacional da Tijuca, Parque Estadual da Pedra Branca, Estação Ecologica Estadual de Paraiso, Reserva Ecologica de Rio das Pedras, Parque Municipal Ecológico da Prainha, Estação Biológica Caratinga, amongst others (see Suppl. material
Discussion
Capsicum campylopodium belongs to the Atlantic Forest clade (
Capsicum campylopodium A flowering branch B eglandular trichome of the leaf C flower D calyx E section of the calyx showing the venation F flower, upper view G sector of opened corolla H gynoecium I fruit J fruit, upper view K fruit, in cross section L anatomical detail of the pericarp (note the giant cell in the mesocarp) M seed N seed, in cross section O structure of seed coat at the seed margin P structure of seed coat at the seed body Q embryo. From Hunziker 25116. Drawn by L. Sánchez. Published in
Capsicum campylopodium A plant B flower C immature fruit D–F diagrams of different stages of fruit development D ovary, in cross section, showing the locules and the number of ovules E young fruit, in cross section (the lateral arrows indicate the fruit is flattened around the centre) F mature depressed fruit (one locule), in longitudinal section, showing the two seeds occupying the whole locule A from Barboza et al. 2057, photo by G.E. Barboza B, C from Bianchetti et al. 511, photos by L. Bianchetti.
Capsicum flexuosum, C. schottianum and C. campylopodium all lack calyx appendages and are sometimes extremely difficult to distinguish from one another due to some characters being poorly preserved in herbarium specimens. Capsicum campylopodium and C. schottianum both have clearly geniculate pedicels, a calyx with five evident nerves and greenish-golden yellow fruits with brownish-black to black seeds. The distinction of C. campylopodium from C. schottianum is based on calyx shape and size (hemispherical and ≤ 1.5 mm in diameter vs. cup-shaped and > 2 mm in diameter in C. schottianum), corolla colour (mostly white with large golden yellow spots within vs. white usually with purple and greenish-yellow spots within), fruit shape (globose-depressed vs. globose or subglobose) and number of seeds (four, very rarely six vs. ≥ six). The separation of C. flexuosum, the most distinctive species of the three, is based primarily on its lack of geniculate pedicels and its having a calyx with ten evident nerves, white corolla with yellowish-green spots within and red fruits (Fig.
For C. salicifolium,
Specimens examined
See Suppl. material
Capsicum carassense
Type
Brazil. Minas Gerais: Catas Altas, RPPN Serra do Caraça, trilha da gruta de Lourdes, após a capelinha, 20°05'41"S, 43°28'52"W, 1386 m elev., 26 Oct 2014, J.R. Stehmann, L.L. Giacomin, G.E. Barboza & S. Knapp 6347 (holotype [two sheets]: BHCB acc.#174038 [BHCB0019940_1, BHCB0019940_2]; isotypes: CORD [CORD00006968], RB [RB01220059, acc. # 674586], MBM).
Description
Erect shrubs (0.8–) 1–2 (–3) m tall, with the main stem somewhat thick and sparsely branched, the branches dichotomous and spreading horizontally. Young stems 3–4-angled, fragile, green, moderately to densely pubescent with uncinate and antrorse, simple, uniseriate, 3–5 (–6)-celled, eglandular trichomes 0.2–0.7 mm long, yellowish-brown when dried; nodes green or purple; bark of older stems brown, striate, pubescent; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous to chartaceous, discolorous, dark green above, paler beneath, moderately pubescent especially on the veins, with simple trichomes like those of the stem and sparse or frequent glandular trichomes (stalk unicellular; head multicellular) adaxially and abaxially; blades of major leaves 6–16 cm long, 0.9–2.5 cm wide, narrowly elliptic to lanceolate, the major veins 6–8 on each side of mid-vein, the mid-vein prominent and the secondary veins obscure, the base attenuate, the margins entire, the apex acute to obtuse; petioles 0.2–0.6 cm long, moderately pubescent; blades of minor leaves 2.9–3.9 cm long, 0.5–0.8 cm wide, narrowly elliptic, the major veins 2–3 (–4) on each side of mid-vein, the base attenuate, the margins entire, the apex obtuse; petioles 0.2–0.4 cm long, moderately pubescent. Inflorescences axillary, 2–4 flowers per axil; flowering pedicels (12–) 15–20 (–22) mm long, slightly angled, erect to spreading, geniculate at anthesis, green, moderately pubescent, the eglandular trichomes short or long, antrorse to spreading; pedicels scars inconspicuous. Buds ellipsoid, cream with greenish-yellow spots. Flowers 5-merous. Calyx 1.2–1.6 mm long, 2.5–3 mm wide, cup-shaped, thin, light green to cream, moderately pubescent with antrorse, curved, 3–5-celled, eglandular trichomes and sparse short glandular trichomes (stalk short, unicellular; head dark, elongate, multicellular), the calyx appendages five, (2.8–) 3–4 (–5) mm long, subequal, thick, erect, cylindrical, inserted very close to the margin. Corolla (8–) 10–12 mm long, 13–20 mm in diameter, thick, white with greenish-yellow spots outside, mostly with large purple spots on the lobes and the throat and cream centre within, stellate with abundant interpetalar membrane, lobed halfway or less of the way to the base, pubescent adaxially with a continuous ring of long glandular trichomes (stalk 2–3-celled; head globose, peltate, unicellular) in the throat and base of the lobes, glabrous abaxially, the tube 4.5–5 mm long, the lobes 4.5–6.5 mm long, 5–8 mm wide, broadly triangular to triangular, the margins densely pubescent, the tips cucullate. Stamens five, subequal; filaments 2.7–3.1 (–4.1) mm long, white, inserted on the corolla ca. 1 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.5–1.9 mm long, ellipsoid, blue, not connivent at anthesis. Gynoecium with ovary 1.3–1.5 mm long, ca. 1.2 mm in diameter, light green, subglobose to ovoid; ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, 4.3–5 (–7) mm long, barely exserted beyond the anthers, white, clavate; stigma ca. 0.2 mm long, ca. 0.7 mm wide, discoid, cream. Berry 6–7 mm in diameter, globose-depressed, green when immature, greenish at maturity, deciduous, pungent, the pericarp with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 18–25 mm long, pendent and slightly curved, slightly angled, widened at the apex, green; fruiting calyx ca. 4 mm in diameter, persistent, not accrescent, discoid, yellowish-green, the appendages spreading, green. Seeds 7–13 per fruit, 3.5–4 mm long, 2.5–3 mm wide, ellipsoid to reniform, brownish-black to black, the seed coat deeply reticulate and slightly tuberculate at margins (SM), reticulate with small pillar-like outgrowths at margins (SEM), the cells polygonal in shape, the lateral walls straight to wavy; embryo imbricate.
Capsicum carassense A flowering branch B–D leaf morphology E eglandular trichome of the stem F glandular trichome of the calyx G, H flower buds in different stages of development I flower J opened corolla K fruit L fruiting calyx. From Bianchetti et al. 1364. Drawn by L. Bianchetti. Published in
Distribution
Ecology
Capsicum carassense inhabits the understorey of the semi-deciduous montane Atlantic Forest, in a shaded and moist environment, between 1,000 and 1,390 m elevation.
Phenology
In flower from October to January, also in May; fruiting in December, February and April.
Chromosome number
Not known.
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (483.4 km2); AOO (32 km2). Capsicum carassense is considered Endangered (EN, B1ab(iii,iv)). We suggest this because of its very restricted geographic distribution, as well as the increasingly degraded habitat quality, especially associated with the extensive iron mining activities in the region (
Discussion
Capsicum carassense belongs to the Atlantic Forest clade (
| Character | C. mirum | C. cornutum | C. carassense | C. mirabile |
|---|---|---|---|---|
| Indument/trichomes (stems and leaves) | Densely pubescent/trichomes antrorse | Densely pubescent/trichome spreading | Moderately to densely pubescent/trichomes antrorse | Glabrate to sparsely pubescent/trichomes antrorse |
| Major leaf shape | Mostly elliptic, apex acute to acuminate | Ovate to widely elliptic, apex acuminate | Narrowly elliptic to lanceolate, apex acute to obtuse | Elliptic to ovate, rarely narrowly elliptic, apex acuminate to long- acuminate |
| Major leaf length/width ratio | 2.5–3 | 2.4–5 | (4–) 5–10 (–16) | (2–) 2.5–4 (–4.9) |
| Petioles length | 0.8–2.5 cm | 0.3–0.8 cm | 0.2–0.6 cm | 0.7–2.5 cm |
| Pedicels length | 12–17 mm | (22–) 25–35 mm | (12–) 15–20 (–22) mm | (13–) 16–25 mm |
| Buds colour | Purple | White with green and purple spots | Cream with greenish-yellow spots | Purple or greenish-purple |
| Calyx appendages | 10, subequal, spreading, long, (1.7–) 2–3.2 mm | (5–) 7–10, unequal, erect or spreading, short to long, 0.5–6 mm | 5, subequal, long, erect, (2.8–) 3–4 (–5) mm | 5, subequal, erect, short to long, (0.4–) 0.5–1.5 (–3) mm |
| Corolla size | 6–8 mm long, 11–14 mm in diameter | (8–) 9–14 mm long, 18–22 mm in diameter | (8–) 10–12 mm long, 13–20 mm in diameter | (6–) 7.5–12 mm long, (9–) 10–13 mm in diameter |
| Corolla colour | Almost entirely purple and a thin white border within | White with small purple or reddish-brown spots within | Mostly with large purple spots and a thin white border within | Mostly with large purple spots and a thin white border within |
Specimens examined
See Suppl. material
Capsicum cardenasii
Type
Cultivated at Indiana University greenhouse from seeds sent by M. Cárdenas from market in La Paz, Bolivia, 15 Aug 1956, C.B. Heiser Jr. 4196 (Paul Smith Ac.-1793) (lectotype, designated here: IND [IND1000063, acc. # 139347]; isolectotype: IND [IND1000064, acc. # 139348]).
Description
Erect shrubs or subshrubs, 0.8–2 (–2.5) m tall, with the main stem 1–1.5 cm in diameter at base, much branched from near the base, the fragile branches in a typical “zig-zag” appearance above. Young stems strongly angled, green, glabrescent with sparse appressed-antrorse, simple, uniseriate, 4–6 (–7)-celled eglandular trichomes 0.08–0.6 mm long and minute, simple, glandular trichomes (stalk short; head dark); nodes green; bark of older stems greyish-white or with light brown-green fissures, glabrescent; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair subequal in size and shape. Leaves membranous, slightly discolorous, glabrescent, with sparse eglandular trichomes similar to those on stems and many small glandular trichomes (stalk unicellular; head dark, multicellular) on both surfaces, the glandular trichomes more abundant along mid-vein abaxially; blades of major leaves 3–5 (–6.5) cm long, 1.4–2.5 cm wide, narrowly ovate or ovate-lanceolate, the major veins 3–4 on each side of mid-vein, the base attenuate, the margins entire, the apex acute; petioles 1.2–2 cm long, glabrous or glabrescent; the blades of minor leaves 2–3.5 cm long, 0.9–1.2 cm wide, narrowly ovate or ovate-lanceolate, the major veins 2–3 on each side of mid-vein, the base attenuate, the margins entire, the apex acute or obtuse; petioles 0.5–0.8 cm long. Inflorescences axillary, 2–3 flowers per axil or flowers solitary; flowering pedicels 8–18 (–22) mm long, angled, erect to slightly spreading, geniculate at anthesis, entirely green, or purple distally, with moderate small glandular trichomes (stalk transparent, uni-bicellular; head dark, multicellular) and sparse short, antrorse eglandular trichomes; pedicels scars inconspicuous. Buds ellipsoid or ovoid, lilac or violet. Flowers 5-merous. Calyx 1–3 mm long, 2–3 mm wide, cup-shaped, thick, green or green with violet spots, moderately pubescent with the same glandular and eglandular trichomes as the pedicels, the calyx appendages five, 1–2 mm long, 0.3 mm wide, subequal, thick, erect or spreading, cylindrical, inserted close to the margin, sparsely pubescent with the same trichomes as the calyx tube. Corolla (6–) 6.5–12 mm long, 8–11 (–13) mm in diameter, thick, almost completely violet or lilac, but white at the base and along the main veins outside and within, sometimes greenish-yellow spots near the base within, campanulate with interpetalar membrane, lobed 1/3 or less of the way to the base, the tube 7–9 mm long, pubescent adaxially with short glandular trichomes (stalk 1–2-celled; head globose, unicellular) up to near its base, glabrous abaxially, the lobes (1.5–) 3–3.2 mm long, 2–2.4 mm wide, triangular, erect or spreading, alternating with five minute interlobes, glabrous adaxially and abaxially, the margins papillate, the tips acute, papillate. Stamens five, equal; filaments (4–) 6–7 mm long, whitish or lilac, inserted on the corolla 1.5–2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.5–1.8 mm long, ellipsoid, lilac or bluish, not connivent at anthesis. Gynoecium with ovary 1.5–1.85 mm long, 1.2–1.6 mm in diameter, green, ovoid or pear-shaped; ovules more than two per locule; nectary 0.4–0.6 mm tall, light green; styles homomorphic, 4.5–5.7 mm long, exserted ca. 1 mm beyond the anthers, lilac or purple, clavate; stigma ca. 0.2 mm long, 0.8 mm wide, discoid or globose, pale green. Berry 6–10 mm in diameter, globose or subglobose, green when immature, orange-red to bright red at maturity, deciduous, pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 10–24 mm long, pendent, strongly angled, slightly widened distally, usually green; fruiting calyx 2–4 mm in diameter, persistent, not accrescent, discoid, green, the appendages 1–2.5 mm long, ca. 0.3 mm wide, appressed to the berry, spreading or reflexed. Seeds (4–) 5–13 per fruit, (2.5-) 3–4.2 mm long, (2.2–) 2.5–2.8 mm wide, C-shaped or subglobose, pale yellow to brownish-yellow, the seed coat reticulate to obscurely reticulate (SM), mostly cerebelloid (SEM), the cells irregular in shape, the lateral walls strongly sinuate in the central zone, rectangular to subpolygonal at margins; embryo imbricate.
Distribution
Capsicum cardenasii is a narrow endemic species restricted mainly to the highlands of La Paz Department (Bolivia, Fig.
Ecology
Capsicum cardenasii is a typical component of the warm and dry hillsides and remnants of forests in the inter-Andean valleys, growing preferentially in open places between cactus and Cassia, at 2,400–3,000 m elevation. It is cultivated by local people on small farms for local or family use of the fruits (Barboza, pers. obs.).
Phenology
Flowering from December to March; fruiting from February to April.
Chromosome number
n = 12 (Heiser and Smith 1958); 2n = 2x = 24 (
Common name
Bolivia. Ulupica (La Paz, Heiser & Smith 4196).
Indigenous name
Bolivia. Uaika (Aymará, La Paz, Barboza 4881).
Uses
The fruits are harvested directly from wild plants and marketed locally on a small scale (
Preliminary conservation assessment
EOO (1,032.864 km2); AOO (32 km2). Capsicum cardenasii is a geographically isolated species from the dry valleys of Luribay (Prov. Loayza), not far from La Paz; based on its extent of occurrence and the number of localities (6), it is assigned a status of Endangered (EN; B1ab(iii,iv)). It is harvested by local people; its area of distribution is poorly known.
Capsicum cardenasii A flowering branch B eglandular trichome of the calyx C glandular trichome of the corolla D glandular trichome of the adaxial surface of the calyx E flower F section of the calyx showing the venation G sector of opened corolla H gynoecium I fruit J anatomical detail of the pericarp (note the giant cell in the mesocarp) K seed L seed, in cross section M structure of seed coat at the seed margin N structure of seed coat at the seed body O embryo A–H from Eshbaugh 1527 I–O from Eshbaugh 2046 J. Drawn by N. de Flury.
Discussion
Capsicum cardenasii is resolved within the Purple corolla clade (
Phytogeographically,
Although the number of collections of C. cardenasii obtained in the field are scarce (8), the ease with which the seeds of this species germinate and produce fertile plants explains the large numbers (> 20) of specimens (and duplicates) gathered from plants in cultivation that are housed in many herbaria (see Specimens Examined).
The type collection of C. cardenasii consists of flowering specimens (two sheets dated 15 Aug 1956 at IND) obtained from seeds bought at the La Paz (Bolivia) marketplace; it is supposed that fruits came from warm, dry places along the Río Abajo, near La Paz, at 2400 m altitude (Heiser and Smith 1958). Of the two specimens in IND, that with barcode 1000063 is the most complete, is labelled type and is here designated as the lectotype. There are specimens distributed in other herbaria (e.g. CORD, IND, LIL, US) with the same collection number as the lectotype (Heiser 4196, Paul Smith Acc. 1793), but these have different dates of collection and should not be considered as duplicates of the lectotype.
Specimens examined
See Suppl. material
Capsicum ceratocalyx
Type
Bolivia. La Paz: Prov. Sud Yungas: 7.5 km (by road) from Huancané on road to San Isidro, 16°21'S, 067°30'W, 2225 m elev., 10 May 2001, M. Nee, L. Bohs, S. Knapp & J.M. Mendoza F. 51778 (holotype: LPB [LPB0003514]; isotypes: CORD [CORD00004289], MO [MO-2078805, acc. # 5959885], NY [01085523], USZ).
Description
Erect shrubs 0.80–3 m tall, much branched above. Young stems angled, green, glabrous to sparsely pubescent, with antrorse, curved, simple, uniseriate, 2–4 (–5)-celled, eglandular trichomes 0.09–0.5 mm long; nodes solid, green; bark of older stems brown, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair markedly unequal in size, similar in shape. Leaves coriaceous, slightly discolorous, glabrescent on both sides with sparse eglandular trichomes similar to the ones of the stems, mainly along the mid-vein abaxially; blades of major leaves 10–22 cm long, 4–7 cm wide, elliptic, the major veins 6–7 on each side of mid-vein, the base attenuate and unequal, the margin slightly revolute, the apex long-acuminate; petioles 1–2 (–3) cm long, glabrous; blades of minor leaves (3–) 5.5–7.5 cm long, 2–2.7 cm wide, elliptic, the major veins 3–4 on each side of mid-vein, the base short-attenuate, the margin slightly revolute, the apex acute; petioles 0.5–0.6 cm long, glabrous. Inflorescences axillary, congested, (4–) 8–10 (–12) flowers on a short rachis; flowering pedicels 10–23 mm long, strongly angled and nearly winged, erect, geniculate at anthesis, green, glabrous; pedicels scars prominent and corky. Buds ovoid, yellow. Flowers 5-merous. Calyx 1.8–2 mm long, ca. 3 mm wide, cup-shaped, slightly 5-nerved, sparsely pubescent, with the same antrorse eglandular trichomes of the young stems and small glandular trichomes (stalk unicellular; head multicellular), the calyx appendages (3–) 5, 0.25–2.5 mm long, subequal, thick, notoriously incurved, spreading, flattened laterally, glabrescent, inserted close to the margin. Corolla 6–8.5 mm long, ca. 5 mm in diameter, yellow with green spots within, stellate to broadly campanulate with interpetalar membrane, lobed nearly or more than the halfway to the base, the tube 3–4 mm long, pubescent adaxially with sparse glandular trichomes (stalk uni-bicellular; head unicellular), glabrous abaxially, the lobes 3.2–3.7 mm long, 2–3 mm wide, triangular, erect, glabrous adaxially and abaxially, the margins involute, the tips acute, papillate. Stamens five, equal; filaments 1–2 mm long, inserted on the corolla 1–1.5 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.4–1.8 mm long, ovoid, not connivent at anthesis. Gynoecium with ovary 1.8–2 mm long, 1.3–1.5 mm wide, ovoid; ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, 5.8–6.5 mm, clavate; stigma 0.1–0.2 mm long, ca. 0.5 mm wide, discoid. Berry 8–11 mm in diameter, globose, slightly flattened at the apex, green when immature, bright red at maturity, persistent, pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels (15–) 25–30 mm long, erect, conspicuously angled, winged and widened distally; fruiting calyx 3–5 (–7) mm in diameter, persistent, not accrescent, discoid, the appendages 2–5 mm long, incurved. Seeds 13–26 per fruit, 4–5 mm long, (2.6–) 3–4 mm wide, C-shaped or teardrop-shaped, brownish-yellow to brown, the seed coat reticulate (SM), reticulate-cerebelloid (SEM), the cells irregular in shape, the lateral walls strongly sinuate in the seed body, wavy at margins to nearly straight near hilum; embryo coiled.
Distribution
Capsicum ceratocalyx is endemic to the Bolivian Departments of La Paz and Cochabamba (Fig.
Ecology
Capsicum ceratocalyx is known from few collections, all from moist montane forest (Yungas) with little disturbance, between 700 and 2,500 m elevation.
Phenology
Flowering and fruiting from November to July.
Chromosome number
Not known.
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (3,612.392 km2); AOO (32 km2). Capsicum ceratocalyx is an endemic with relatively small EOO and AOO from the montane forests of the Bolivian Yungas, an ecoregion that deserves urgent special conservation efforts (
Discussion
Capsicum ceratocalyx has been assigned to the Bolivian clade (
Specimens examined
See Suppl. material
Capsicum chacoense
Capsicum chacoense var. tomentosum Hunz., Darwiniana 9(2): 235. 1950. Type. Argentina. Chaco: [Dept. Independencia], Colonia J.J. Mármol, 31 Dec 1946, F. Buratovich 117 (holotype: LIL [acc. # 173409); isotype: B [B10-1067916]).
Type
Argentina. Chaco: [Dept. Primero de Mayo] entre Colonia Benítez y Resistencia, 12 Mar 1945, A.T. Hunziker 7340 (lectotype, designated here: CORD [CORD00003920]; isolectotypes: CORD [CORD00003919, CORD00087962]).
Description
Low compact shrubs or subshrubs 0.40–1 (–2.5) m tall, much branched from a thick basal rootstock, with the main stem 2–3 cm in diameter at base, the branches expanded and divaricated, in a typical “zig-zag” appearance. Young stems strongly 3–4-angled, fragile, green or purple, sparsely to densely pubescent with antrorse and more or less rigid or spreading and flexuous, simple, uniseriate, 3–7-celled, eglandular trichomes 0.03–0.8 (–1.4) mm long, rarely branched trichomes 1–7 mm long; nodes green; bark of older stems brown, glabrescent to glabrous; lenticels sparse. Sympodial units unifoliate or difoliate, the leaves geminate; leaf pair more or less similar in shape and size. Leaves membranous, slightly discolorous or concolorous, glabrescent to densely pubescent with eglandular trichomes similar to the stems on both surfaces and margins; blades of all leaves 2–6 (–8) cm long, 1–3.5 (–5) cm wide, ovate, narrowly ovate or rarely elliptic, the major veins 3–4 on each side of midvein, the base attenuate and asymmetric, the margins entire, the apex long-acuminate; petioles 0.5–2 (–3.5) cm long, glabrescent to densely pubescent. Inflorescences axillary, flower solitary; flowering pedicels (5–) 10–25 (–40) mm long, strongly angled, erect, geniculate at anthesis, green, scarcely to moderately pubescent; pedicels scars inconspicuous. Buds globose or ovoid, white. Flowers 5-merous. Calyx 1.2–2 mm long, 2–2.5 mm wide, cup-shaped, thick, green, moderately pubescent with the same eglandular trichomes as stems, the calyx appendages 7–10 (rarely 5), unequal, rarely subequal, the five main appendages longer, 0.5–1.5 mm long, 0.3 mm wide, alternating with 2–5 shorter secondary appendages up to 0.7 mm long, thick, erect or slightly spreading, cylindrical or slightly compressed, inserted close to the margin, sparsely pubescent with the same trichomes as calyx tube. Corolla 4–6 (–9) mm long, 9–11 mm in diameter, thick, entirely white, stellate with interpetalar membrane, lobed 1/2 or less of the way to the base, glabrous adaxially and abaxially, the tube 2–4 mm long, the lobes 2–2.6 (–3.1) mm long, 2–2.4 mm wide, triangular, spreading, the margins papillate, the tips acute, papillate. Stamens five, equal; filaments 0.8–1.5 mm long, white, inserted on the corolla 1–1.3 mm from the base, with conspicuous auricles free, not fused to the corolla at the point of insertion; anthers (0.9–) 1.2–1.5 mm long, ellipsoid, yellow or cream, not connivent at anthesis. Gynoecium with ovary 1.5–2.3 mm long, ca. 1.7 mm in diameter, light green, ovoid; ovules more than two per locule; nectary ca. 0.4 mm tall, light green; styles homomorphic, 2.4–2.8 (–4) mm long, exserted ca. 1–1.5 mm beyond the anthers, white, cylindrical; stigma ca. 0.2 mm long, 0.2 mm wide, cream or light green, globose. Berry 7–10 mm in diameter, globose or (6–) 8–14 mm long, 5–8 mm in diameter, ellipsoid, green or green with blackish spots when immature, orange to bright red at maturity, deciduous, pungent, in some populations, non-pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 15–20 mm long, erect, strongly angled, widened distally, green; fruiting calyx 4–5 mm in diameter, persistent, not accrescent, discoid, green, the appendages 0.5–1.6 mm long, spreading or slightly recurved. Seeds 14–25 per fruit, 3.4–4 mm long, 2.8–3 mm wide, C-shaped, rarely subglobose or reniform, pale yellow, the seed coat smooth to reticulate (SM), reticulate-cerebelloid (SEM), the cells irregular in shape, the lateral walls strongly sinuate in seed body, nearly straight at margin; embryo imbricate or coiled.
Distribution
Capsicum chacoense is a widespread species usually confined to Chaco vegetation, which extends from Bolivia and Paraguay to central Argentina (Fig.
Ecology
Capsicum chacoense is a typical component of the dry and subhumid Gran Chaco forests ranging from the inter-Andean valleys at higher elevations to the lower Chaco forests in northern Argentina and Paraguay. Capsicum chacoense is found in the undergrowth, beneath the shade of nurse shrubs or trees, from 50–2,700 m elevation.
Phenology
Flowering from late October to March and April, fruiting from January to May or June.
Chromosome number
n = 12 (
Common names
Argentina: Ají (Catamarca, Brizuela 1127; Chaco, Aguilar 568; Córdoba, Fernández 17; Corrientes, Nicora s.n.; Santiago del Estero, Soriano & Barret 3592), Bolita (Santiago del Estero, Perrone s.n.), Cumbaré (Córdoba, Castellanos 217), Cumbarí (Catamarca, de Ance 81; Corrientes, Ibarrola 2988; La Rioja, Hunziker 4807), Lají (Córdoba, Lorentz s.n.), Pipí (Formosa, Morel 910), Putaparió (Chaco, Gaillard s.n.; Córdoba, Cocucci 4966; Tucumán, Schreiter 1927), Putaqueteparió (Córdoba, Hawkes et al. 3308), Uchuca (Catamarca, Vervoorst 3535), Ají cumbarí (Catamarca, Schickendantz 74; Chaco, Schulz 2; Córdoba, Castagnino s.n.; La Rioja, Giacomelli 130), Ají chuca (Chaco, de Ance 81), Ají fuerte (Córdoba, Villafañe 400), Ají picante (Córdoba, Botta 115), Ají quitucho (Catamarca, Capparelli 158), Ají uchiquita (La Rioja, Hunziker 4807), Ají uchuco (Catamarca, Falcone & Castellanos 262), Ají del campo (Chaco, Schulz 92; Córdoba, Castellanos 217; La Rioja, Hunziker 5088; San Luis, Anderson 1447; Santa Fe, Krapovickas 762), Ají del monte (Catamarca, Cabrera 1132; Chaco, Fortunato 1445; Córdoba, Spegazzini s.n.; Salta, Ayarde & Sidán 299; Santiago del Estero, Bartlett 20435; Tucumán, Schreiter 1927), Ají mala palabra (Santiago del Estero, Crespo s.n.), Ají puta parió (San Juan, Cortez 156), Ají quitucho dulce (Salta, Hunziker 1578), Picante del monte (Jujuy, Joergensen s.n.), Pimiento del monte (Chaco, Meyer 8561); Bolivia: Aribibi (Santa Cruz, Navarro & Vargas C. 261), Ají del zorro (Chuquisaca, Saravia Toledo 10343); Paraguay: Ají (Boquerón, August 18), Cambarí (Central, Rojas 10821), Cumbary (Capital, Rojas 14325).
Indigenous names
Argentina: Atéshuk (Chorote,
Uses
The pungent fruits are locally used as spicy food additives for both local and indigenous people (
Preliminary conservation assessment
EOO (1,724,002 km2); AOO (952 km2). Capsicum chacoense is widespread across subtropical Chaco forests from Bolivia to Paraguay and is assigned the Least Concern (LC) category.
Discussion
Capsicum chacoense belongs to the Baccatum clade (
A second variable feature is the number and degree of development of the calyx appendages. The most common condition is the presence of calyces with 10 unequal appendages, the five main ones longer and alternating with five shorter, all of them usually well-developed (that is, appendages exceed the truncate calyx edge). In some cases, the shorter appendages vary from 2–5, with some exceeding the calyx edge and others scarcely noticeable, reduced to a mucro. Calyces with this second pattern create confusion in the identification of fruiting specimens, especially when all short appendages are not well-developed. Often, these specimens are annotated in herbaria as C. baccatum from which C. chacoense can be distinguished by its smaller and entirely white corollas.
The fruits of C. chacoense are locally very appreciated for their flavour and high pungency (see references in Uses). However, C. chacoense is naturally polymorphic for the production of capsaicinoids, such that completely pungent and completely non-pungent individuals co-occur in some Bolivian populations (Tewskbury et al. 2006); non-pungent fruits have also been recorded in Argentinean populations (e.g. Salta, Hunziker 1578).
There is little information about which birds, the most effective dispersers of Capsicum seeds, eat C. chacoense fruits. Information from a herbarium label (Bolivia, Debouck 3016) suggests that small parrots eat the fruits of this species. Tewskbury et al. (2006) mentioned Elaenia parvirostris ‘fiofío pico corto’ (Fam. Tyrannidae) and Turdus amaurochalinus ‘zorzal chalchalero’ (Fam. Turdidae) as the major dispersers of Capsicum seeds in Bolivia.
In the protologue of C. chacoense,
Capsicum chacoense A root B fruiting branch C eglandular trichome of the leaf D flower E sector of opened corolla F gynoecium G fruit H seed I seed, in cross section J embryo A, C–F from Hunziker 18572 B, G–J from Hunziker et al. 25388. Drawn by N. de Flury. Published in
Specimens examined
See Suppl. material
Capsicum chinense
Capsicum cerasiforme Mill., Gard. Dict. ed. 8, no. 5. 1768. Type. Cultivated at the Chelsea Physic Garden, London, seeds from Spanish West Indies (no specimens cited).
Capsicum cerasiforme Lam., Tabl. Encycl. 2: 26. 1794, nom. illeg., not Capsicum cerasiforme Mill. (1768). Type. Brazil. “E Brafilia” (lectotype, designated here: P [P00357733]).
Capsicum luteum Lam., Tabl. Encycl. 2: 26. 1794. Type. India. “Ex India. Sonnerat” P. Sonnerat s.n. (lectotype, designated here: P [P00357731]).
Capsicum cerasiforme Willd., Enum. Pl. [Willdenow] 1: 242. 1809, nom. illeg., not Capsicum cerasiforme Mill. (1768). Type. Cultivated in the Berlin Botanic Garden, Germany, “Habitat…..” Capsicum cerasiforme [sheet] 1, herb. Willdenow (lectotype, designated here: B [B-W04427-01-0]).
Capsicum milleri Roem. & Schult., Syst. Veg., ed. 15 bis [Roemer & Schultes] 4: 563. 1819, nom. illeg. superfl. Type. Based on Capsicum cerasiforme Mill. (cited in synonymy).
Capsicum indicum var. luteum (Lam.) Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 24. 1829. Type. Based on Capsicum luteum Lam.
Capsicum indicum var. humifusum Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 26. 1829. Type. Based on Capsicum cerasiforme Mill. (cited in synonymy).
Capsicum indicum var. ochranthum Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 27. 1829. Type. Based on Capsicum cerasiforme Willd. (cited in synonymy).
Capsicum dichotomum
Vell., Fl. Flumin.: 61. 1829 (“1825”); Fl. Flumin. Icon. 2: t. 9. 1831 (“1827”), as “Capsicum conicum”. Type. Brazil. [Rio de Janeiro]: “Colitur hortis” (lectotype, designated by
Capsicum odoriferum
Vell., Fl. Flumin.: 61. 1829 (“1825”); Fl. Flumin. Icon. 2: t. 8. 1831 (“1827”). Type. Brazil. [Rio de Janeiro]: “Colitur hortis” (lectotype, designated by
Capsicum conicum Vell., Fl. Flumin. Icon. 2: t. 9. 1831. (“1827”), nom. illeg., not Capsicum conicum G.Mey. (1818). Type. Based on Capsicum dichotomum Vell.
Capsicum toxicarium Poepp., Not. Natur- Heilk. 32: 228. 1832. Type. No locality cited (no specimens cited, no original material found). Peru. “Peruvian hispan., Maynas alto”, Jan 1830, E.F. Poeppig 2220 (neotype, designated here: W [acc. # 0102196].
Capsicum cereolum Bertol., Hort. Bonon. Pl. Nov. 1: 6. t. 2. 1838. Type. Cultivated in Bologna, Italy “Nascitur in Brasilia unde semina ad nos attulit, et comiter dedit Eq. NUNNEZIUS” (lectotype, designated here [illustration]: Bertoloni, Hort. Bonon. Pl. Nov. 1: 6. t. 2. 1838).
Capsicum cordiforme var. subangulosum Fingerh., Monogr. Capsic.: 29. 1832. Type. No locality cited (no specimens cited; lectotype, designated here: [Fingerhuth, Monogr. Capsic. Tab. IX d. 1832]).
Capsicum cordiforme var. majus Fingerh., Monogr. Capsic.: 30. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. IX e. 1832).
Capsicum cordiforme var. minus Fingerh., Monogr. Capsic.: 30. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. X a. 1832).
Capsicum cordiforme var. olivaeforme Fingerh., Monogr. Capsic.: 30. 1832. Type. No locality cited (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. X b. 1832).
Capsicum cordiforme var. globosum Fingerh., Monogr. Capsic.: 30. 1832. Type. “Crescit in Indiis et America meridionali” (no specimens cited; lectotype, designated here [illustration]: Fingerhuth, Monogr. Capsic. Tab. X c. 1832).
Capsicum ustulatum Paxton, Paxton’s Mag. Bot. 5: 197. 1838. Type. Cultivated at Chatwsworth House, England (no specimens cited; lectotype, designated here: [illustration], Paxton, Paxton’s Mag. Bot. 5: [un-numbered plate opposite] 197. 1838).
Capsicum cerasiforme var. maurocarpum Dunal, Prodr. [A. P. de Candolle] 13(1): 422. 1852. Type. No locality cited (no specimens cited; lectotype designated here: [illustration] “SolanumCapsicum dictum perenne minuserectum” Weinemann, Phytanthoza Iconogr. 4: 349, t. 930 c. 1745).
Capsicum oxycarpum Dunal, Prodr. [A. P. de Candolle] 13(1): 426. 1852. Type. Brazil. Rio de Janeiro, 1831, M. Blanchet 90 (holotype: G-DC [G00200070]; isotype: MPU [MPU023041]).
Capsicum cordiforme var. subsulcatum Dunal, Prodr. [A. P. de Candolle] 13(1): 427. 1852. Type. No locality cited (no specimens cited; lectotype designated here: [illustration] Fingerhuth, Monogr. Capsic. Tab. IX c. 1832).
Capsicum annuum var. chinense (Jacq.) Alef., Landw. Fl.: 132. 1866. Type. Based on Capsicum chinense Jacq.
Capsicum annuum var. luteum (Lam.) Alef., Landw. Fl.: 132. 1866. Type. Based on Capsicum luteum Lam.
Capsicum annuum var. milleri (Roem. & Schult.) Alef., Landw. Fl.: 133. 1866. Type. Based on Capsicum milleri Roem. & Schult.
Capsicum grossum var. cerasiforme (Lam.) C.B.Clarke, Fl. Brit. India 4: 239. 1883. Type. Based on Capsicum cerasiforme Lam.
Capsicum annuum var. cerasiforme (Mill.) Irish, Rep. (Annual) Missouri Bot. Gard. 9: 92. 1898. Type. Based on Capsicum cerasiforme Mill.
Capsicum frutescens var. cerasiforme (Mill.) L.H.Bailey, Gentes Herbarum 1: 129 1923. Type. Based on Capsicum cerasiforme Mill.
Capsicum assamicum J.Purkay. & Lok.Singh, Ozean J. Appl. Sci. 5(1): 56. 2012. Type. India. Assam: Tezpur, 157 m, 25 May 2008, Purkayastha & Singh, DRLT 12 (holotype: CAL [by error, BSI in protologue, n.v.]; isotype: CAL [by error, DRLT and BSI in protologue, n.v.]).
Type
Cultivated in Vienna, Austria [“Hort. Bot. Vindob.”], N.J. von Jacquin s.n. (lectotype, designated here: W [acc. # 0080115]).
Description
Low, erect, short-lived subshrubs or rarely shrubs, 0.5–1.5 (–2.5) m tall, with the main stem (0.5–) 0.8–1.5 cm in diameter at base, few to much branched from near the base. Young stems 4-angled, fragile, green or greenish-brown, glabrous to glabrescent, with spreading, simple, uniseriate, (2–) 4–5-celled, eglandular trichomes 0.07–0.7 mm long; nodes solid, green or purple; bark of older stems light brown or green with light brown stripes, glabrous to sparsely pubescent; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous, concolorous or slightly discolorous, dark green above, pale green below, glabrescent to sparsely pubescent, with simple, 4–9-celled, eglandular trichomes 0.6–1.2 mm long, especially along the mid-vein or with a tuft of trichomes in the basal vein axils abaxially; blades of major leaves (4–) 5.25–10 (–15.5) cm long, 2.4–4 (–7) cm wide, ovate to elliptic, the major veins 4–5 (–6) on each side of mid-vein, the base attenuate or truncate and rather unequal, the margins entire, the apex short-acuminate, acuminate or acute; petioles 0.5–3 (–3.5) cm, glabrous to sparsely pubescent; blades of minor leaves similar in shape, 4–5.5 cm long, 2–2.5 cm wide; petioles 0.7–1.5 cm long, with the same pubescence as the major leaves. Inflorescences axillary, 2–4 (–5) flowers per axil, occasionally solitary flower; flowering pedicels 12–20 (–30) mm long, angled, erect or slightly spreading, geniculate at anthesis (wild forms) or pendent and non-geniculate (domesticated forms), green or green with purple lines, glabrous to moderately pubescent, the eglandular trichomes short, antrorse; pedicels scars conspicuous, slightly corky. Buds ellipsoid, cream or greenish-white. Flowers 5–6-merous. Calyx 1–2.5 (–3) mm long, 3–4 mm wide, cup-shaped, green, pentagonal or hexagonal in outline, the main veins strongly marked, the calyx appendages absent or with 5–6 mucro-like appendages, glabrous to moderately pubescent with similar short or long eglandular trichomes as the stems. Corolla 5 (–6)-merous, (5–) 6.5–8 mm long, 10–15 (–20) mm in diameter, dull white or greenish-white, occasionally with purple spots outside and within, stellate with interpetalar membrane, lobed nearly 2/3 of the way to the base, glabrous adaxially and abaxially, the tube (2–) 2.5–3 mm long, the lobes 3.5–5 mm long, 2–3.5 mm wide, triangular, spreading, the margins papillate, the tips acute, cucullate, papillate. Stamens 5 (–6), equal; filaments 1–1.3 mm long, white, cream or purple, inserted on the corolla 1–1.5 mm from the base, with auricles fused to the corolla at the point of insertion; anthers (1–) 1.38–2.05 mm long, blue or bluish-grey, very rarely yellow or greenish-white, broadly ellipsoid or ellipsoid, connivent or not connivent at anthesis, the connective sometimes wide and clearer. Gynoecium with the ovary 2–2.5 mm long, 2.5–3.5 mm in diameter, 2–3 (–4)-carpelar, light green, subglobose; ovules more than two per locule; nectary ca. 0.5 mm tall; styles heteromorphic, 3–4.1 mm, included, at the same level to the stamen length or exserted ca. 2 mm beyond the anthers, lilac or white, cylindrical; stigma ca. 0.15 mm long, ca. 0.5 mm wide, minute, discoid, pale green or pale yellow. Berry < 10 mm in diameter, subglobose and orange to red (in wild populations), highly variable in size, shape and colour (in semi-domesticated or domesticated cultivars): subglobose or triangular, 10–20 mm in diameter, to long-triangular or campanulate, 30–60 (–100) mm long, 20–30 mm in diameter, some blocky, with the apex pointed, blunt or long-acuminate and upcurved and the base obtuse or truncate, green, yellow, brown or purple when immature, pale yellow, yellow, dark brown, orange, red or vermilion-scarlet at maturity, deciduous or persistent, very pungent (sometimes non-pungent), the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 15–45 (–55) mm long, thick, angled, strongly widened distally, erect and rigid (wild) or pendent and curved (domesticated); fruiting calyx 5–10 mm in diameter, persistent, not accrescent, discoid or shallowly cup-shaped, sometimes reflexed, with a strong annular constriction at junction with the pedicel (wild and domesticated), sometimes the margin ripped, green. Seeds 14–35 per fruit, (2.7–) 3–4 mm long, (2.5–) 3–3.5 mm wide, C-shaped or subglobose, pale yellow or nearly white, the seed coat smooth (SM), cerebelloid (SEM), the cells irregular in seed body, polygonal at margins, the lateral walls sinuate in the seed body, straight at margins; embryo imbricate.
Capsicum chinense (A–H from wild plants I–M from domesticated plants) A plant B flower buds C flower on geniculate pedicel D flower, in front view E flower, in full anthesis F immature fruit G fruiting pedicel H mature fruit I flowering branch J flowers on pendent pedicels K, L immature fruits M mature fruit A–H no specimen vouchers (cult. in Banco de Germoplasma de Hortaliças, Embrapa/Hortaliças, Brasília-DF, Brazil), photos taken in situ by L. Bianchetti I–M no specimen vouchers (cult. in Pairumani, Cochabamba-Bolivia), photos taken in situ by G.E. Barboza.
Distribution
Capsicum chinense wild forms were thought to originally occur in the north-central Amazon Basin lowlands (Brazil), where domestication is thought to have occurred (
Capsicum chinense has been introduced into United States of America (
Ecology
Capsicum chinense grows in wet tropical and subtropical forests where native communities cultivate it around their chakras or in-home gardens, between 100 and 800 (–1,800) m elevation. Wild populations in northern Brazil grow in lowlands and disperse spontaneously in fallow agriculture areas, before the beginning of the rainy period (
Phenology
Flowering and fruiting all year in most parts of its range.
Chromosome number
n = 12 (
Common names
Argentina: Ají bolita (Salta, Hilgert 2060). Belize: Jabanero pepper (Corozal, Balick 2211); Bolivia: Ají (Beni, Williams 937; La Paz, Williams 774), Aji mote (Santa Cruz, Krapovickas & Schinini 32488), Ají soliman (Cochabamba, Thomas 1093), Ají trompillo (Cochabamba, Thomas 704), Ají grande rojo (Beni, Rivero 428); Brazil: Baianinha (Rondônia, Teles Walter 579), Canaimé, Chumbinho, Malaguetão, Morangão, Moranguinho, Murici, Murupi, Pimentãozinho, Peão-amaerlo, Peão-vermelho, Peixe-boi, Pimenta amarela, Pimenta-moranga, Pimenta murici, Pimenta ornamental, Pimenta-roxa, Chifre-de-carneiro, Chifre-de veado, Cumari-do-Pará, Esporão-de-galo, Olho-de-cará, Olho-de-carangueijo, Olho-de-mutum, Olho-de-peixe (falsa), Olho-de-peixe (verdadeira), Pimenta-vermelha longa, Unha-de-gato, Pimenta-de-cherio amarela (ardosa), Pimenta-de-cheiro longa, Pimenta-de mesa-ardosa (Roraima,
Indigenous names
Bolivia: Bidó (Tacana, La Paz, Williams 1177), Cheti (Trinitario, Cochabamba, Thomas 704), Ta’ (Beni, Davis 1066), Hchetgi (Trinitario, Cochabamba, Thomas 1092), Tata (Yuqui, Cochabamba, Martínez et al. 246), Uchu (Quechua, La Paz, Williams 772), Winno (Yuracare, Cochabamba, Thomas 1093), Winno manera (Yuracare, Cochabamba, Thomas 704); Brazil: Pimi’ró (Macuxi, Roraima,
Uses
Capsicum chinense has medicinal, ornamental and food uses. Indigeneous communities and rural people widely cultivated this species around their homes to consume the fruits raw, cooked or used as a spice (
In addition to medicinal properties indicated in Table
Preliminary conservation assessment
EOO (18,657,305 km2); AOO (712 km2). Capsicum chinense is a very widespread cultivated species worldwide; we assign the status of Least Concern (LC).
Discussion
Capsicum chinense belongs to the Annum clade (
Ever since Smith and Heiser (1957) first recognised C. chinense as a cultivated species in the genus, its recognition as an independent entity and as a member of the C. annuum complex (C. annuum-C. frutescens-C. chinense) has been debated in literature (see
An unequivocal morphological delimitation for C. chinense is difficult for two reasons: first, intermediate well-established types occur between wild and domesticated forms, probably due to intraspecific hybridisation (
Morphologically,
The differentiation of C. chinense from C. annuum (wild or domesticated specimens) is sometimes also difficult, especially at the fruiting stage. Wild forms of C. chinense with small red fruits and erect pedicels could be confused with some domesticated (or semi-domesticated) specimens of C. annuum var. glabriusculum, which share the same pedicel position and fruit characteristics (size, shape and colour), although wild forms of this variety have smaller fruits than the typical C. chinense. The presence/absence of the constriction in the pedicel/calyx junction (lacking in C. annuum var. glabriusculum) allows the assignment to one or other taxon. Furthermore, C. chinense is a more robust plant with larger leaves and flowers than C. annuum var. glabriusculum.
During its long history of cultivation, C. chinense fruits have developed a broad range of variation due to active selection by growers, differing cultivation methods and adaptation to the environment (
The pungency and aroma of this species are also remarkable. The “Carolina Reaper” cultivar is reputed to be the world’s hottest chile, which rates at an average of 1,641,183 Scoville Heat Units (SHU) (https://www.guinnessworldrecords.com/world-records/hottest-chili, accessed on 29 October 2019) and is a favourite amongst hot-pepper lovers. At the other extreme, the fruits of the low pungency varieties (pimenta-doce, pimenta-de-cheiro longa, pimenta-de-cheiro amarela) found in Brazil (Roraima) are used by indigenous and non-indigenous communities and are preferentially used in stews or salads, for preparing jellies or liqueurs or for food dish decoration (
Capsicum cerasiforme was published by
For C. luteum,
There are three sheets of original material labelled C. cerasiforme in Willdenow’s Herbarium held at Berlin. All of them contain reproductive branches; one of these (B-W04427-01-0) consists of a fruiting branch that matches Willdenow’s description exactly (
In describing C. toxicarium,
In the protologue of C. cerasiforme var. maurocarpum,
The original material of C. assamicum could not be found and is apparently lost. According to the protologue (
Specimens examined
See Suppl. material
Capsicum coccineum
Brachistus coccineus
Rusby, Bull. New York Bot. Gard. 8(28): 117. 1912. Type. Bolivia. La Paz: [Prov. A. Iturralde]. San Buena Ventura, 1400 ft, 18 Nov 1901, R.S. Williams 634 (lectotype, designated by
Lycianthes coccinea (Rusby) Rusby, Bull. Torrey Bot. Club 53: 210. 1926. Type. Based on Brachistus coccineus Rusby
Type
Based on Brachistus coccineus Rusby.
Description
Sprawling vines or scrambling shrubs, (1–) 1.5–7 m tall, with the main stem thick, 2–3 cm in diameter at base, few to much branched, the branches scandent. Young stems strongly angled, fragile, brown or green, glabrous, glabrescent or moderately pubescent, with antrorse, curved, simple, uniseriate, 4–6-celled, eglandular trichomes 0.3–0.9 (–1.2) mm long; nodes solid, green; bark of older stems green or brown with lighter stripes, glabrescent; lenticels absent. Sympodial units difoliate, the leaves geminate, rarely solitary; leaf pair unequal in size, similar in shape. Leaves membranous, concolorous, sometimes brilliant, glabrous to moderately pubescent on both sides and especially along the main veins abaxially, with simple trichomes like those of the stems, sometimes trichomes in tufts in the vein axils beneath; blades of major leaves 4.7–13 (–15) cm long, (1.7–) 2.1–5 cm wide, ovate to elliptic, the major veins 5–7 on each side of mid-vein, prominent abaxially, the base attenuate or short-attenuate and barely unequal, the margins entire, the apex acuminate; petioles 1–1.8 cm long, moderately pubescent; blades of minor leaves 3.2–4.8 cm long, 1.4–1.6 cm wide, elliptic or ovate, the major veins 3–4 on each side of mid-vein, the base short-attenuate, the margins entire, the apex acute; petioles 0.3–0.4 cm long, moderately pubescent. Inflorescences axillary, 4–13 (–18)-flowers on a short rachis 1.5–3 mm long; flowering pedicels 5–15 mm long, angled, erect, geniculate at anthesis, green, glabrescent to moderately pubescent, the eglandular trichomes short, antrorse; pedicel scars prominent, corky. Buds ovoid, colour unknown. Flowers 5-merous. Calyx 1–2.2 mm long, ca. 2 mm wide, cup-shaped, thick, slightly 5-nerved, green, sparsely pubescent with the same eglandular trichomes as the stems, calyx appendages absent or 2–10 unequal, the five longer appendages, 0.8–1.3 (–2) mm long, the shorter appendages 0.2–0.5 mm, filiform, erect, sparsely pubescent with the same trichomes as the calyx tube. Corolla (5–) 6.5–8 mm long, ca. 7–8 mm in diameter, yellow outside, usually yellow with yellowish-green or purple-brown spots within, stellate with interpetalar membrane, lobed nearly halfway to the base, pubescent adaxially with abundant long glandular trichomes (stalk 2–4-celled; head peltate, unicellular) in the throat and the lobes, the tube 2.5–3.5 mm, glabrous abaxially, the lobes 2.3–3.8 mm long, 2.4–3.4 mm wide, broadly triangular or ovate, the margins involute, sparsely pubescent (2–3-celled eglandular trichomes) abaxially, the tips cucullate and papillate. Stamens five, equal; filaments 0.9–1.9 mm long, purple, lilac or greyish, inserted on the corolla 1.2–1.4 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.3–2 mm long, ellipsoid, dull yellow or light brown, not connivent at anthesis. Gynoecium with ovary ca. 1.5 mm long, 1 mm in diameter, light green, ovoid; ovules more than 2 per locule; nectary ca. 0.3 mm tall; styles homomorphic, 3–4 mm, barely exserted beyond the anthers, purple or white, clavate; stigma ca. 0.2 mm long, 0.6 mm wide, discoid or globose, light green. Berry (6–) 7–9 mm in diameter, globose, green to brown when immature, turning to orange, glossy red or reddish-orange at maturity, only 2–4 fruits per inflorescence, deciduous, pungent, pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells 0–4; fruiting pedicels 10–18 mm long, erect, faintly angled, widened distally, green; the fruiting calyx 4–5.5 mm in diameter, persistent, not accrescent, discoid, rotate or reflexed, green, margin entire or sometimes ripped, the appendages up to 2.2 mm long, spreading or reflexed. Seeds (2–) 4–9 (–13) per fruit, 4–4.6 mm long, (2.8–) 3.2–3.75 mm wide, C-shaped or teardrop-shaped, yellow to brownish-yellow, the seed coat faintly reticulate (SM), reticulate-cerebelloid (SEM), the cells irregular and elongate near hilum, the lateral walls sinuate to straight at seed margin; embryo annular or imbricate.
Distribution
Capsicum coccineum grows from eastern Peru (Ayacucho, Cuzco, Huánuco, Junín, Loreto, Madre de Dios, San Martín and Ucayali Departments) to north-central Bolivia (Beni, Cochabamba, La Paz, Pando and Santa Cruz Departments) and western Brazil (Acre, Amazonas and Rondônia States) (Fig.
Ecology
Capsicum coccineum is a common species of the Amazon Basin lowlands, found in tropical evergreen moist forests or in deciduous subtropical forests along the eastern Andes. It grows on scrubby banks of roads through disturbed forests, along river edges and streams or in flooded areas, usually in the sun, between 150 and 800 m elevation.
Phenology
Flowering mainly from September to March; fruiting all year.
Chromosome number
Not known.
Common names
Bolivia: Pimienta (Santa Cruz, Steinbach 5373), Ají del monte (Beni, Killen et al. 3415; Cochabamba, Thomas & Berdeja 1428; Sud Yungas, Seidel & Vaquiata 7689); Brazil: Pimentinha (Acre, Daly 9979); Peru: Ají silvestre (Loreto, Mc Daniel 14075; Huánuco, Woytkowski 7516), Ajicillo (Huánuco, Schunke V. 10620), Ajisillo (San Martín, Schunke V. 3415), Charapilla (Huánuco, Schunke V. 9984), Charapillo (Huánuco, Schunke V. 12469), Chintillo (San Martín, Plowman & Schunke V. 11523), Sacha Ají (Huánuco, Schunke V. 9984), Sacha charapillo (Ucayali, Schunke & Graham 16612), Ají silvestre liana (Loreto, Mc Daniel & Santiago 2556).
Indigenous names
Bolivia: Meñu winno (Yuracaré, Cochabamba, Thomas & Berdeja 1428), Hpochetgi (Trinitario, Cochabamba, Thomas & Berdeja 1428); Tá yejti (Mosetén, La Paz, Vargas et al. 1310), Neshita’mo (Beni, Guareco 457).
Uses
The fruits are used as condiments in Bolivia (Steinbach 5373, Rivero 218), but on a lesser scale than other wild species (‘ulupica’ or ‘aribibi’). In Peru, fruits are used to prepare curare (Woytkowski 7516). The entire plant is used as a medicine (see Table
Preliminary conservation assessment
EOO (987,169.745 km2); AOO (336 km2). Capsicum coccineum occupies a large range along the lowlands of the Amazon Basin in well preserved areas, as well as in fragmented forests. The species inhabits in reduced subpopulations along the main effluents of the Amazon drainage basin (Rivers Beni, Ucayali, Purús, Huallaga, Apurimac, Madre de Dios, Río Acre, Surutú and others) and in many conservation units where is expected that it is not in a serious risk of threat. However, due to the continuing decline of the quality of the habitat outside of natural reserves, we assign C. coccineum the Near Threatened (NT) category.
Discussion
Capsicum coccineum has been placed in the Bolivian clade, although such placement is only moderately supported (
Two morphs of C. coccineum can be distinguished, based on the number of calyx appendages. The first morph, which includes the type collection, has a calyx with 10 unequal appendages or 2–10 appendages; the second morph has a calyx lacking appendages. Populations from Bolivia generally have calyx appendages, while populations from Peru and Brazil exhibit both morphs. Further fieldwork throughout the range of C. coccineum is needed to elucidate if the variability observed in the calyx morphology is an intraspecific variation or if a cryptic species is involved. Similarly, corolla colour is also variable in this species and deserves more attention in the field. Some collector labels mention a uniform corolla colour (yellow, cream, brilliant greenish-yellow), while others indicate dull yellow corollas with yellowish-green spots within or with purple-brown or violet centre. Variations in corolla colour and degree of development of the calyx appendages are observed in other species from the Brazilian Atlantic Forest, for example, C. schottianum.
In Bolivia, C. coccineum is sympatric with C. minutiflorum and C. baccatum var. baccatum. All three of these entities share red globose pungent fruits on erect pedicels. Capsicum coccineum differs from C. minutiflorum in its scrambling vine habit vs. erect shrubby habit, multi-flowered inflorescence (up to 18 flowers) vs. few-flowered inflorescence (up to five flowers) and calyx appendages absent or 2–10 vs. usually five appendages. Capsicum baccatum var. baccatum differs from C. coccineum in the same set of contrasting characters and in its corolla colour that is white (vs. yellow or cream) and the dimorphic styles (vs. homomorphic styles). The fruits of C. baccatum var. baccatum can also be ellipsoid.
Capsicum coccineum A reproductive branch B flower C calyx with appendages D section of the calyx showing the venation E eglandular trichome of the calyx F opened corolla G, H anthers, dorsal and ventral views, respectively I gynoecium J node with fruits K anatomical detail of the pericarp (note the giant cell in the mesocarp) L seed, in cross section M structure of seed coat at the seed margin N structure of seed coat at the seed body O embryo A, B, D–I, K from Woytkowski 1176 C, J from Nee & Saldías 35956 L–O from Nee 44503. Drawn by J. de Ugarte.
Specimens examined
See Suppl. material
Capsicum cornutum
Bassovia cornuta Hiern, Vidensk. Meddel. Naturhist. Foren. Kjøbenhavn: 59. 1877. Type. Brazil. Rio de Janeiro: Rio de Janeiro, [no date], A.A.W. Lund s.n. (lectotype, designated here: C [C10019145]; isolectotypes: [K000585893], P [P00410056]).
Capsicum dusenii Bitter, Abh. Naturwiss. Vereine Bremen 24: 520. 1919. Type. Brazil. São Paulo: Serra do Mar, Alto da Serra, 30 Sep 1912, P.K.H. Dusén 14227 (lectotype, designated here: S [acc. # S04-2813]; isolectotypes: CORD [CORD00006948 fragment ex S], RB [RB01413389], S [acc. # S18-42558]).
Type
Based on Bassovia cornuta Hiern.
Description
Erect shrubs or subshrubs, (0.80–) 1–3.5 m tall, with the main stem thick, ca. 2.5 cm in diameter at base, much branched above, the branches dichotomously spreading in a typical “zig-zag” appearance. Young stems angled, fragile, green or brown, densely pubescent, with spreading, more or less rigid, brilliant and ferruginous, simple, uniseriate, (3–) 5–9-celled, eglandular trichomes 0.5–3 mm long, rarely glabrescent; nodes solid, green; bark of older stems dark brown, sparsely pubescent; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous, slightly discolorous, green above, light green beneath, densely pubescent adaxially with appressed-antrorse eglandular trichomes similar to those of the stems and abundant spreading, uniseriate, 5–8-celled, eglandular trichomes abaxially, especially on the veins and margins; blades of major leaves 6–16 (–18) cm long, 4–8 cm wide, ovate or widely elliptic, the major veins 6–8 on each side of mid-vein, the base attenuate, the margins entire, the apex acuminate; petioles 0.3–0.5 (–0.8) cm long, densely pubescent with spreading eglandular trichomes; blades of minor leaves (3–) 4–6 cm long, 1.5–3.2 cm wide, elliptic or ovate, the major veins 3–5 on each side of mid-vein, the base attenuate, the margins entire, the apex acute; petioles 0.2–0.4 cm long, same pubescence as the major leaves . Inflorescences axillary, 2–5 (–7) flowers per axil or flowers solitary; flowering pedicels (22–) 25–35 mm long, delicate, angled, erect, geniculate at anthesis, green or green with purple lines, moderately pubescent, the eglandular trichomes long, spreading; pedicels scars inconspicuous. Buds ovoid, inflated, white with green and purple spots. Flowers 5-merous. Calyx 1–2 mm long, 4–5 mm wide, cup-shaped, thin, green, densely pubescent with spreading eglandular trichomes, the calyx appendages (5–) 7–10 unequal, the five main appendages 2.5–5 (–6), the five secondary appendages shorter 0.5–1.5 (–2) mm long, thin, erect or spreading, linear or subulate, inserted very close to the margin. Corolla (8–) 9–14 mm long, 18–22 mm in diameter, white with purple and yellowish-green spots outside, white with intense small purple or reddish-brown spots amongst the veins and in the throat and yellowish-cream centre within, stellate with thin interpetalar membrane, lobed halfway or less of the way to the base, the tube 4–5 mm long, pubescent adaxially with a continuous ring of glandular trichomes (stalk long, 2–3-celled; head globose, peltate, unicellular), glabrous abaxially, the lobes 3.5–6.8 mm long, 3–5.5 mm wide, broadly triangular, spreading, glabrous adaxially and with eglandular trichomes 3–6-celled on the veins abaxially, the margins papillate, the tips acute, cucullate, papillate. Stamens five, equal; filaments 1.4–2.2 (–2.5) mm long, white or cream, inserted on the corolla ca. 1.75 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.2–2.3 mm long, ellipsoid, pale grey or grey, not connivent at anthesis. Gynoecium with ovary ca. 2 mm in diameter, light green, globose; ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, 4–6.8 mm long, exserted ca. 1.3 mm beyond the anthers, white or cream, clavate; stigma 0.3 mm long, 0.6–0.8 mm wide, globose or discoid, pale green. Berry 7–10 mm in diameter, globose, green when immature, greenish-golden yellow at maturity, deciduous, slightly pungent, the pericarp thin, translucent, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels (25–) 30–38 mm long, pendent and curved, angled, widened distally, green; the fruiting calyx 4–5 mm in diameter, persistent, not accrescent, discoid, green, the appendages 1–6 mm long, spreading. Seeds (4–) 6–18 (–20) per fruit, 3–3.5 mm long, 2.5–3.5 mm wide, teardrop-shaped, black, the seed coat reticulate and tuberculate at margins (SM), reticulate with pillar-like outgrowths at margins (SEM), the cells polygonal in shape, the lateral lateral walls straight; embryo imbricate.
Distribution
Ecology
Capsicum cornutum occurs in the Atlantic Forest (Mata Atlântica), in the Dense Ombrophilous Forest (Floresta Ombrófila Densa), in semi-shade, on the edge of steep, open ravines or along forest roadsides, between 500 and 900 m elevation.
Phenology
Flowering from September to May. Fruiting from December to May and June.
Chromosome number
n = 13 (
Common name
Brazil. Pimentinha-do-mato (Rio de Janeiro, Bovini & Giordano 363).
Uses
None recorded.
Preliminary conservation assessment
EOO (17,682.480 km2); AOO (72 km2). Capsicum cornutum occurs exclusively in the Brazilian Atlantic Forest, one of the world’s biological diversity hotspots that is increasingly threatened by the rapid destruction (deforestation) and fragmentation of its natural areas. This species inhabits the Serra do Mar, in much reduced subpopulations mainly in officially protected areas, such as Parque Nacional do Itatiaia and APA-Cairuçu (Rio de Janeiro), Parque Estadual da Serra do Mar and Reserva Biológica do Alto da Serra de Paranapiacaba (São Paulo). Based on the EOO, the continuing decline observed in the quality of its habitat outside of the natural reserves and decline in number of locations, we assign the threat status of Vulnerable (VU; B1ab(iii,iv)).
Discussion
Capsicum cornutum is a member of the Atlantic Forest clade (
Capsicum villosum is superficially similar to C. cornutum in its pubescence, geniculate pedicels and corolla and fruit colour, but differs in having five subequal calyx appendages, a smaller corolla (7–9 mm long, 14–14.5 mm in diameter vs. 8–14 mm long, 18–22 mm in diameter in C. cornutum), a different design of purple pigmentation in the corolla lobes and throat (two large spots at the base of each lobe forming a more or less ring-like purple centre within the corolla vs. many small purple or reddish-brown spots amongst the veins in C. cornutum) and filaments 1.6–2.4 longer than the anthers (vs. filaments somewhat longer than the anthers in C. cornutum).
Capsicum cornutum has also been confused in herbaria with another species of the Atlantic Forest clade, C. recurvatum. Capsicum cornutum differs in having dense pubescence of long spreading trichomes, longer and erect or spreading calyx appendages and purple pigmentation within the corolla; C. recurvatum is much less pubescent with shorter antrorse trichomes, has shorter and recurved calyx appendages and has a corolla with greenish-yellow spots within and no purple pigmentation. Capsicum cornutum is sympatric, but cannot be confused, with C. schottianum. The presence of calyx appendages, long trichomes and a larger corolla distinguish C. cornutum from C. schottianum.
When describing B. cornuta,
In his description of C. dusenii,
Capsicum cornutum A leaf B eglandular trichomes of the leaf C flowering branch D sector of opened corolla E gynoecium F fruit G fruit (one carpel), in longitudinal section H anatomical detail of the pericarp (note the giant cell in the mesocarp) I seed J seed, in cross section K structure of seed coat at the seed margin L embryo A–G from Hunziker 19557 H–L from Kuhlmann 4321. Drawn by L. Sánchez. Modified from
Specimens examined
See Suppl. material
Capsicum dimorphum
Brachistus dimorphus Miers, Ann. Mag. Nat. Hist., ser. 2, 3(16): 267. 1849. Type. Colombia. Quindio: Region froid en Las Tapias, Dec 1814, J. Goudot s.n. (holotype: K [K000585926]; isotypes: BM [BM000777291, pro parte only fragments at bottom right side], P [P00410061]).
Type
Based on Brachistus dimorphus Miers.
Description
Erect or scandent shrubs or subshrubs, (0.4–) 0.8–2 (–3) m tall, profusely branched above, the branches dichotomously spreading in a typical “zig-zag” appearance. Young stems terete or slightly angled, fragile, greenish-purple or maroon, densely pubescent with ochraceous or white, spreading or antrorse, flexuous or hirsute, simple, uniseriate, 2–8-celled, eglandular trichomes 0.3–1 (–1.5) mm long, sometimes glabrous or glabrescent; nodes green; bark of older stems pale brown or golden-brown; lenticels light brown, sometimes absent. Sympodial units difoliate, the leaves geminate; leaf pair markedly unequal in size and shape. Leaves membranous, rarely coriaceous, with sparse or abundant antrorse eglandular trichomes adaxially and abaxially, the trichomes spreading on the veins abaxially; blades of major leaves 4.2–12 (–17) cm long, 0.8–4 (–8) cm wide, elliptic, the major veins 4–6 on each side of mid-vein, sometimes purple-coloured abaxially, the base attenuate, asymmetric or not, the margins entire, the apex long-acuminate; petioles 0.5–1.2 cm long, moderately pubescent or glabrescent; blades of minor leaves sessile, 0.5–4 (–8) cm long, 0.4–1.7 (–5) cm wide, orbicular or ovate or rarely elliptic, the major veins 2–3 on each side of mid-vein, the base short-attenuate or rounded, asymmetric, the margins entire, the apex obtuse or rounded, with same pubescence as major leaves. Inflorescences axillary, 2–5 flowers or flowers solitary; flowering pedicels (3–) 5–14 mm, angled, green or green with purple lines, curved to pendent, non-geniculate at anthesis, glabrescent to moderately pubescent, the eglandular trichomes short or long, spreading or antrorse; pedicels scars inconspicuous. Buds ovoid, purple or yellowish. Flowers 5-merous. Calyx 1.75–2.6 mm long, 3–3.3 mm in diameter, cup-shaped, circular or pentagonal in outline, fleshy, green, greenish-purple or purple, calyx appendages absent or 1–3, as mucro-like structures, 0.5–1 mm long, spreading and laterally flattened, emerging 0–0.2 mm below the margin, glabrescent to densely pubescent with antrorse or spreading trichomes. Corolla 6–9.5 (–11) mm long, 8–12 mm in diameter, entirely yellow or yellow with dark purple or maroon spots outside and within, stellate with interpetalar membrane, lobed nearly halfway to the base, glabrous adaxially and abaxially, the tube 2–3.5 mm long, the lobes (1.75–) 2–4 mm long, 1.5–2.8 (–3) mm wide, triangular or ovate, spreading or slightly reflexed at anthesis, the margins and tips papillose. Stamens five, equal; filaments (1.5–) 2–2.5 mm long, cream or light lilac, inserted on the corolla ca. 2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.5–2.3 mm long, ovoid, slightly apiculate, pale purple, bluish-purple, dark yellow or cream, connivent at anthesis. Gynoecium with ovary 1.37–1.6 mm long, 1.3–1.5 mm in diameter, cream, ovoid; ovules more than two per locule; nectary ca. 0.6 mm tall; styles homomorphic, 4.5–6.7 mm long, exserted 1.3–2 mm beyond the anthers, white near the base and purple or pale lilac to the apex, clavate, slightly curved distally; stigma 0.2–0.3 mm long, 0.6 mm wide, usually discoid, green or yellowish-green. Berry 5–11 mm in diameter, globose or subglobose, bright or opaque light green when immature, bright reddish-orange or bright red at maturity, deciduous, non-pungent, the pericarp thick, opaque, lacking giant cells (endocarp smooth); stone cells absent; fruiting pedicels 6–15 mm long, usually erect, rarely curved to pendent, sometimes angled, widened distally, green or purple; fruiting calyx 4–5 (–7) mm in diameter, persistent, not accrescent, discoid, sometimes ripped at the margin, greenish-white or purple, the appendages if present, reflexed or spreading, purple. Seeds (6–) 10–36 per fruit, 1.9–2.7 mm long, 1.8–2.1 mm wide, C-shaped or teardrop-shaped, brownish-black to black, the seed coat reticulate (SM, SEM), the cells polygonal in shape, the lateral walls straight; embryo annular.
Capsicum dimorphum A flowering branch B flower; C section of the calyx showing the venation; D sector of opened corolla; E gynoecium; F fruit G anatomical detail of the pericarp (note the absence of giant cells in the mesocarp) H seed; I seed, in cross section J structure of seed coat at the seed margin K structure of seed coat at the seed body L embryo A, B, D, E from Jaramillo et al. 2698 C from Sneidern 3044 F–L from Cuatrecasas 8572. Drawn by L. Ochoa.
Capsicum dimorphum A plant B, C flower buds of different colour D, E flowers, in lateral view, with different corolla colour outside F, G flowers, in front view, with different colouration patterns in the corolla within H immature fruit I mature fruit A, H, I from Beltrán 140, photos by G. Beltrán B–G from Orejuela R. et al. 2685, photos by G.E. Barboza.
Distribution
Ecology
Capsicum dimorphum is an Andean species of premontane or montane moist forests. It is found in the margins, understorey or interior of primary or secondary (sometimes disturbed) forests, between 950 and 3,000 m elevation.
Phenology
Flowering and fruiting all year.
Chromosome number
Not known.
Common names
Colombia: Ahuyamo (Quindío, Bernal 1828), Mirtico de monte (Cauca, Pittier 737; Cundinamarca, Duque-Jaramillo 3330).
Uses
None recorded.
Preliminary conservation assessment
EOO (738,784.034 km2); AOO (452 km2). Capsicum dimorphum is a widespread Andean species in northern South America; considering the large EOO and its presence in officially protected areas in Colombia, Ecuador and Peru, we suggest a status of Least Concern (LC).
Discussion
Capsicum dimorphum is a member of the Andean clade, recovered as sister to C. longifolium (
Capsicum dimorphum is sympatric with C. geminifolium which is distinguished by having long-acuminate leaves, 2–5 long and thin calyx appendages and campanulate corollas. Populations of C. dimorphum from Peru (Department Pasco) are glabrescent to glabrous plants and the leaves are somewhat coriaceous, but the flowers and fruits match those of the pubescent populations of Colombia and Ecuador.
Specimens examined
See Suppl. material
Capsicum eshbaughii
Capsicum eximium var. tomentosum Eshbaugh & P.G.Sm., Baileya 18: 15. 1971. Type. Cultivated in the Indiana University greenhouses, from seeds collected in Bolivia (Santa Cruz: Prov. Florida: 158 km W of Santa Cruz, on road to Cochabamba, 1300 m elev., P.G. Smith C281), 29 Nov 1960, C.B. Heiser C301 (lectotype, designated here: IND [IND-0105969, acc. # 139720]; isolectotypes: IND [IND-0105968, acc. # 113590, IND-0153285, acc. # 139721]).
Type
Based on Capsicum eximium var. tomentosum Eshbaugh & P.G.Sm.
Description
Erect shrubs or subshrubs, 1–3 m tall, with the main stem 1.5–3 (–4) cm at base, few or much branched from near the base and with fragile and tangled branches. Young stems strongly 3–4-angled, fragile, pale green, densely pubescent with white or ochraceous, brilliant, spreading, simple, glandular trichomes (stalk 3–7-celled, head unicellular, stipitate or not stipitate) 0.1–0.8 mm long or furcate trichomes with both branches ending in an unicellular head or one branch eglandular and the other longer and glandular; nodes green; bark of older stems greyish-white or pale brown, fissured, glabrescent; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair more or less similar in size and shape. Leaves membranous, concolorous or discolorous, intense green adaxially and whitish-grey abaxially, densely pubescent on both surfaces, especially along the veins, with the same glandular trichomes like those on the stems and a few simple 3–4-celled eglandular trichomes; blades of major leaves 3.7–5.7 (–6.5) cm long, (1.6–) 2–4 cm wide, ovate, major veins 3–4 on each side of mid-vein, the base attenuate or cuneate and somewhat asymmetric, the margins entire, the apex acuminate; petioles 1.3–2.5 cm long, densely pubescent; blades of minor leaves 3–4.3 cm long, 1.4–2 cm wide, ovate, the major veins 2–3 on each side of mid-vein, the base attenuate, the margins entire, the apex acute; petioles 0.6–0.7 cm long, densely pubescent. Inflorescences axillary, 2–3 (–4) flowers per axil or more rarely flowers solitary; flowering pedicels 8–17 mm long, strongly angled, erect, geniculate at anthesis, densely glandular pubescent, the trichomes spreading; pedicel scars inconspicuous. Buds globose, white or white-purple. Flowers 5-merous. Calyx ca. 2 mm long, ca. 2 mm wide, cup-shaped, thick, green, densely glandular pubescent as stems and leaves, calyx appendages (5–) 10 (–12), 1.5–2.7 (–3) mm long, 0.1 mm wide, unequal, thin, erect, linear, inserted close to the margin, densely pubescent with the same trichomes as calyx tube. Corolla (5–) 6–7 mm long, 6–7 mm in diameter, white with greenish-yellow spots outside and within, sometimes corollas nearly white or with pale purple spots in the lobes, stellate with interpetalar membrane, lobed nearly halfway to the base, pubescent adaxially with short glandular trichomes (stalk 1–2-celled; head globose, unicellular) in the throat and base of the lobes, the tube 2–4 mm long, glabrous abaxially, the lobes 3–3.5 mm long, 3.5–4 mm wide, triangular or widely ovate, spreading, with eglandular trichomes abaxially, the margins finely ciliate, the tips acute, papillate. Stamens five, equal; filaments 2.5–3 mm long, white, inserted on the corolla 0.9–1.2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.5–1.7 mm long, ellipsoid, light yellow, bluish post-dehiscent, not connivent at anthesis. Gynoecium with ovary 1–1.2 mm long, ca. 1.5 mm in diameter, green, ovoid; ovules more than two per locule; nectary ca. 0.4 mm tall; styles dimorphic, 3.25–4.3 mm long, at the same level or sparsely exserted beyond the anthers, purple, clavate; stigma ca. 0.2 mm long, 0.35 mm wide, discoid, pale green. Berry (4–) 6–8 mm in diameter, globose or subglobose, dark green turning to dark brown when immature, bright red at maturity, pungent, pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels (10–) 15–20 mm long, erect, strongly angled, widened distally, brownish-green; the fruiting calyx 4–5 mm in diameter, persistent, not accrescent, discoid, green, the appendages 1.5–3.5 mm long, spreading. Seeds 8–20 per fruit, 3–3.5 mm long, 2.5–3.2 mm wide, C-shaped, yellow to brownish-yellow, the seed coat faintly reticulate (SM), reticulate-cerebelloid (SEM), the cells irregular in seed body, polygonal at margins, the lateral walls sinuate in seed body, straight to wavy at margins; embryo imbricate.
Distribution
Capsicum eshbaughii is an endemic species restricted mainly to central Bolivia (Santa Cruz Department), at mid-elevation (1,300–1,800 m), with only one collection in the Department of Cochabamba, at 3,000 m (Fig.
Ecology
Capsicum eshbaughii is an uncommon species in dry deciduous and degraded marginal forests close to urbanised areas.
Phenology
Flowering from November to April. Fruiting from February to April.
Chromosome number
2n = 2x = 24 (
Common name
Bolivia. Ulupica (Santa Cruz, Eshbaugh 1943; Nee 43483).
Uses
Fruits are used as condiments (Nee 43483).
Preliminary conservation assessment
EOO (365.297 km2); AOO (24 km2). Capsicum eshbaughii has a small geographical extent and area of occupancy and is known from only 10 collections, the majority of them from Samaipata and surroundings. As far as we know, this species is only found in anthropogenically disturbed areas (
Capsicum eshbaughii A flowering branch B eglandular trichome of the leaf C flower D, E furcate glandular trichomes of the pedicel F fruiting calyx G glandular trichome of the abaxial surface of the calyx H, I glandular trichomes of the adaxial surface of the calyx J sector of opened corolla K gynoecium L fruit M seed N embryo A–E, G–K from Eshbaugh 1943 b F, L–N from Nee 36164. Drawn by P. Peralta. Published in
Discussion
Capsicum eshbaughii was assigned to the Purple corolla clade (sister to C. eximium,
Capsicum eshbaughii A plant B flower buds C flower, in pre-anthesis D–G stellate corollas with different colouration patterns H flower buds and flower in anthesis, seen from behind I immature fruit J mature fruits A, E, H–J from Carrizo García 67, photos by G.E. Barboza and C. Carrizo García B, C, D, F, G from Palombo 19, photos by N. Palombo.
Experimental studies have shown that C. eshbaughii hybridises freely with C. eximium and C. cardenasii, the others ‘ulupicas’, producing highly fertile hybrids (
Specimens examined
See Suppl. material
Capsicum eximium
Type
Argentina. Salta: Dpt. Guachipas, Quebrada de San Antonio, Pampa Grande, 1600 m elev., semillas del ejemplar A. T. Hunziker 1907, cultivadas en el Jardín Botánico de la Facultad de Agronomía y Veterinaria de Buenos Aires, 4 Mar 1943, A.T. Hunziker 7346 (lectotype, designated by
Description
Erect shrubs or subshrubs, 0.5–3 (–4) m tall, the main stem thick, up to 6 cm in diameter at base, much branched from near the base, the branches fragile, flexuous, fragile in a typical “zig-zag” appearance above. Young stems strongly angled, fragile, green, moderately pubescent with antrorse, flexuous, simple, uniseriate, 4–7-celled, eglandular trichomes 0.5–1.6 mm long and sparse minute glandular trichomes (stalk short, translucent, 1–2-celled; head dark, multicellular); bark of older stems greyish-white, brown or dark green, fissured, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair more or less similar in size and shape. Leaves membranous, slightly discolorous or concolorous, glabrescent on adaxial and abaxial surfaces and margins with sparse eglandular trichomes like those on stems, but abundant in a tuft of trichomes in the basal vein axils and spreading along the mid-vein abaxially; blades of major leaves 3.4–12.5 cm long, 2.1–5 (–6) cm wide, ovate or elliptic, the major veins 4–5 on each side of mid-vein, the base attenuate or cuneate and asymmetric, the margins entire, the apex acuminate; petioles 1–2.5 cm long, glabrescent or glabrous; blades of minor leaves 3–4 cm long, 1.3–2 cm wide, ovate or elliptic, the major veins 3–4 on each side of mid-vein, the base attenuate, the margins entire, the apex acute; petioles 0.3–0.5 cm long, with the same pubescence as the major leaves. Inflorescences axillary, 2–5 flowers per axil or flowers solitary; flowering pedicels 6–18 mm long, strongly angled, erect, geniculate at anthesis, green, scarcely to moderately pubescent with eglandular and glandular trichomes; the eglandular trichomes short, antrorse; pedicels scars inconspicuous. Buds globose or ovoid, fairly inflated, lilac, purple or yellowish-green. Flowers 5-merous, occasionally perianth 4-merous. Calyx 2–2.5 mm long, ca. 3 mm wide, cup-shaped, thick, green, green with purple spots or purple, moderately pubescent with the same eglandular and glandular trichomes as the stem, the calyx appendages (4–) 5, 1.2–2.7 (–3) mm long, subequal, thick, erect or slightly spreading, cylindrical or laterally compressed, inserted close to the margin, sparsely pubescent with the same trichomes as calyx tube or glabrescent. Corolla 5–8.5 mm long, 9–11 mm in diameter, lilac or purple or white with lilac and greenish-yellow spots outside, lobes marginally or completely lilac, purple or magenta, tube greenish-yellow or ochre and white centre within, sometimes the purple pigmentation is lacking, stellate with interpetalar membrane, 5 (4–)-lobed, halfway or less of the way to the base, pubescent adaxially with a continuous ring of long glandular trichomes (stalk 2–3-celled; head globose, unicellular) in the throat and up to near the base of the lobes, glabrous abaxially, the tube 3–4 mm long, the lobes 3–4.4 mm long, 2.4–3.6 mm wide, triangular, spreading, the margins finely ciliate, the tips acute, papillate. Stamens five, equal; filaments 2.7–3.8 mm long, white or lilac, inserted on the corolla 1–1.5 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.7–2.1 mm long, ellipsoid or ovoid, yellow or purplish, not connivent at anthesis. Gynoecium with ovary 1.3–1.8 mm long, 0.9–1.5 mm in diameter, green, ovoid; ovules more than two per locule; nectary ca. 0.4 mm tall; styles homomorphic, 3.5–4.5 mm long, barely exserted beyond the anthers, lilac or white, clavate; stigma ca. 0.2 mm long, 0.5 mm wide, discoid, pale green. Berry 7–10 mm in diameter, globose, green or green with black or violet spots turning to dark brown when immature, bright red at maturity, deciduous, pungent (in some populations non-pungent), the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 14–24 mm long, erect, strongly angled, widened distally, green or purplish-green; fruiting calyx 3–5 mm in diameter, persistent, not accrescent, discoid, green or purple, the appendages 1–3.3 mm long, spreading or reflexed. Seeds 7–17 per fruit, 2.8–4.2 mm long, 2.1–3 mm wide, C-shaped or subglobose, brownish-yellow, the seed coat faintly reticulate (SM), reticulate-cerebelloid (SEM), the cells irregular in seed body and polygonal at margins, the lateral walls sinuate in seed body, straight to wavy at margins; embryo imbricate.
Capsicum eximium A flowering branch B flower C eglandular trichome of the calyx D opened corolla E, F anthers, dorsal and ventral views, respectively G gynoecium H fruit I seed J seed, in cross section K embryo A from Hunziker 7346 B–K from Hunziker 1907. Drawn by N. de Flury. Published in
Capsicum eximium A plant (wet habitat) B plant (dry habitat) C main fissured stem D young inflorescence E–G flower buds with different colouration H–K flowers, in front view, with different corolla colouration within L flower, in lateral view M, N flowers seen from behind O mature fruits A, G, N from Barboza et al. 4914 B, E, M from Barboza et al. 4885 C, K from Barboza et al. 4903 D from Wageningen Netherlands University germplasm collection F, I from Barboza et al. 4895 H, O from Barboza 1919 (cult.) J from Barboza et al. 4896 L from Barboza et al. 3543 A–C, E–O photos by G.E. Barboza, D photo by P. Bosland.
Distribution
Capsicum eximium occupies a continuous area from northern Bolivia (La Paz, Potosí, Cochabamba, Santa Cruz, Chuquisaca and Tarija Departments) to northern Argentina (Jujuy, Salta and Tucumán Provinces) (Fig.
Ecology
Capsicum eximium grows preferentially in dry mesothermic and sub-Andean valleys with deciduous forest and scrub (Chaco), in pastures or on the edge of cultivated fields; it is often found on steep or gentle slopes, along dried watercourses or in remnant of forests dominated by Mimosoideae, Schinopsis or cacti, between 1,000 and 3,000 m elevation.
Phenology
Flowering from November to April; fruiting from late December to May.
Chromosome number
n = 12 (Heiser and Smith 1958); 2n = 2x = 24 (
Common names
Argentina: Ulapuca (Salta, Lahitte s.n.), Ulupica (Salta, Schinini et al.34774), Ají cobincho (Salta, Hilgert 2061); Bolivia: Ulupica (Cochabamba, Peñaranda 458; La Paz, Beck 25261; Potosí: Zamora 193; Santa Cruz, Vargas C. 1382), Ají ulupica (Tarija, Manchego CEP T21), Ulupica muruchi, Ulupica negra semiosca, Ulipica grande hosca, Ulupica tuna, Ulupica negra neta, Ulupica blanca, Ulupica verde, Ulupica camba, Ulupica negra con flor blanca (Chuquisaca,
Uses
The fruits are very pungent and are used as spices or in pickles in the Bolivia (
Preliminary conservation assessment
EOO (278,222.889 km2); AOO (396 km2). Capsicum eximium is found over a large extent of occupancy from northern Bolivia to northern Argentina and is common in the inter-Andean valleys. We suggest the status of Least Concern (LC). It is a species of open areas, forming small populations and is sometimes cultivated on farms for self-consumption of the fruits.
Discussion
Capsicum eximium is a member of the Purple corolla clade (
The pungency of the fruits is polymorphic in C. eximium as it is in C. chacoense and C. baccatum (
Capsicum eximium is a self-compatible species (
Specimens examined
See Suppl. material
Capsicum flexuosum
Capsicum schottianum var. leptophyllum Dunal, Prodr. [A. P. de Candolle] 13(1): 416. 1852. Type. Brazil. “In Brasiliâ australiore. (Sellow e Sendtn. l.c.)” (no herbaria cited; no original material found).
Capsicum parvifolium var. sellowianum Dunal, Prodr. [A. P. de Candolle] 13(1): 419. 1852. Type. Brazil. “In Brasilia australiore. (Sellow.)” (no herbaria cited; no original material found).
Capsicum campylopodium forma magis-puberula
Chodat, Bull. Herb. Boissier ser. 2, 2: 815. 1902. Type. Paraguay. [Canindeyú]: Sierra de Maracayú, in silva Ipé hú, Oct 1898–1899, É. Hassler 5134 (lectotype, designated by
Capsicum campylopodium forma laurifolium Chodat, Bull. Herb. Boissier ser. 2, 2: 815. 1902. Type. Paraguay. [Itapuá]: in silva pr. fl. Capibary, 5 Dec 1898–1899, É. Hassler 5893 (lectotype, designated here: G [G00390270]; isolectotypes: BM [BM000074083], G [G00390269], K [K000648539], P[P00410127], S [S16-27826], UC [UC950167]).
Capsicum mositicum Toledo, Arq. Bot. Estado São Paulo 3(2): 64, f. 2. 1953. Type. Brazil. São Paulo: Mun. de Amparo, Monte Alegre, margem da estrada para Socorro, 740 m elev., 17 Dec 1942, M. Kuhlmann 144 (holotype: SP [001631, acc. # 47939]; isotypes: CORD [CORD00006628, CORD00006629]).
Capsicum ramosissimum Witasek, Denkschr. Kaiserl. Akad. Wiss., Wien. Math.-Naturwiss. Kl. 79(2): 320. 1910. Type. Brazil. “Prov. São Paulo: Prope “Fazenda bella vista”, in districtu urbis Santa Cruz ad flumen Rio Pardo, ca. 500 m elev., VII, leg. v. Wettstein et Schiffner”, Jul 1901, R. v. Wettstein & V. Schiffner [338] (lectotype, designated here: WU [acc. # 0037945]; isolectotypes: CORD [CORD00006633], F [v0093723F, acc. # 871103, fragment], W [1922-0001510], WU [acc. # 0037946], Z [Z-000038683]).
Capsicum schottianum var. flexuosum (Sendtn.) Hunz., Huitième Congr. Int. Bot. Paris. Comptes Rend. Séances Rapp. & Commun. 1954, sect.4: 73. 1956. Type. Based on Capsicum flexuosum Sendtn.
Type
Brazil. “In Brasilia australiore: Sellow” (no original material located; Brazil. Paraná: Mun. Curitiba, Parque Iguaçu, 27 Dec 1979, R. Kummrow 1307; neotype, designated here: MBM [MBM064252]; isoneotype, CORD [CORD00101762]).
Description
Erect subshrubs or shrubs, (0.3–) 0.9–2 m tall, more rarely small trees 2–2.5 m tall, with the main stem thick 2–2.5 cm in diameter at base, much branched above, the branches dichotomously spreading in a typical “zig-zag” appearance. Young stems terete or slightly ridged, fragile, green, glabrous to moderately or densely pubescent, with antrorse simple, uniseriate, 2–6-celled, eglandular trichomes 0.2–0.9 mm long; nodes solid, green; bark of older stems dark brown, glabrous, rarely pubescent; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal or more or less equal in size, similar in shape. Leaves membranous, discolorous, dark green above, light green or greenish-grey beneath, moderately pubescent on both sides or, if glabrescent, with an evident tuft of trichomes in the basal vein axils abaxially, the trichomes appressed-antrorse similar to those of the stems; blades of major leaves 5.2–14 cm long, 1.5–4.5 (7.5–) cm wide, ovate to elliptic, the major veins 4–7 on each side of mid-vein, the base attenuate or short-attenuate, the margins entire, the apex acuminate; petioles 0.5–1.5 cm, glabrous to moderately pubescent; blades of minor leaves 1.7–3.5 (–4.5) cm long, 0.5–2 cm wide, ovate to elliptic, the major veins 3–4 on each side of mid-vein, the base attenuate, the margins entire, the apex acute to acuminate; petioles 0–0.5 cm, with same pubescence as the major leaves . Inflorescences axillary, 2–3 (–6)-flowers per axil or flowers solitary; flowering pedicels 10–21 mm long, terete, pendent, more rarely spreading, non-geniculate at anthesis, glabrous to moderately pubescent, the eglandular trichomes short, antrorse; pedicels scars inconspicuous. Buds globose, inflated, white with light green spots. Flowers 5-merous. Calyx 1.3–2 mm long, 3–3.5 mm wide, cup-shaped, green or greenish-yellow, hyaline at the margin, glabrous to moderately pubescent, the calyx appendages five, inconspicuous, umbo-like and the calyx pentagonal in outline with a tuft of short uniseriate eglandular trichomes on each angle, if the calyx appendages absent, the calyx circular in outline. Corolla 6–11 mm long, ca. 13 mm in diameter, white with yellowish-green pigmentation outside, white with variously yellowish-green spots in the base of lobes and throat within, exceptionally also with purple spots, stellate or rotate-stellate, with thin interpetalar membrane, lobed less than or up to nearly halfway to the base, the tube 3–6 mm long, pubescent adaxially with a continuous ring of glandular trichomes (stalk long 1–3-celled; head globose, peltate, unicellular), glabrous abaxially, the lobes 3–5 mm long, 2–4.5 (–5) mm wide, broadly triangular, spreading, glabrous adaxially and abaxially, the margins involute, papillate, the tips cucullate, densely papillate. Stamens five, equal, filaments 2.5–3.5 mm long, cream, inserted on the corolla 1–1.3 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.1–1.9 mm, ellipsoid or ovoid, yellow, not connivent at anthesis. Gynoecium with ovary 1.3–2 mm long, 1.2–1.4 mm in diameter, light green, ovoid; ovules more than two per locule; nectary ca. 0.4 mm tall; styles homomorphic, (3.8–) 4–5.7 mm, exserted 0.8–1 mm beyond the anthers, white, clavate; stigma 0.2 mm long, 0.8 mm wide, discoid, pale green. Berry 5–8 mm in diameter, subglobose or globose, brilliant green when immature, reddish-orange or red at maturity, deciduous, pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 15–26 mm long, pendent, terete, widened distally, green; fruiting calyx 4–5 mm in diameter, persistent, not accrescent, discoid, light green, sometimes the margin ripped. Seeds (4–) 5–25 per fruit, 2.8–3.4 mm long, 2.2–3 mm wide, C-shaped, subglobose or teardrop-shaped, brownish-black, the seed coat faintly reticulate (SM), reticulate (SEM), the cells polygonal to irregular in shape, the lateral walls sinuate in the seed body and straight to wavy at margins; embryo imbricate or coiled.
Distribution
Capsicum flexuosum is a widespread species occupying a more or less continuous range from north-eastern Argentina (Corrientes and Misiones Provinces) and southern Paraguay (Alto Paraná, Amambay, Caaguazú, Caazapá, Canindeyú, Central, Guairá, Itapuá, Paraguarí and San Pedro Departments) to south and south-eastern Brazil (Minas Gerais, Paraná, Rio Grande do Sul, Santa Catarina and São Paulo States) (Fig.
Ecology
Capsicum flexuosum is very common in the interior and margins of primary and secondary forests, in remnants of forests or disturbed areas. In Brazil, it occurs in a wide range of vegetation types, primarily in the Atlantic Rainforest (Floresta Estacional Decidual, Floresta Estacional Semidecidual Montana and Submontana, Floresta Ombrófila Densa Submontana and Montana, Floresta Ombrófila Mista Montana, Floresta Ombrófila Densa das Terras Baixas), at low and medium altitudes, between 30 and 1,300 m.
Phenology
Flowers nearly all year around; fruiting occurs from January to July and from October to December.
Chromosome number
n = 12 (
Common names
Argentina: Cumbarí (Misiones, Montes 2139), Pimentina (Misiones, Montes 2375), Pimentiña (Misiones, Montes 2375), Ai Jesu (Misiones, Buchinger & Rodríguez 3222), Ají Cumbarí (Corrientes, Bonpland s.n.), Pimenta silvestre (Misiones, Montes 2140), Pimienta del monte (Misiones, Schwindt 4310), Pimiento del monte (Misiones, Buchinger & Rodríguez 3222), Pimentón del monte (Misiones, Schwindt 1871); Brazil: Pimenteira (Rio Grande do Sul, Santos et al. 2869), Pimenta-braba (Rio Grande do Sul, Abruzzi 576), Pimenta de passarinho (Paraná, Lleras Pérez et al. 1947), Pimenta do bugni (Santa Catarina, Neubert 216), Pimento-do-mato, pimenta-braba (Santa Catarina, Schwirkowski 1519); Paraguay: Locote (Canindeyú, Montes 3280), Pimiento silvestre (Canindeyú, Montes 3280).
Indigenous names
Argentina: Guachu ky’i (Guabyrá poty, Misiones, Keller & Ferreira 1349), Guach ky’yi (Takuapi, Misiones, Keller 2957), ‘Ka’a ete’y’ (Guabyrá poty, Misiones, Keller & Ferreira 288); Paraguay: Ke-jiú (Canindeyú, Montes 3280).
Uses
Herbarium labels record that fruits are used as condiments in Argentina and Paraguay due to their high pungency. There are no records of uses from Brazil.
Species conservation assessment
EOO (778,640.645 km2); AOO (1,348 km2). Capsicum flexuosum is quite abundant throughout its distribution. Based on the EOO, the AOO and its presence in many conservation units, we consider C. flexuosum is not under risk and assign the category of Least Concern (LC).
Discussion
Capsicum flexuosum is the single member of the Flexuosum clade (
Capsicum flexuosum A flowering branch B flower C calyx D glandular trichome of the abaxial surface of the calyx E eglandular trichome of the adaxial surface of the calyx F sector of opened corolla G glandular trichome of the abaxial surface of the corolla H gynoecium I fruit J seed K seed, in cross section L embryo A from Hatschbach 18030 B–H from Subils & Moscone 4273 I–L from Hunziker et al. 24993. Drawn by N. de Flury. Published in
Another variable trait is the greenish-yellow pigmentation of the white corolla; this colouration can be present as many small spots interrupted by white lines covering the throat and the base of the lobes or it can be present as five large spots covering the surface (Fig.
Capsicum flexuosum A fruiting branch B flowering node C stellate corolla with greenish spots D, H mature fruits E plant F flower bud G rotate-stellate corolla with purple and greenish spots A–D from Barboza et al. 1034 E Barboza et al. 3631 F–H from Carrizo García 84. Photos by C. Carrizo García.
A striking population from around Monteiro Lobato (São Paulo State, Brazil) is morphologically very similar to C. flexuosum in its habit, pendent, non-geniculate flowering pedicels, pentagonal calyx, orange-red globose pungent fruits and black seeds. However, unlike other C. flexuosum populations in which the corolla is clearly stellate and white with greenish-yellow spots (Fig.
Capsicum schottianum and C. campylopodium are the most morphologically similar species to C. flexuosum. All three of these species lack calyx appendages and are extremely difficult to distinguish from one another in herbarium material, when the corolla colour is not stated on the labels or only fruit is present. Capsicum flexuosum differs from C. campylopodium and C. schottianum in its non-geniculate (vs. geniculate) pedicels, yellowish-green spots on the adaxial surface of the corolla (absent in C. campylopodium and in combination with purple spots in C. schottianum) and edible reddish-orange or red fruit (vs. greenish-golden yellow fruits).
For over 100 years, C. flexuosum has been misinterpreted in both literature and herbaria.
Specimens examined
See Suppl. material
Capsicum friburgense
Type
Brazil. Rio de Janeiro: Mun. Nova Friburgo, subindo o Morro da Caledônia, a 6.45 km do Camping Club do Brasil (RJ.2), 22°17'S, 42°32'W, 1800 m elev., 6 Apr 1986, L. Bianchetti, A.T. Hunziker, V. Casali & G.P. Silva 393 (holotype: CEN [CEN00010214]; isotypes: CORD [CORD00003939, CORD00003940, CORD00004164]).
Description
Erect shrubs 0.8–2.5 (–3) m tall, with the main stem 0.8–1.5 cm in diameter at base, few to much branched above, the branches pendulous dichotomously spreading in a typical “zig-zag” appearance. Young stems angled, fragile, green, glabrescent, with sparse appressed-antrorse, simple, uniseriate, 2–3-celled, eglandular trichomes 0.2–0.5 mm long and abundant minute glandular trichomes (stalk translucent, unicellular, head dark and multicellular); nodes solid, green or slightly purple; bark of older stems dark brown, smooth, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous, concolorous to slightly discolorous, glabrescent on both sides, especially on the margins and veins, with antrorse, curved, 3–5-celled, eglandular trichomes 0.3–0.7 mm long; blades of major leaves (5.5–) 8.5–13 (–21) cm long, (1.5–) 2.5–4.5 (–7.5) cm wide, ovate to elliptic, the major veins 6–7 on each side of mid-vein, the base short-attenuate or attenuate, unequal, the margins entire, the apex acuminate; petioles 0.6–1.2 (–1.5) cm, glabrescent; blades of minor leaves (1.8–) 2.2–3.3 (–5) cm long, 0.9–2 (–2.5) cm wide, elliptic or ovate, the major veins 3–5 on each side of mid-vein, the base rounded, the margins entire, the apex acute; petioles 0.2–0.5 cm, glabrescent. Inflorescences axillary, 2-flowered or flowers solitary; flowering pedicels (17–) 21–49 (–65) mm long, angled, erect or slightly spreading, geniculate at anthesis, green, glabrous or glabrescent, the eglandular trichomes minute, antrorse; pedicels scars inconspicuous. Buds ovoid, entirely purple or fuchsia. Flowers 5-merous. Calyx 2–3 mm long, 3–4 mm wide, cup-shaped, green, thin, strongly 5-nerved, glabrescent with short, uniseriate, eglandular trichomes on the margin and minute glandular trichomes on the tube similar to those of the stem and leaves, the calyx appendages five, 1.2–3 (–3.5) mm long, subequal, erect or spreading, glabrescent. Corolla (7–) 9–12 (–15) mm long, 7.5–10.5 mm in diameter, entirely violet or fuchsia at anthesis; campanulate-urceolate without interpetalar membrane, lobed at the apex, glabrous adaxially and abaxially, the tube 4–6 mm long, the lobes (1.5–) 2–3 (–4) mm long, (1.5–) 2–3 (–4) mm wide, broadly triangular, spreading to strongly recurved at anthesis, the margins and tips densely papillate or with short eglandular trichomes, the tips cucullate. Stamens five, equal; filaments (4–) 5–6 (–7) mm long, lilac, inserted on the corolla 1.5–1.75 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.5–2 (–2.5) mm, ellipsoid, yellowish-white, grey-purple post-dehiscent, not connivent at anthesis. Gynoecium with ovary ca. 1.8 mm long, 1.2 mm in diameter, light green, globose to ovoid; ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, (5–6) 8–11 mm, barely exserted beyond the anthers, white, clavate; stigma ca. 0.3 mm long, 1 mm wide, discoid-depressed, pale green. Berry (4–) 6–10 mm in diameter, globose or globose-depressed, light green and pungent when immature, dark green to greenish-golden yellow and scarcely pungent to rather sweet at maturity, deciduous, the pericarp thin, translucent, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 35–65 mm long, pendent, angled, widened distally, green, the receptacle inflated and forming a weak annular constriction in the limit with the calyx; fruiting calyx 4–4.5 mm in diameter, persistent, not accrescent, green, discoid, the appendages 2–4 mm long, spreading or slightly reflexed. Seeds (2–) 4–9 per fruit, 2.5–3.2 mm long, 2–2.5 mm wide, C-shaped, brownish-black to black, the seed coat reticulate and tuberculate at margins (SM), reticulate-cerebelloid with pillar-like outgrowths at margins (SEM), the cells polygonal in seed body and elongate at hilum zone, the lateral walls straight, sometimes wavy in seed body; embryo imbricate.
Distribution
Ecology
Capsicum friburgense grows in one of the most protected areas of the Atlantic Forest (Mata Atlântica), which has the highest elevations of the Serra do Mar. It is found in the understorey of primary forests of the Floresta Pluvial Montana (Floresta Ombrófila Densa Altomontana), from 1,750 to 1,950 m elevation.
Phenology
Flowering from December to May. Fruiting from February to May.
Chromosome number
n = 13 (
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (123.163 km2); AOO (24 km2). Capsicum friburgense grows in two forested areas that have been set aside as Environmental Protection Areas in Rio de Janeiro, the APA do Caledônia (Maciço da Caledônia) and the Parque Estadual dos Três Picos. It has a small population (< 250 mature individuals) with not more than 10 subpopulations per site. Observers are seeing a continuing decline of subpopulations size, due to serious environmental pressures caused by deforestation, development and changes in hydrology. In recent years, these protected areas faced important natural disasters, including forest fires (2007, 2011 and 2019), great floods and mudslides (2011, see
Discussion
Capsicum friburgense is a beautiful species of the Brazilian Atlantic Forest clade (
Capsicum friburgense is sympatric in Nova Friburgo (Rio de Janeiro) with C. mirabile; both species have a calyx with five appendages and geniculate pedicels and they have similar colour and morphology of fruit and seeds. Capsicum friburgense differs from C. mirabile in having one or two flowers per node, campanulate-urceolate corollas without spots and fruiting pedicels up to 65 mm long (vs. 2–5 flowers per node, multi-coloured stellate corollas and fruiting pedicels up to 32 mm long in C. mirabile).
Specimens examined
See Suppl. material
Capsicum frutescens
Capsicum conoide Mill., Gard. Dict. ed. 8, Capsicum no. 8. 1768. Type. Cultivated at the Chelsea Physic Garden (England), seeds from Antigua (Antilles) (no specimens cited).
Capsicum conicum G.Mey., Prim. Fl. Esseq.: 112. 1818, nom. illeg., non Capsicum conicum Lam. (1794). Type. “In plantationibus” (no specimens cited) [Guyana]. Rio Essequebo, Sept, E.K. Rodschied 30 (lectotype, designated here: GOET [GOET003418]).
Capsicum minimum Roxb., Fl. Ind., ed. Carey & Wall. 2: 261. 1824, nom. illeg., not C. minimum Mill. (1768). Type. India. “East India” (no specimens cited), 18 Dec 1814, W. Roxburgh s.n. [Wallich Cat. No. 2641A) (lectotype, designated here: K [K001116724]).
Capsicum fastigiatum Blume, Bijdr. Fl. Ned. Ind. 13: 705. 1826. Type. “Crescit: in hortis et locis incultis … Nomen: Tjabe rawiet” [Indonesia]. Java, C.L. Blume s.n. (lectotype, designated here: L [L 0003564, acc. # 202560]).
Capsicum indicum var. conoide (Mill.) Dierb., Arch. Apotheker-Vereins Nördl. Teutschl. 30(1): 28. 1829. Type. Based on Capsicum conoide Mill.
Capsicum indicum var. berberideum Dierb., Arch. Apotheker-Vereins Nördl. Teutschl. 30(1): 29. 1829. Type. Based on Capsicum frutescens Mill. (= C. frutescens L.)
Capsicum baccatum
Vell., Fl. Flumin.: 60. 1829 (“1825”); Fl. Flumin. Icon. 2: t. 3. 1831 (“1827”), nom. illeg., non Capsicum baccatum L. (1753). Type. Brazil. [Rio de Janeiro]: “Sponte crescit, et colitur hortis” (lectotype, designated by
Capsicum conoide var. sulcatum Fingerh., Monogr. Capsic.: 15. 1832. Type. No locality cited (no specimens cited; lectotype, designated here: [illustration] Fingerhuth, Monogr. Capsic. Tab. III c. 1832]).
Capsicum conoide var. chordale Fingerh., Monogr. Capsic.: 15. 1832. Type. “Crescit in America et Indiis”; no specimens cited; lectotype, designated here: [illustration] Fingerhuth, Monogr. Capsic. Tab. III d. 1832]).
Capsicum flexuosum var. perrottetti Dunal, Prodr. [A. P. de Candolle] 13(1): 413. 1852. Type. French Guiana. Sin. loc., 1820, S. Perrottet 218 (holotype: G-DC [G00131855]).
Capsicum frutescens var. multilobatum Dunal, Prodr. [A. P. de Candolle] 13(1): 413. 1852. Type. No locality indicated, possibly from plants in cultivation (holotype: G-DC [G00131856]).
Capsicum conicum var. orientale Dunal, Prodr. [A. P. de Candolle] 13(1): 415. 1852. Type. Sudan. [Kurdufan] “ad pagum Cordofan in Milbes”, 4 Dec 1839, G.C.T. Kotschy 292 (lectotype, designated here: G-DC [G00131905]; isolectotypes: BM [BM001016438, BM001016449], E [E00687052], G [G00442768], LE).).
Capsicum conicum G.Mey. [unranked “variat”] latifolium Dunal, Prodr. [A. P. de Candolle] 13(1): 415. 1852. Type. Sudan. “Senaar”, 1831, G. Acerbi s.n. (lectotype, designated here: G-DC [G00131885]).
Capsicum conicum G.Mey. [unranked “variat”] angustifolium Dunal, Prodr. [A. P. de Candolle] 13(1): 415. 1852. Type. Oman. Muscat “in horto Mascate”, P.M.R. Aucher-Eloy 5039 (lectotype, designated here: G [G00390279]; isolectotype: G [G00390280]).
Capsicum crispum Dunal, Prodr. [A. P. de Candolle] 13(1): 415. 1852. Type. Republic of Mauritius. “Cult. au Jardin des Pamplemousses”, 1839, L. Bouton s.n. (holotype: G-DC [G00131904]; isotype: MPU [MPU023045]).
Capsicum crispum var. piper-rabiosum Dunal, Prodr. [A. P. de Candolle] 13(1): 416. 1852. Type. France. Île de Bourbon [= Réunion Island], 1821, Anonymous s.n. (holotype: G-DC [G00131903]; isotype: MPU [MPU023044]).
Capsicum conoide var. oblongoconicum Dunal, Prodr. [A. P. de Candolle] 13(1): 415. 1852. Type. No locality cited (no specimens cited; lectotype designated here: [illustration] Fingerhuth, Monogr. Capsic. Tab. III, b. 1832).
Capsicum curvipes Dunal, Prodr. [A. P. de Candolle] 13(1): 423. 1852. Type: French Guiana. “In Guianâ (h. Moric.)”, Anonymous s.n. (holotype: G [G00390275]; isotype: MPU [MPU023048].
Capsicum annuum var. frutescens (L.) Kuntze, Revis. Gen. Pl. 2: 449. 1891. Type: Based on Capsicum frutescens L.
Capsicum annuum var. conicum (G.Mey.) Alef., Landw. Fl.: 132. 1866. Type: Based on Capsicum conicum G.Mey.
Capsicum annuum var. subconicum Alef., Landw. Fl.: 133. 1866. Type: Based on Capsicum conoide Mill.
Capsicum annuum var. conoide (Mill.) Irish, Rep. (Annual) Missouri Bot. Gard. 9: 65. 1898, as “conoides”. Type: Based on Capsicum conoide Mill.
Capsicum frutescens var. conoide (Mill.) L.H.Bailey, Gentes Herbarum 1: 129. 1923. Type: Based on Capsicum conoide Mill.
Capsicum annuum var. parvo-acuminatum Makino, J. Jap. Bot. 3(8): 30. 1926. Type. Cultivated in Japan “Hab. JAPAN, cultivated” (no specimens cited, no original material located).
Capsicum frutescens var. queenslandicum Domin, Biblioth. Bot. 89: 572. 1928. Type: Australia. Queensland: “Nordost-Queensland: Harweys Creek”, Jan 1910, K. Domin 8247 (holotype: PR [acc. # 530853]).
Capsicum annuum var. oblongo-conicum (Dunal) Cufod., Bull. Jard. Bot. État Bruxelles 33 (Suppl.): 860. 1963. Type: Based on Capsicum conoide var. oblongo-conicum Dunal.
Type
“Habitat in India” Herb. A. van Royen n. 908.244–150 (lectotype, designated by Heiser and Pickersgill 1969, pg. 280: L [L 0053043, acc. # 902560, branch in the lower half of the sheet]).
Description
Low subshrubs or shrubs, herbaceous or woody at the base, 0.3–1.5 (–2.5) m tall, much branched from near the base. Young stems slightly angled, fragile, green or purple or green with purple ridges, glabrous to sparsely pubescent with simple, uniseriate, 4–9 (–11)-celled, eglandular trichomes 0.3–1.4 mm long; nodes solid, green or purple; bark of older stems brown; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair more or less similar in size and shape. Leaves membranous, concolorous or slightly discolorous, glabrescent or glabrous on both surfaces, sometimes with eglandular trichomes on the main veins or a tuft of trichomes in the vein axils abaxially; blades of all leaves (4–) 4.4–8.2 (–12.5) cm long, 2–4.5 (–6) cm wide, ovate or narrowly elliptic, the major veins 5–7 on each side of mid-vein, the base asymmetric, cuneate or attenuate, the margins entire, the apex acuminate to long-acuminate; petioles 0.5–3 cm, glabrescent or glabrous. Inflorescences axillary, 2–4 (–5) flowers per axil, rarely flowers solitary; flowering pedicels 9–30 (–40) mm long, erect, geniculate at anthesis, glabrous or sparsely pubescent; the eglandular trichomes minute, antrorse; pedices scars inconspicuous. Buds ovoid, cream or greenish-white. Flowers 5–7-merous, spreading or pendent. Calyx 1.2–2.5 mm long, cup-shaped, thick, green, glabrous or sparsely pubescent, the calyx appendages absent or five, ca. 0.5 mm long. Corolla 3.75–6.5 mm long, 5–15 mm in diameter, usually dull white or greenish-white outside and within, stellate with interpetalar membrane, lobed nearly the halfway to the base, pubescent adaxially with small glandular trichomes (stalk 1–2-celled; head unicellular) in the throat and base of the lobes, glabrous abaxially, the tube 3–4 mm long, the lobes 3–5 mm long, 2–3.5 mm wide, the margins finely ciliate, the tips acute, papillate. Stamens five (–seven), equal; filaments 1–1.5 mm long, cream or purple, inserted on the corolla 1–1.6 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.5–2.85 mm long, ellipsoid, bluish-grey or purplish or (rarely) dark green or yellow, connivent at anthesis. Gynoecium with ovary 2.5–4 mm long, 1.3–1.8 mm in diameter, oblong-ovoid, pale green; ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, 3–4 mm, exserted 1.5–2 mm beyond the anthers, cream or purple, cylindrical; stigma 0.1 mm long, 0.25 mm wide, discoid, light green. Berry usually 9–30 (–60) mm long, 4–12 (–15) mm in diameter, usually elongate and narrowly triangular, with the apex pointed or blunt and the base narrowed, usually green and yellowish-green when immature with transitions to yellow, orange-red, orange-yellow, orange to red at maturity, deciduous or persistent, pungent, rarely non-pungent, pericarp thick and opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 16–35 (–50) mm long, usually erect, sometimes pendent, terete to strongly angled, widened distally, green; fruiting calyx (3–) 4–6 (–7) mm in diameter, persistent, somewhat accrescent, deeply cup-shaped, without an evident constriction in its base and the junction with the pedicel, sometimes the margin ripped, green. Seeds 10–52 per fruit, 2–4 mm long, 2.2–3.3 (–4) mm wide, C-shaped to subglobose, pale yellow, the seed coat smooth to obscurely reticulate (SM), reticulate-cerebelloid (SEM), the cells irregular in shape, the lateral walls slightly to strongly sinuate; embryo imbricate.
Capsicum frutescens A reproductive branch B eglandular trichome of the leaf C flower D section of the calyx showing the venation E glandular trichome of the abaxial surface of the calyx F opened corolla G glandular trichome of the abaxial surface of the corolla H, I anthers, dorsal and ventral views, respectively J gynoecium K anatomical detail of the pericarp (note the giant cells in the mesocarp) L seed M seed, in cross section N structure of seed coat at the seed margin O structure of seed coat at the seed body P embryo. From Hunziker 25489. Drawn by N. de Flury.
Capsicum frutescens A plant B flower buds C flower showing the calyx D flower, in lateral view E flower, in front view (note the connivent anthers) F flower after anthers dehiscence G immature (green) and mature (red) fruits H fruit, in longitudinal section showing the seeds A, B, E–G from Barboza & Leiva González 4829 C, D, H from Barboza et al. 2041. Photos by G.E. Barboza.
Distribution
Capsicum frutescens has a broad distribution in lowland tropical America (Fig.
Ecology
Capsicum frutescens grows in low semi-deciduous forests or in disturbed areas and agricultural clearings; it is commonly cultivated near homesites and in the chakras of many local communities, between 10 and 1,500 (2,000) m elevation.
Phenology
Flowering and fruiting all year in different parts of its range.
Chromosome number
2n = 2x = 24 (
Common names
Argentina: Tabasco (Salta, Hunziker 25489); Antilles: Bird pepper (
Indigenous names
Belize: Mash-ík (Mayal, Cayo, Balick et al. 2272), Smash-ík (Cayo, Balick et al. 2391); Bolivia: Bido (Tacana, La Paz, Williams 764), Naris (Chiquitano, Santa Cruz, Del Aguila et al. 662); Colombia: Bee-a’ (Tucano, Amazonas, Schultes & Cabrera 13046), Biaá (Yucana, Amazonas, Cárdenas 9405), Cog (Puinave, Guainía, Triana 18), Eviviaa (Tucano, Guainía, Marín & Rodríguez 508), Fekorai (Huitoto-Mɨnɨka, Amazonas, Henao 167), Ferocoi (Huitoto, Caquetá, Cárdenas et al. 9300), Ichiriay (Matapi, Amazonas, Cárdenas et al. 9413), Jibirai (Caquetá, Cárdenas 9345), Jichiri (Yucuna, Amazonas, Cárdenas et al. 9441), Lehirihay (Yucuma, Caquetá, Cárdenas et al. 9325), Meniray (Huitoto, Caquetá, Cárdenas 9307), Mèe (Colona, Amazonas, Torres & Rodríguez 2020), Pidootú (And, Amazonas, Castro & Andoke 608), Pipitatu (And, Amazonas, Castro & Andoke 606), Pxrxtú (And, Amazonas, Castro & Andoke 597), Tsirrerreji (Sikuare guahibo, Guainía, Rodriguez 2), Viaa (Kuboes, Guainía, Marín & Rodríguez 509); Ecuador: Aatyu (Chapalaachi, Esmeraldas, Yañez et al. 1407), Ampy (Shuar, Zamora-Chinchipe, Ortega et al. 51), Chimidu dio (Cayapa, Esmeralda, Kvist & E. Asanza 40456), Ma pipi pia (Siona and Secoya Indians, Napo, Vickers 208), Ocoma (Cofán, Napo, Cerón 194), Panduchu (Achuar Jívaro, Pastaza, Lewis et al. 14011), Pía (Secoya, Sucumbíos, Miranda-Moyano 225), Pipetio (Cayapa, Esmeraldas, Kvist 40565), Pucuitape (Cayapa, Esmeraldas, Kvist & Asanza 40356), Sun’nyo pipi pia (Siona & Secoya Indians, Napo, Vickers 226), Tun (Colorado, Pichincha, Kvist 40201), Uchu (Pastaza, Lewis et al. 14011); Guatemala: Ich (Ch’orti, Chiquimula, Kufer 100); Paraguay: Ki’ï (Central, Schinini & Bordas 24511); Peru: Shunaru’ca (Chayahuita, Loreto, Odonne 641), Tsukagka (Amazonas, Salaün 177), Yancuru’ca (Chayahuita, Loreto, Odonne 664). Mexico: Guiiña xcuuchu (Zapoteco, Oaxaca, Sánchez L. et al. 953).
Uses
Capsicum frutescens has medicinal, ornamental, ritual and food applications. The fresh or cooked fruits are an important component of daily meals in India (soups, sauces, jams, pepper powder). The famous hot Tabasco sauce is made from tabasco peppers, a varietal of C. frutescens. In the Brazilian Amazon, herbarium labels report plants with sweet fruits (Walter et al. 575) that are used for decorating dishes and salads and in smoking rituals (
Preliminary conservation assessment
EOO (19,757,841 km2); AOO (1,336 km2). Capsicum frutescens is a widespread cultivated species across the Americas and can be assigned the Least Concern (LC) status.
Discussion
Capsicum frutescens belongs to the Annuum clade (
Morphoagronomic and molecular characterisation studies have been carried out on many accessions of C. frutescens from different germplasm banks (
Many authors have recognised close affinities between C. annuum, C. frutescens and C. chinense (e.g.
Flowering specimens of C. frutescens and C. chinense are sometimes difficult to distinguish in herbaria when fruits are missing. Venation of flowering calyx is a good distinguishing feature between both species since, in C. frutescens, the main nerves are often completely immersed in the calyx surface (Fig.
Capsicum conoide was coined by
For C. conicum, Georg
When Roxburgh used the name Capsicum minimum for the first time (
The description of C. fastigiatum (
Capsicum conoide var. sulcatum var. chordale were both coined by
In describing C. frutescens var. multilobatum,
In describing C. conoide var. oblong-conicum, Dunal cited no herbarium material, but he referred to the Fingerhuth’s illustration “Tab 3, f. b” as the element he has seen. Therefore, he is clearly differentiating this illustration from C. conoides and it is the original material for the varietal name. We select this illustration as the lectotype.
Specimens examined
See Suppl. material
Capsicum galapagoense
Brachistus pubescens
Stewart, Proc. Calif. Acad. Sci., ser. 4, 1: 137. 1911. Type. Ecuador. Galápagos: Albemarle Island [= Isabela], Villamil, 450–600 ft elev., 3 Jan 1906, A. Stewart 3352 (lectotype, designated by
Capsicum galapagense Heiser & P.G.Sm., Brittonia 10: 200. 1958, nom. illeg., not Capsicum galapagoense Hunz. (1956). Type. Based on Brachistus pubescens Stewart
Type
Based on Brachistus pubescens Stewart.
Description
Erect low shrubs, 0.5–1 m tall, much branched from near the base. Young stems terete to slightly 2–3-angled, fragile, densely white or yellowish-white (when dry) pubescent, with spreading, simple, uniseriate, 3–7-celled, eglandular trichomes 0.3–1.5 mm long; nodes green; bark of older stems brown, glabrescent; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous, slightly discolorous, densely pubescent on both surfaces, especially on the veins, with similar eglandular trichomes to the stems, plus sparse small glandular trichomes and occasionally furcate eglandular trichomes; blades of major leaves 2–6 (–8) cm long, 0.9–2.8 (–3.5) cm wide, ovate, the major veins 3–5 on each side of mid-vein, the base cuneate or truncate and asymmetric, the margins entire, the apex slightly acuminate; petioles 0.5–1.5 cm long, densely pubescent; blades of minor leaves 1.1–1.95 cm long, 0.3–0.7 cm wide, ovate, the major veins 3–4 on each side of mid-vein, the base cuneate or truncate and asymmetric, the margins entire, the apex acute or obtuse; petioles 0.2–0.7 cm long, densely pubescent. Inflorescences axillary, 1–2-flowered; flowering pedicels 6–8 mm long, short, angled, erect to slightly spreading, geniculate at anthesis, densely pubescent, the eglandular trichomes long, spreading; pedicel scars conspicuous. Buds ovoid, white. Flowers 5-merous. Calyx 1.3–1.6 (–2) mm long, 1.8–2 mm wide, cup-shaped, circular in outline, thin, green, densely pubescent with the same trichomes as stems and leaves, the calyx appendages absent. Corolla 4–5 mm long, ca. 6 mm in diameter, entirely white or dull white outside and within, stellate without interpetalar membrane, lobed nearly halfway to the base, the tube 2–2.5 mm long, pubescent adaxially with sparse short glandular trichomes (stalk unicellular; head globose, unicellular), glabrous abaxially, the lobes 2.3–2.5 mm long, ca. 2.5 mm wide, ovate, spreading, glabrous adaxially and abaxially, the margins papillate, the tips acute, papillate. Stamens five, equal; filaments 1–1.3 mm long, white, inserted on the corolla ca. 0.7–1 mm from the base, with auricles fused to the corolla at point of insertion; anthers 0.9–1.3 mm long, ellipsoid, yellow, connivent at anthesis. Gynoeciumm with ovary 0.9–1.3 mm long, 0.8–1 mm in diameter, ovoid; ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, 2.25–2.5 mm long, exserted 0.5–0.8 mm beyond the anthers, cylindrical, white; stigma 0.09 mm long, ca. 0.24 mm wide, discoid. Berry 5–7 mm in diameter, globose or somewhat ellipsoid, dark green when immature, red-orange or bright red at maturity, deciduous, pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 8–18 (–22) mm long, erect, slightly angled or terete, widened distally, green; fruiting calyx 2.8–4 mm in diameter, persistent, not accrescent, discoid, green. Seeds 8–9 per fruit, 3.5–4 mm long, 2.5–3 mm wide, flattened, C-shaped to reniform, pale yellow, the seed coat smooth (SM), cerebelloid (SEM), the cells irregular in seed body and polygonal at superior margin, the lateral walls sinuate in seed body, wavy at margins; embryo annular or imbricate.
Distribution
Capsicum galapagoense is endemic to the Galápagos Archipelago of Ecuador (Islands of Bartolomé, Fernandina, Isabela, Santa Cruz, Santiago, Rabida and Pinta) (Fig.
Ecology
Capsicum galapagoense grows in shade under shrubs or small trees in the arid lowlands to moist uplands of the islands (Pisonia, Scalesia or Croton forests); 15–900 m elevation.
Phenology
Flowering and fruiting from December to August and likely all year.
Chromosome number
n = 12 (Heiser and Smith 1958); 2n = 2x = 24 (
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (ca. 8000 km2); AOO (56 km2). Based on the number of locations and the area of occupancy, these suggest an Endangered (EN; B2ab(iii)) category for Capsicum galapagoense. The threats in the Galápagos, such as land-use activities, introduced alien plants in the inhabited islands and the population explosion of goats and pigs in the uninhabited ones (e.g. Islas Bartolomé, Fernandina or Rabida), have caused serious ecological problems that need to be addressed urgently to protect the rare and endemic species of the islands (
Discussion
Capsicum galapagoense belongs to the Annuum clade (
Capsicum galapagoense A flowering branch B eglandular trichome of the leaf C flower D calyx E section of the calyx showing the venation F sector of opened corolla G gynoecium H fruit I anatomical detail of the pericarp (note the giant cell in the mesocarp) J seed K seed, in cross section L structure of seed coat at the seed margin M structure of seed coat at the seed body N embryo A, B from Stewart 3351 C–G from Taylor G11 H–N from Schimpf 20. Drawn by L. Ochoa.
Capsicum galapagoense is self-compatible and has been experimentally crossed with members of the Annuum clade (C. annuum, C. chinense, C. frutescens) and the Baccatum clade (C. baccatum wild, C. rabenii, C. chacoense); no incompatibilities were found in any direction. Based on this breeding evidence, there is the potential for hybridisation between C. annuum or C. frutescens and C. galapagoense, although
Specimens examined
See Suppl. material
Capsicum geminifolium
Acnistus geminifolius
Dammer, Bot. Jahrb. 36(4): 384. 1905. Type. Ecuador. Pichincha: “in silv. Monte Corazón”, Sep 1873, P.L. Sodiro 114/84 (lectotype, designated by
Capsicum scolnikianum Hunz., Kurtziana 1: 213. 1961. Type. Peru. Piura: Canchaque, en el camino de Piura a Huancabamba, 1200 m elev., 1 Dec 1948, R. Scolnik 1389 (lectotype, designated here: CORD [CORD00006647]; isolectotype: CORD [CORD00006648]).
Type
Based on Acnistus geminifolius Dammer.
Description
Erect shrubs or subshrubs, 1–4 (–6) m tall, with the main stem 1–1.5 cm in diameter at base, profusely branched above, rarely herbs 0.30–0.80 m, the branches sometimes scandent. Young stems angled, fragile, green, sometimes with purple ridges, glabrous or sparsely to moderately pubescent with spreading or antrorse, white to yellowish-white, simple, uniseriate, 4–6-celled, eglandular trichomes 0.3–0.6 mm long, sometimes sparse branched trichomes; nodes solid, green; bark of older stems dark brown or greyish, glabrous or moderately pubescent; lenticels abundant, light brown. Sympodial units difoliate, the leaves geminate; leaf pair markedly unequal in size and similar or dissimilar in shape. Leaves membranous, concolorous or discolorous, deep green above, pale green or purple below, with sparse or abundant, simple, eglandular trichomes 0.5–1.2 mm long adaxially and abaxially, sometimes also branched trichomes along mid-vein and occasionally a tuft of simple and branched trichomes in the main vein axils abaxially; blades of major leaves 4–13 cm long, 0.4–2.2 (–3.57) cm wide, elliptic to narrowly elliptic, sometimes ovate or falcate, the major veins 4–6 on each side of mid-vein, the base attenuate, somewhat asymmetric, the margins entire, the apex acuminate or long-acuminate; petioles 0.4–0.8 cm long, moderately pubescent or glabrescent with simple trichomes; blades of minor leaves 0.8–5 (–5.5) cm long, 0.4–1.5 (–2.7) cm wide, ovate or elliptic, the major veins 3–5 on each side of mid-vein, the base short attenuate, rarely asymmetric, the margins entire, the apex obtuse; petioles 0.1–0.5 cm, glabrescent. Inflorescences axillary, 2–5 (–6) flowers per axil, rarely flowers solitary; flowering pedicels (10–) 15–25 (–27) mm, thin, terete, pendent, non-geniculate at anthesis, green or purple-tinged, glabrous to moderately pubescent, the eglandular trichomes short or long, antrorse; pedicels scars inconspicuous. Buds ovoid, yellow or dark purple. Flowers 5-merous. Calyx 1.5–3 mm long, 2–2.5 mm wide, cup-shaped, thin or somewhat fleshy, green, greenish-purple or dark purple (nearly black), glabrescent to moderately pubescent with antrorse eglandular trichomes 0.3–0.7 mm long, the calyx appendages (2–) 3–5, 3–6.5 mm long, 0.3–0.6 mm wide, subequal, erect or spreading, subulate. Corolla 7–12 (–15) mm long, 13–18 mm in diameter, dull yellow or yellow with sparse to abundant maroon or purple spots within and outside or nearly completely purple outside, campanulate, stellate in outline, with a thin interpetalar membrane connecting the lobes in the proximal half, lobed nearly 1/3 of the way to the base, glabrous abaxially and adaxially, the tube 6–7 mm long, 8–10 mm in diameter, the lobes (3–) 5–8 mm long, (3–) 4.5–5.5 mm wide, ovate, erect or spreading at anthesis, the margins and tips papillate. Stamens five, equal; filaments 2–3 mm long, pale green, white or lilac, glabrous, inserted on the corolla ca. 2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers (1.6–) 2–2.5 mm long, ovoid, cream, yellow or rarely white, slightly connivent at anthesis. Gynoecium with ovary (1.5–) 1.8–2 mm long, ca. 1.5 mm in diameter, light green or cream, ovoid; ovules more than two per locule; nectary 0.5–0.8 mm tall, cream; styles homomorphic, 5–7 mm long, exserted 0.8–1.3 mm beyond the anthers, slightly curved distally, cream, clavate; stigma 0.2–0.3 mm long, ca. 0.8 mm wide, usually discoid, sometimes slightly bilobed, light green. Berry 7–12 mm in diameter, globose or globose-depressed, light green when immature, pale orange or orange at maturity, deciduous, non-pungent, the pericarp thick, opaque, lacking giant cells (endocarp smooth); stone cells 1–5 or absent, subglobose or irregular, 1–1.5 mm in diameter; fruiting pedicels 18–30 mm long, pendent, terete or distally ribbed, scarce to strongly widened distally, green, greenish-purple or purple; fruiting calyx 4–5 mm in diameter, persistent, not accrescent, discoid, greenish-purple or green, the appendages 3–8 mm long, 1 mm wide, spreading or reflexed, green, greenish-purple or purple. Seeds (8–) 12–70 per fruit, 1.8–2.3 mm long, 1.3–1.5 mm wide, teardrop-shaped, black, the seed coat reticulate (SM and SEM), the cells polygonal or irregular in shape, the lateral walls straight or wavy; embryo annular.
Distribution
Capsicum geminifolium is widely distributed over north-western South America, from Colombia and Ecuador to central Peru (Fig.
Ecology
Capsicum geminifolium grows in margins or interior of primary or secondary Andean to sub-Andean montane rain forests, somewhat exposed to light, at (100–) 950–3,500 m elevation.
Phenology
Flowering and fruiting all year.
Chromosome number
Not known.
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (1,120,997.593 km2); AOO (380 km2). Capsicum geminifolium is very common across its range and it is also found in many protected areas. We assign the status of Least Concern (LC).
Discussion
Capsicum geminifolium is a member of the Andean clade (
Capsicum geminifolium A flowering branch B flower C glandular trichome of the abaxial surface of the calyx D eglandular calyx of the leaf and calyx E branched trichome of the leaf and calyx F section of the calyx showing the venation G sector of opened corolla gynoecium H gynoecium I fruit J anatomical detail of the pericarp (note the absence of giant cells in the mesocarp) K seed, in cross section L structure of seed coat at the seed margin M structure of seed coat at the seed body N embryo. From Scolnik 1389. Drawn by L. Sánchez.
Capsicum geminifolium A plant B main stem with lenticels C flowering branch D flower bud E flower, in lateral view, with long calyx appendages F flower, with short calyx appendages G flower, in pre-anthesis H, I corollas, in front view, with different colouration patterns within J immature fruit K mature fruit A, B, D, J from Barboza & Leiva González 4845 C, G from Deanna & Leiva González 3 E, I from Barboza & Leiva González 4852 F, H from Deanna & Leiva González 77 K from Orejuela R. 2688 A, B, D, E, I, J photos by G.E. Barboza C, F–H photos by R. Deanna K photo by A. Orejuela.
When
The type collection of C. scolnikianum, housed at CORD, is mounted on two sheets (CORD00006647, CORD00006648). A label in Hunziker’s hand stating “C. scolnikianum, Typus!, Scolnik 1389” on CORD 00006647 indicates his intent to consider this sheet as the type; therefore, we here designate it the lectotype.
Specimens examined
See Suppl. material
Capsicum hookerianum
Brachistus hookerianus
Miers, Ann. Mag. Nat. Hist., ser. 2, 3(16): 268. 1849. Type. Ecuador. Guayaquil: “Cerro of Lantana”, Jan 1846, W. Jameson s.n. (lectotype, designated by
Bassovia brachypoda Dunal, Prodr. [A. P. de Candolle] 13(1): 411. 1852. Type. Peru. J.A. Pavon s.n. (holotype: G; isotypes: CORD [CORD00084676 fragment ex G]; MPU [MPU023058]).
Capsicum brachypodum (Dunal) Kuntze, Revis. Gen. Pl. 2: 450. 1891. Type. Based on Bassovia brachypoda Dunal
Capsicum eggersii Bitter, Repert. Spec. Nov. Regni Veg. 18: 126. 1922. Type. Ecuador. Manabi: Agua Amarga, near El Recreo, 15 Jan 1897, H.F.A. von Eggers 15555 (holotype: B [destroyed, F neg. 2867]; lectotype, designated here: M [M-0171548]; isolectotypes: CORD [CORD00084677 fragment ex L, CORD00087961 fragment ex K), F [F0072805F, acc. # 143187, fragment], K [K000585902], L [L.2881993, acc. # 602560], P [P00482081], US [01919835, acc. # 939121]).
Type
Based on Brachistus hookerianus Miers.
Description
Climbing erect subshrubs or shrubs, (0.5–) 1–3 m tall, with the main stem 1.5–2 cm in diameter at base, profusely branched above. Young stems, angled, fragile, flexuous, glabrous to moderately pubescent with white, simple, uniseriate, 4–6-celled, eglandular trichomes 0.4–0.9 mm long; nodes green; bark of older stems longitudinally striped, dark brown, glabrous; lenticels abundant, elliptic or rounded, cream or light brown. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size and shape. Leaves membranous, discolorous, deep green above, paler below, with sparse white simple eglandular trichomes adaxially and moderate to abundant trichomes abaxially, especially on main veins, the trichomes 0.4–1.2 mm long, sometimes branched trichomes 0.6–1 mm long; blades of major leaves 4–12.5 cm long, 2–5.5 cm wide, ovate or broadly ovate, the major veins 4–6 on each side of mid-vein, the base long-attenuate, strongly asymmetric, the margins entire, the apex acuminate; petioles 0–1.3 cm long, somewhat winged due to the decurrence of the base leaf, pubescent or glabrescent; blades of minor leaves 2.5–4.2 (–7) cm long, 1.2–2.7 cm wide, ovate, the major veins 3–4 on each side of mid-vein, the base short, attenuate, rarely asymmetric, the margins entire, the apex acute, obtuse or rounded; petioles 0–0.5 cm, glabrescent or pubescent. Inflorescences axillary, 2–7 flowers per axil, rarely flowers solitary; flowering pedicels 8–15 mm long, thin, pendent, non-geniculate at anthesis, glabrous to glabrescent, the eglandular trichomes antrorse; pedicels scars conspicuous, corky. Buds not seen. Flowers 5–merous. Calyx 1.3–2 mm long, 2.2–2.7 mm wide, cup-shaped, green, glabrescent to densely pubescent with eglandular trichomes, the calyx appendages usually 8–10, unequal, the five main appendages longer 4–6 (–7) mm long, the secondary appendages shorter 1.3–4 mm, erect or somewhat spreading, linear-subulate, ca. 1 mm below the margin. Corolla 7.5–9 mm long, 8–10 mm in diameter, yellow, campanulate with abundant interpetalar membrane, lobed nearly 1/3 of the way to the base, the tube 4.5–6 mm long, glabrous adaxially and abaxially, the lobes 2–2.5 (–3) mm long, ca. 3.5–5.5 mm wide, broadly triangular, glabrous adaxially and with abundant eglandular trichomes near the apex abaxially, the margins papillate, the tips cucullate, papillate. Stamens five, equal; filaments (1.5–) 1.8–2 mm long, inserted on the corolla ca. 2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers (1.5–) 1.7–2.1 mm long, ellipsoid, yellow, not connivent at anthesis. Gynoecium with ovary 1.5–1.6 mm long, ca. 1.3 mm in diameter, ovoid or subglobose; ovules more than two per locule; nectary 0.5 mm tall; styles homomorphic, ca. 4 mm long, exserted less than 1 mm beyond the anthers, clavate; stigma ca. 0.5 mm long, 0.8–1 mm wide, capitate or bilobed, light green. Berry 5–9 mm in diameter, globose or subglobose, green or yellow when immature, bright red at maturity, non-pungent, the pericarp thick, opaque, lacking giant cells (endocarp smooth); stone cells 2–4, irregular in shape; fruiting pedicels 12–18 mm long, pendent, angled, widened distally, green; fruiting calyx 4–6 mm in diameter, persistent, not accrescent, discoid, greenish-white, the appendages 3–6 (–7.5) mm long, spreading or reflexed, greenish-white and purple tinged. Seeds (10–) 22–45 per fruit, 2.2–2.8 mm long, 1.5–1.9 mm wide, ellipsoid or C-shaped, yellow or brown, the seed coat reticulate (SM), cerebelloid (SEM), the cells irregular in shape, the lateral walls sinuate to strongly sinuate; embryo imbricate.
Distribution
Capsicum hookerianum is restricted to extra-Andean western Ecuador (Guayas, El Oro, Loja and Manabí Provinces) and northern Peru (Tumbes Department) (Fig.
Ecology
Capsicum hookerianum grows in dry deciduous forests at low elevations (100–1,000 m).
Phenology
Flowering from November to April (July); fruiting from late December to July.
Chromosome number
Not known.
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (32,802.812 km2); AOO (136 km2). Although C. hookerianum has a relatively large extent of occurrence and is found in many localities, some of them protected areas, we observed a continued decline in the area of occupancy outside of official natural reserves; for these reasons, we assign a category of Near Threatened (NT).
Discussion
Capsicum hookerianum is a member of the Andean clade (
Capsicum hookerianum A flowering branch B eglandular trichome of the leaf C flower D section of the calyx showing the venation E glandular trichome of the abaxial surface of the calyx F sector of opened corolla G gynoecium H fruiting branch I fruit J anatomical detail of the pericarp (note the absence of giant cells in the mesocarp) K seed L seed, in cross section M structure of seed coat at the seed margin N structure of seed coat at the seed body O embryo A–G from Asplund 15241 H–O from Asplund 15363. Drawn by L. Ochoa.
Capsicum hookerianum is sympatric with the widespread C. rhomboideum which is a more vigorous plant with dense, branched pubescence and multi-flowered inflorescences with up to 12 flowers (Fig.
Duplicates of the type collection of C. eggersii are distributed in several Herbaria (CORD, F, K, L, M, P, US). At CORD and F, there are small fragments taken from other specimens (e.g. fragments accompanying F neg. 2867 from B and fragments in CORD taken from the duplicate in L), whereas in the remaining herbaria, the specimens consist of good fertile material. We have selected the most complete and best-preserved sheet of Eggers 15555 (M-0171548) as the lectotype for C. eggersii.
Specimens examined
See Suppl. material
Capsicum hunzikerianum
Type
Brazil. São Paulo: Salesópolis, Boracéia, 30 Nov 1951, M. Kuhlmann 2785 (holotype [2 sheets]: CORD [CORD00003944 (sheet A) and CORD00003945 (sheet B); isotypes: CEN [CEN00109451], SP [SP001623, acc. # 79274], SPSF [acc. # 30213]).
Description
Robust erect shrubs 1–3 m tall, much branched above, the branches much leafy. Young stems terete or slightly 3-angled, rigid, green with violet spots, glabrous; nodes solid, green or purple; bark of older stems fissured, brown, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar or dissimilar in shape. Leaves coriaceous, discolorous, dark green above, light green beneath, glabrous on both sides; blades of major leaves (7.5–) 9.5–20 (–25) cm long, 2.5–7 (–8.5) cm wide, ovate to elliptic, the major veins (4–) 5–7 on each side of mid-vein, the base attenuate and unequal, the margins entire, slightly revolute, the apex long-acuminate; petioles (0.5–) 0.8–2 (–3.5) cm long, green with dark lilac spots, glabrous; blades of minor leaves 2–4.5 cm long, (0.8–) 1–2 cm wide, ovate, the major veins 3–4 on each side of mid-vein, the base attenuate, the margins entire, the apex acute; petioles 0.2–0.5 cm long, glabrous. Inflorescences axillary, 2–4 (–5) flowers per axil, rarely flowers solitary; flowering pedicels (13–) 20–38 (–48) mm long, terete or slightly angled, erect or slightly spreading, geniculate at anthesis, green, glabrous or sparsely pubescent with minute, few glandular trichomes (stalk unicellular; head dark, multicellular) and some sparse antrorse eglandular trichomes; pedicels scars conspicuous, corky. Buds ovoid, cream or white. Flowers 5-merous. Calyx 3.5–5 (–6.5) mm, 4–4.5 mm wide, cup-shaped, membranous and cream amongst the veins, the veins thick and pale green, glabrous, the calyx appendages 5 (6–10), unequal, pale green, erect to spreading, cylindrical, inserted very close to the margin, the five main appendages longer 2.5–4.5 (–5) mm long, the 1–5 secondary appendages shorter 1–2 mm long. Corolla 10–14 (–16) mm long, (10–) 15–23 mm in diameter, thick, white with greenish-yellow lines outside, mostly white with diffuse brown-purple spots at the base of each lobe and throat and a narrow greenish-yellow centre within, stellate with thin interpetalar membrane, lobed 1/3 of the way to the base, glabrous abaxially and adaxially, the tube 5–7.5 mm long, the lobes 6–8 mm long, (3–) 3.5–5 (–6) mm wide, broadly triangular, spreading, the margins involute and finely ciliate, the tips strongly cucullate forming a hood-like structure, papillate. Stamens five, equal; filaments (1.5–) 2–3 (–3.5) mm long, white or cream, inserted on the corolla ca. 1.5–2 mm from the base, with auricles fused to the corolla at point of insertion; anthers (2–) 2.5–4 mm, ellipsoid, grey, not connivent at anthesis. Gynoecium with ovary 1–2 mm long, 1–1.5 mm in diameter, light green, subglobose; ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, (4–) 5–6 mm long, exserted ca. 1 mm beyond the anthers, cream, clavate; stigma 0.3 mm long, 0.8–0.9 mm wide, somewhat discoid-bilobulate, light green or cream. Berry 6–10 mm in diameter, globose, slightly depressed, light green when immature, greenish-golden yellow at maturity, deciduous, pungent, the pericarp thin, translucent, with giant cells (endocarp alveolate); stone cells 3–5, spherical, 0.17–0.22 mm in diameter; fruiting pedicels (23–) 35–50 mm long, curved or pendent, terete, strongly widened at the apex, green; fruiting calyx 4–7 mm in diameter, persistent, not accrescent, green, discoid, the appendages 3–6 (–7) mm long, green, spreading or reflexed. Seeds 10–20 per fruit, 2.5–3.2 mm long, 2–2.5 mm wide, C-shaped or ellipsoid, black, the seed coat reticulate and tuberculate at margins (SM), reticulate with pillar-like outgrowths at margins (SEM), the cells polygonal in shape, the lateral walls straight; embryo imbricate.
Capsicum hunzikerianum A flowering branch B flower C calyx D sector of opened corolla E, F anthers, dorsal and ventral views, respectively G stigma H fruit I seed A–G from Kuhlmann 2785, H, I from Mattos and Mattos 14254. Drawn by L. Ribulgo. Published in
Capsicum hunzikerianum A plant B flowering branch C flower bud D, E flowers, in front view, with different corolla colour patterns F flower, seen from behind G immature fruit H mature fruit I seed A, E from Bianchetti et al. 1537, photos by L. Bianchetti B, C, D, F, H, I no specimen vouchers, photos taken in situ by C. dal Zovo (Associazione PepperFriends) G from Barboza 5041, photo by G.E. Barboza.
Distribution
Ecology
Capsicum hunzikerianum inhabits the montane forests of the Atlantic Forest (Mata Atlântica), in Dense Ombrophilous Forest (Floresta Ombrófila Densa), in wet, shady or semi-shady or marshy places, at medium elevations (800–1,100 m).
Phenology
Flowering from November to May. Fruiting from late January to May.
Chromosome number
2n = 2x = 26 (Barboza et al. 5041, see Table
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (1,456.094 km2); AOO (40 km2). Capsicum hunzikerianum grows in two rain forest reserves in the State of São Paulo, the Parque Estadual da Serra do Mar-Núcleo Cunha and the Estação Biológica da Boracéia. In both protected areas, few subpopulations have been recorded. Based on the small extent of occurrence and area of occupancy, the low number of the different locations (= 5) and a decline in the area of occupancy, we assign C. hunzikerianum the threat status of Endangered (EN; B1ab(ii).
Discussion
Capsicum hunzikerianum is probably a member of the Atlantic Forest clade, but has not yet been included in phylogenetic analyses. It shares corolla shape and colour, fruit colour and pungency, seed colour, seed coat ornamentation, chromosome number, habitat and distribution of the members of this clade. It is a distinct species of the montane forest of Serra do Mar distinguished by its general glabrescence, large and coriaceous leaves and large flowers. The calyx is the largest in the genus, reaching 6.5 mm long, with five long appendages (up to 5 mm long) and 1–5 unequal, shorter appendages (1–2 mm long). The corolla is unusual for its large size and white colour with few brown-purple spots (Fig.
Capsicum hunzikerianum is sympatric with the morphologically similar C. schottianum. The two species share geniculate pedicels, stellate corollas and fruit and seed features, but they differ in the corolla size (10–16 mm long in C. hunzikerianum vs. 7–10 mm long in C. schottianum), number of calyx appendages (5–10 vs. 0–5 minute appendages), consistency of the leaves (coriaceous vs. membranous) and general indumentum (plants glabrous vs. plants glabrescent to pubescent).
Specimens examined
See Suppl. material
Capsicum lanceolatum
Brachistus lanceolatus Greenm., Bot. Gaz. 37(4): 212. 1904. Type. Guatemala. Alta Verapaz: Chucaneb, 1850 m elev., Apr 1889, J. Donnell Smith 1837 (holotype: US [00027406, acc. # 1335158]).
Capsicum guatemalense Bitter, Repert. Spec. Nov. Regni Veg. 20: 377. 1924. Type. Guatemala. Suchitepequez: Las Nubes, Nov 1877, C.G. Bernoulli & R. Cario 2339 (lectotype, designated here: GOET [GOET003421]; isolectotype: GOET [GOET003422]).
Type
Based on Brachistus lanceolatus Greenm.
Description
Erect shrubs or subshrubs, 1–3 (–5) m tall, much branched from near the base, the branches flexible. Young stems terete or slightly angled, fragile, green, glabrous or glabrescent with sparse white antrorse, curved, simple, uniseriate, 4–6-celled, eglandular trichomes 0.4–1.3 mm long; nodes green; bark of older stems brown, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair markedly unequal in size and shape. Leaves membranous to coriaceous, concolour, glabrous adaxially and with simple, 2–5-celled, eglandular trichomes abaxially, especially on veins and margins; blades of major leaves 6.5–16 cm long, 1.4–3.8 cm wide, elliptic to lanceolate, the major veins 5–7 on each side of mid-vein, sometimes more evident only on one side, the base long-attenuate or attenuate, asymmetric, the margins entire, the apex long-acuminate; petioles 0.5–0.8 cm long, glabrescent; blades of minor leaves (1–) 2–5.5 cm long, (0.6–) 0.8–2.7 cm wide, ovate or elliptic, the major veins 2–3 on each side of mid-vein, the base slightly asymmetric, the apex acute or obtuse; petioles 0–0.2 cm long, glabrescent. Inflorescences axillary, 1-flowered, rarely 2-flowered; flowering pedicels (15–) 25–43 mm long, thin, curved to pendent, non-geniculate at anthesis, green or purple, glabrous; pedicels scars inconspicuous. Buds ovoid, yellow-purple or intense purple. Flowers 5-merous, rarely perianth 4-merous. Calyx 1.2–2 mm long, 2–4 mm wide, cup-shaped, green or greenish-purple, glabrous or glabrescent, the calyx appendages (4–) 5, (2–) 3–5 mm long, ca. 0.7 mm wide, subequal, spreading or strongly reflexed, linear or subulate, 0.1–0.4 mm below the margin, glabrous or glabrescent. Corolla 9.8–14 mm long, 10–15 mm in diameter, purple and marginally white outside and within, campanulate with abundant interpetalar membrane, lobed 1/3 of the way to the base, glabrous abaxially and adaxially, the tube 8–11 mm, the lobes (1.8–) 2–3 mm long, 3.5–4 mm wide, triangular to broadly triangular, the margins and tips papillate or with short eglandular trichomes. Stamens five, equal or subequal; filaments 2.5–4.2 (–4.6) mm long, white or yellowish-white, inserted on the corolla 2–2.5 mm from the base, with auricles fused to the corolla at the point of insertion; anthers (1.2–) 1.5–2 mm long, ellipsoid, yellowish-white, connivent at anthesis. Gynoecium with ovary ca. 2 mm long, 1.5–1.8 mm in diameter, pale green, ellipsoid or ovoid; ovules more than two per locule; nectary 0.5 mm tall; styles homomorphic, 4.5–6 mm long, exserted 1–2 mm beyond the anthers, white; stigma ca. 0.2 mm long, ca. 0.8 mm wide, slightly bilobed, light green. Berry 7–13 mm in diameter, globose or globose-compressed, green when immature, orange or orange-red at maturity, non-pungent, the pericarp thick, opaque, lacking giant cells (endocarp smooth); stone cells absent; fruiting pedicels (25–) 30–55 mm long, pendent, terete or slightly angled, widened distally, green or greenish-purple; fruiting calyx 4–7 mm in diameter, persistent, not accrescent, green, discoid, with a strong annular constriction at junction with the pedicel, the appendages 4–9 mm long, 0.8–1 mm wide, green, strongly reflexed. Seeds (20–) 29–60 (–93) per fruit, 2–2.5 mm long, (0.8–) 1.2–1.8 mm wide, C-shaped or teardrop-shaped, brownish-black to black, the seed coat reticulate (SM and SEM), the cells polygonal to slightly irregular in shape, the lateral walls straight or slightly sinuate; embryo annular.
Distribution
Capsicum lanceolatum is distributed in southern Mexico, Guatemala and Honduras (Fig.
Ecology
Capsicum lanceolatum grows in primary forest remnants or disturbed montane rain forests on steep slopes, ravines, or in stream canyons at (100–) 1,000–3,000 m elevation.
Phenology
Flowering from May to December and fruiting all year.
Chromosome number
2n = 2x = 26 (
Common names
Guatemala: Pajarito del río (Quezaltenango, Steyermark 33429), Yerba de pajarito (Quezaltenango, Steyermark 33357).
Indigenous names
Mexico: Tumattez (Tzeltal, Chiapas, Méndez Girón 7745), Chuj ch ‘ul tumaltez (Tzeltal, Chiapas, Ton 7803).
Uses
None recorded.
Preliminary conservation assessment
EOO (255,067.790 km2); AOO (252 km2). Capsicum lanceolatum has a large extent of occurrence; however, based on its small area of occupancy, the continuing decline of the number of locations, the extreme fluctuations observed in the number of mature individuals in the subpopulations and the demonstrated extinction of this species in many natural habitats (Bosland and González 2000), we assign this species the Endangered (EN; B2b,c(ii,iii,iv)) status.
Capsicum lanceolatum A flowering branch B flower C calyx (appendages cut off) D eglandular trichome of the adaxial surface of the calyx E glandular trichome of the abaxial surface of the calyx F sector of opened corolla G gynoecium H fruit I anatomical detail of the pericarp (note the absence of giant cells in the mesocarp) J seed K seed, in cross section L structure of seed coat at the seed margin M structure of seed coat at the seed body N embryo A from Ventura A. 798 B–G from Skutch 1450 H–N from Beaman 6014. Drawn by L. Ochoa. Published in
Discussion
Capsicum lanceolatum is a member of the Andean clade (
The other wild Capsicum species of the Andean clade that partially overlaps in distribution with C. lanceolatum is C. rhomboideum; this latter species is found in more disturbed areas. Capsicum lanceolatum differs from C. rhomboideum by having less vigorous habit, mostly glabrous (vs. densely pubescent) plant body, solitary flowers (vs. many-flowered inflorescences), white-purple corollas (vs. yellow) and larger fruits (berries 7–13 mm vs. 5–9 mm in diameter in C. rhomboideum).
Capiscum guatemalense was described, based on a collection made by Bernoulli and Cario held in GOET (
Specimens examined
See Suppl. material
Capsicum longidentatum
Type
Brazil. Bahia: Mun. Itatim, Morro da Pedra Grande, base do Morro, 12°42'57"S, 39°45'46"W, 285 m elev., 8 Apr 2006, E. Melo, F. França, C.T. Lima & C. Cunha 4344 (holotype: HUEFS [HUEFS000001093, acc. # 109249]).
Description
Erect, slender or semi-scandent shrubs (1–) 1.5–4 m tall, much branched from near the base. Young stems angled, fragile, greyish or brown, glabrous, the youngest stems densely pubescent with furcate and dendritic eglandular trichomes 0.2–0.9 mm long; nodes green; bark of older stems greyish, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous, discolorous, green above, light green or yellowish-green beneath, densely pubescent on both surfaces, especially abaxially, with simple uniseriate eglandular trichomes and branched trichomes similar to those of the stems; blades of major leaves 3.5–5.4 cm long, 1.5–2.5 cm wide, ovate, the major veins 4–5 on each side of mid-vein, the base short-attenuate or truncate and unequal, the margins entire, the apex acuminate; petioles (0.3–) 0.5–1.5 cm, densely pubescent; blades of minor leaves 2.5–3 cm long, 1–1.3 cm wide, ovate, the major veins 3–4 on each side of mid-vein, the base attenuate, the margins entire, the apex acute; petioles 0.2–0.5 cm long, densely pubescent. Inflorescences axillary, 2–5 flowers, rarely flowers solitary; flowering pedicels (6.5–) 8–23 mm long, strongly angled, pendent, non-geniculate at anthesis, with abundant spreading dendritic trichomes; pedicels scars inconspicuous. Buds ovoid, whitish-green. Flowers 5-merous. Calyx 2–4 mm long, 3–3.5 mm wide, cup-shaped, thick, greenish-yellow, strongly 5 (–6)-nerved, densely pubescent with branched trichomes, the calyx appendages 5 (–6), (4.5–) 5–8.5 mm long, 0.1–0.2 mm wide, subequal or unequal, erect, greenish-yellow, linear, inserted very close to the margin, with the same indumentum as calyx tube. Corolla 6–7.5 mm long, white outside, white with greenish-yellow spots in the lobes and throat and white centre within, stellate with interpetalar membrane, lobed nearly halfway to the base, pubescent adaxially with small glandular trichomes (stalk long, 2-celled; head globose, unicellular) in the throat and base of the lobes, glabrous abaxially, the tube 3–3.5 mm long, the lobes 3.3–3.6 (–4) mm long, 2–2.5 mm wide, broadly triangular, the margins involute densely pubescent, the tips cucullate densely papillate. Stamens five, equal; filaments 1.25–2 mm long, cream, inserted on the corolla ca. 1.5 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 2–2.5 mm long, ellipsoid, cream or light brown, not connivent at anthesis. Gynoecium with ovary ca. 2 mm long, 1.3–1.4 mm in diameter, greenish-white, ovoid; ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, 3.5–3.75 mm, exserted ca. 1 mm beyond the anthers, greenish-white, clavate; stigma ca. 0.3 mm long, 0.6 mm wide, somewhat discoid, light green. Berry 7.5–9.5 mm long, 7–8.5 mm in diameter, globose or subglobose, slightly flattened at the apex, light green when immature, colour at maturity unknown, probably yellowish-green, deciduous, non-pungent, the pericarp with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 15–20 mm long, pendent, angled, slightly widened distally, green; fruiting calyx 5–6 mm in diameter, persistent, not accrescent, green, discoid, strongly 5(–6)-nerved, the appendages 5–8.5 mm long, ca. 0.3 mm wide, spreading, green. Seeds (3–) 5–17 per fruit, 3–3.7 mm long, 2.5–2.8 mm wide, C-shaped or ellipsoid, brown to brownish-black, faintly reticulate (SM), cerebelloid (SEM), the cells irregular in shape, the lateral walls wavy to strongly sinuate; embryo imbricate.
Distribution
Capsicum longidentatum is endemic to the core of the Brazilian Caatinga (Bahia, Mina Gerais, and Pernambuco States) (Fig.
Ecology
Capsicum longidentatum is common at the base of the inselbergs, on granitic hillsides with shrubby open vegetation and in gallery forests along small rivers, between 250 and 900 m.
Phenology
Flowering from October to April; fruiting from November to May.
Chromosome number
2n = 2x = 24 (
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (275,449.384 km2); AOO (92 km2). Capsicum longidentatum is a species restricted to the Caatinga, an ecosystem with significant human disturbance and habitat fragmentation (
Capsicum longidentatum A reproductive branch B detail of a flowering node C flower bud D flower, upper view E opened corolla (one stamen has been removed) F, G anthers, ventral and dorsal views, respectively H gynoecium I fruit J fruiting calyx K seed L embryo A, D, E, F–H from Melo et al. 4344 B, C, I–L from Agra & Barboza 7086. Drawn by L. Ribulgo. Published in
Discussion
In phylogenetic analyses, C. longidentatum was resolved as an isolated branch and assigned to the monotypic Longidentatum clade, but the species placement is not strongly supported (
Capsicum longidentatum A habitat (Caatinga) B plant C typical aspect of the plant in the arid caatinga D flowering branch E pendent flower F flower, in front view G immature fruit H fruiting calyx I seed A–C, G–I from Agra & Barboza 7086 D–F from Tabosa et al. 55 A–C, G–I photos by G.E. Barboza D–F photos by J.R. Stehmann.
Capsicum longidentatum is distinguishable from C. parvifolium and C. caatingae, the other members of the Caatinga clade, in its long calyx appendages (4.5–8.5 mm long), white corolla with greenish-yellow spots within, densely branched pubescence (though furcate or dendritic trichomes appear occasionally in C. caatingae and C. parvifolium, respectively) and non-pungent fruits. Capsicum caatingae and C. parvifolium have corollas mostly purple, usually simple pubescence and pungent fruits. Capsicum caatingae lacks of calyx appendages (vs. five, rarely six, in C. longidentatum) and has arborescent habit and multi-flowered inflorescences (vs. shrubby habit and few-flowered inflorescences in C. longidentatum), while C. parvifolium has similar habit and inflorescences as C. longidentatum, but the 5 (–7) calyx appendages are shorter (0.7–2.2 mm long).
There are few reports on labels of the fruit colour of Capsicum longidentatum at full maturity; it may be yellow (Gonçalves et al. 175, Pastore & Harley 2603, Socorro 150, Melo 4693) or orange (Pastore & Harley 2603). Both colour and consistency of the pericarp need to be checked in the field to observe whether it is similar to Brazilian species with greenish-golden yellow gelatinous fruits (Atlantic forest species and also C. parvifolium) or to the red fruited species, such as C. caatingae.
Specimens examined
See Suppl. material
Capsicum longifolium
Type
Ecuador. Zamora-Chinchipe: Area of Estación Científica San Francisco, road Loja-Zamora, ca. 35 km from Loja, transect Q2, 03°58'S, 79°04'W, 1900 m elev., 12 Jun 2005, F.A.Werner 1548 (holotype: QCA [160608]; isotypes: LOJA, NY [01130066]).
Description
Erect, scandent shrubs (0.60–) 1.40–3 m tall, laxly branched above, the branches bending down. Young stems angled, fragile, green, glabrous; nodes green or purplish-green; bark of older stems striate, dark green, glabrous; lenticels ovoid, whitish to light brown. Sympodial units difoliate, the leaves geminate; leaf pair markedly unequal in size and shape. Leaves coriaceous, slightly discolorous, adaxial surface dark green and shiny, abaxial surface light green and opaque, glabrous on both surfaces and margins; blades of major leaves (7–) 8.5–17 (–18) cm long, (0.8–) 1–2.5 cm wide, narrowly elliptic, the major veins (11–) 13–17 on each side of mid-vein, the base asymmetric and attenuate, the margins entire, the apex acuminate; petioles (0.2–) 0.5–1.4 cm long, glabrous; blades of minor leaves 2.5–5.7 cm long, 1–2 cm wide, ovate or broadly elliptic, the major veins 4–5 on each side of mid-vein, the base short-attenuate, sometimes asymmetric, the margins entire, the apex obtuse; petioles 0.1–0.5 cm long, glabrous. Inflorescences axillary, 3–7 (–9) flowers per axil or sometimes on a short rachis, rarely flowers solitary; flowering pedicels 3–8 mm long, filiform, terete, pendent, slightly curved, non-geniculate at anthesis, green, glabrous; pedicels scars inconspicuous. Buds ovoid, yellow or purplish-yellow. Flowers 5-merous. Calyx 2.5–3 mm long, 2.8–3 mm wide, cup-shaped, membranous, translucent, light green or greenish-purple, glabrous, the calyx appendages 2–3, 2–2.5 mm long, 1.8–2.2 mm wide, subequal, thick, green or purple, oblique or spreading, triangular-compressed, wing-like, glabrous. Corolla 6–8.5 mm long, 8–11 mm in diameter, thick, entirely yellow or yellow with red-brown pigmentation in the throat or at margin lobes within, campanulate-stellate, without or with a thin interpetalar membrane, lobed 1/3 to nearly halfway to the base, glabrous adaxially and abaxially, the tube (3–) 4–5 mm long, the lobes 3–3.5 (–4) mm long, ca. 3 mm wide, broadly ovate, erect or spreading, the tips cucullate and papillate. Stamens five, equal; filaments 2–2.6 mm long, white or red-brown, inserted on the corolla ca. 2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 2–2.75 mm long, ellipsoid, purplish-white or brown, connivent at anthesis. Gynoecium with ovary 1.6–1.8 mm long, 1.2 mm in diameter, white or light green, subglobose; ovules more than two per locule; nectary 0.3–0.5 mm tall; styles homomorphic, 5–5.8 mm long, exserted 1–1.4 mm beyond the anthers, white and lilac at the apex or red-brown, clavate; stigma 0.3 mm long, 0.2–0.4 mm wide, somewhat bilobed, light green. Berry 8–13 mm in diameter, globose, slightly flattened at the apex, green when immature, orange at maturity, deciduous, non-pungent, the pericarp thick, opaque, lacking giant cells (endocarp smooth); stone cells absent; fruiting pedicels 10–16 mm long, pendent, terete, widened distally, green; fruiting calyx 4–5.5 mm in diameter, persistent, not accrescent, discoid, green-purple or green, the appendages short and wide (2–2.8 mm long, 2.4–2.6 mm wide at base) or long and more slender (4.5–5.5 mm long, ca. 1.5 mm wide at base), spreading or reflexed, green-purple or green. Seeds ca. 24 per fruit, 1.7–2.3 mm long, 1.7–2.2 mm wide, D- or teardrop-shaped, black, the seed coat reticulate (SM and SEM), the cells rectangular or polygonal in shape, the lateral walls straight or slightly sinuate; embryo annular.
Distribution
Capsicum longifolium is endemic to northern Peru (Amazonas, Cajamarca, Junín and Piura Departments) and southern Ecuador (Zamora-Chinchipe Province) (Fig.
Ecology
Capsicum longifolium is a plant of Andean montane wet forests, in the interior of primary forests in shady areas at mid-elevations (1,800–2,200 m).
Phenology
Flowering and fruiting from December to August (probably all year).
Chromosome number
2n = 2x = 26 (
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (67,266.225 km2); AOO (24 km2). Although C. longifolium has been collected many times in San Francisco Biological Reserve (SFBR, Zamora-Chinchipe, Ecuador), it is known from only five other locations in areas not included in the National System of Protected Areas where the quality of its habitat probably will decline. Based on this, we suggest a status of Vulnerable (VU; B2ab(iii).
Discussion
Capsicum longifolium is strongly resolved within the Andean clade (
Capsicum longifolium A reproductive branch B flower C opened corolla D, E, F anthers, ventral, lateral and dorsal views, respectively G gynoecium H ovary, in cross section I fruit J seed. From Barboza & Leiva González 4849. Drawn by S. Leiva González. Published in
Capsicum longifolium A plant B internode with lenticels C, D flower buds E Flower, in longitudinal section F flowers showing corolla yellow with brownish centre G flower, in lateral view H, I flowers with completely yellow corollas, upper and lateral views, respectively J, K flowers with yellow corollas and red-brown edges, upper and lateral view, respectively L, M immature fruits N mature fruit A, C, H, I from Barboza & Leiva González 4821 B, D, E, L, N from Barboza & Leiva González 4846 F, G from Barboza & Leiva González 4850 J, K, M from Barboza & Leiva González 4851. Photos by S. Leiva González and G.E. Barboza. Published in
Capsicum longifolium is morphologically most similar to C. dimorphum, its sister species and to C. regale with which it shares the stellate yellow corollas, the non-pungent fruits and the black seeds. Capsicum longifolium can be distinguished from C. dimorphum by having completely glabrous vegetative organs and calyces, long and narrow (ratio 6–10.8), coriaceous, major leaves, flowers 3–7 (–9) on a short rachis and calyces with 2–3 thick triangular-compressed appendages that look like wings. In contrast, C. dimorphum has pubescent vegetative organs and calyces, shorter and wider (ratio 4–5.25), membranous, major leaves, solitary or up to five axillary flowers and no calyx appendages or, if appendages are present, they are three and minute (Fig.
Specimens examined
See Suppl. material
Capsicum lycianthoides
Type
Ecuador. Chimborazo: secus v. Chasuán, Jul 1860, R. Spruce s.n. (holotype: W [acc. # 1889-0222993]; isotypes: CORD [CORD00101757, fragment ex K], K [K000201917]).
Description
Erect slender or scandent shrubs or subshrubs, (0.50–) 1–3 (–4) m tall, few branched above, the branches dichotomously spreading in a typical “zig-zag” appearance. Young stems angled, fragile, flexuous, dark green or purple, glabrous, the new growth with few antrorse, simple, uniseriate, 3–4-celled, eglandular trichomes 0.1–0.3 mm long; nodes solid, green or purple; bark of older stems brown, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair markedly unequal in size and shape. Leaves coriaceous, discolorous, dark green above, pale green or purple or green with purple spots below, glabrous on both surfaces, sometimes with some antrorse eglandular trichomes on mid-vein abaxially; blades of major leaves (10–) 11–22.5 cm long, (3–) 4–8.5 cm wide, ovate or broadly ovate, rarely elliptic, the major veins (6–) 7–10 on each side of mid-vein, the base attenuate to strongly asymmetric, the margins entire, the apex acuminate or long-acuminate; petioles 0.8–2.3 (–3.5) cm long, glabrous; blades of minor leaves 2.5–5.5 (–8) cm long, 1.8–4.5 cm wide, ovate or orbicular, the major veins 3–5 on each side of mid-vein, the base truncate or rounded, the margins entire, the apex acute or obtuse; petioles 0–0.4 cm, glabrous. Inflorescences axillary, (2–) 3–8 (–10) flowers on a short rachis; flowering pedicels 8–15 mm, thin, terete, curved to pendent, non-geniculate at anthesis, green or purple, glabrous or moderately pubescent, the eglandular trichomes short, antrorse; pedicels scars conspicuous, corky. Buds ovoid, yellow or yellow-purple. Flowers 5-merous. Calyx 2–4 mm long, 3–5 mm in diameter, cup-shaped, thin or somewhat fleshy, green, greenish-purple or purple, the calyx appendages (2–) 3–5, 2–3.5 mm long, subequal, linear or subulate, sometimes like horns, spreading or reflexed, 0.3–0.5 mm below the margin, tube and appendages glabrescent with antrorse trichomes 0.3–0.5 mm long. Corolla 8–15 mm long, 15–18 mm in diameter, yellow or yellow with a purple star or dark brown spots outside and within, broadly campanulate, with a thin and wide interpetalar membrane connecting the lobes to the distal end, pentagonal in outline, shallowly lobed, glabrous adaxially and abaxially, the tube 7.5–14.5 mm long, the lobes ca. 0.5 mm long, the margin and the tips papillate. Stamens five, subequal or filaments unequal, with three filaments 1.5–2.5 mm long and two filaments 2.5–4 mm long, pale yellow or yellow-brown or whitish, inserted on the corolla ca. 2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers (2.2–) 2.5–2.7 mm long, ellipsoid, whitish, yellow or light brown, not connivent at anthesis. Gynoecium with ovary 1.5–2 mm long, ca. 1.2 mm in diameter, greenish-yellow, ovoid or ellipsoid; ovules more than two per locule; nectary 0.5–0.7 mm tall; styles homomorphic, 6–7.2 mm long, exserted ca. 1.5 mm beyond the anthers, white or cream, clavate; stigma 0.3–0.4 mm long. 0.7 mm wide, usually discoid, sometimes slightly bilobed, light green or cream. Berry 7–12 mm in diameter, globose or globose-depressed, white or light green when immature, bright orange or red at maturity, deciduous, non-pungent, the pericarp thick, opaque, lacking giant cells (endocarp smooth); stone cells 1–6 or absent, ellipsoid, 0.8–1.1 mm long, 0.5–1.2 m in diameter; fruiting pedicels 13–30 mm long, pendent, terete, slightly widened distally, green or greenish-purple; fruiting calyx 5–6 mm in diameter, persistent, not accrescent, greenish-purple or purple, discoid, the appendages 3–6 mm long, 1.5 mm wide, reflexed, greenish-purple or purple. Seeds 34–75 per fruit, 1.5–1.8 mm long, 1.1–1.4 mm wide, teardrop-shaped, brownish-black to black, the seed coat reticulate (SM and SEM), the cells polygonal in shape, the lateral walls straight; embryo annular.
Capsicum lycianthoides A flowering branch B reproductive nodes with pedicel scars C flower, in pre-anthesis D, E flowers, in lateral view, with variations in calyx morphology and corolla colouration F, G flowers, in front view H immature fruit I mature fruit A, I from Beltrán 85, photos by G. Beltrán B, D, H from Deanna et al. 144 C, E–G from Deanna et al. 133, photos by R. Deanna.
Distribution
Ecology
Capsicum lycianthoides is a typical element of tropical montane rain forests growing in the margins or interior of primary or secondary forests in sunny or shady areas at (300–) 500–3,500 m elevation.
Phenology
Flowering and fruiting all year.
Chromosome number
2n = 2x = 26 (
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (224,147.836 km2 – LC); AOO (492 km2 – EN). Capsicum lycianthoides is widely distributed along the Colombian and Ecuadorian Andes and is a common species where it occurs. Based on the EOO and AOO and that many collections have been made in different official and private protected areas in recent years, we assign this species the Least Concern (LC) status.
Discussion
Capsicum lycianthoides is a member of the Andean clade (
The name C. lycianthoides has been ignored in literature and the species has been confused with C. geminifolium (
Specimens examined
See Suppl. material
Capsicum minutiflorum
Bassovia minutiflora Rusby, Mem. New York Bot. Gard. 7: 343. 1927. Type. Bolivia. La Paz: Prov. Sud Yungas, Vic. Huachi, head of Beni River, 3000 ft elev., 21 Aug 1921, H.H. Rusby 680 (holotype: NY [00138548]; isotypes: GH [GH00077005], MICH [1109880], MO [MO-1642901, acc. # 5468836], US [US00027424, acc. # 1284151]).
Type
Based on Bassovia minutiflora Rusby.
Description
Erect slender shrubs or subshrubs (1–) 1.5–3 (–5) m tall, with the main stem thick 3–4 cm in diameter at base, few to much branched above. Young stems angled, fragile, green, sparsely pubescent, with antrorse, curved, simple, uniseriate, 3–4-celled, eglandular trichomes 0.05–0.6 mm long; nodes solid, green; bark of older stems fissured, light brown, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar or dissimilar in shape. Leaves membranous, concolorous or slightly discolorous, glabrous or sparsely pubescent on both surfaces, with simple, eglandular trichomes like those of the stems on margins and especially along the main veins abaxially, sometimes trichomes in tufts in the vein axils abaxially; blades of major leaves 3.5–9 (–10.5) cm long, (0.8–) 2.5–3.5 (–4.5) cm wide, ovate to elliptic, the major veins 3–4 (–6) on each side of mid-vein, the base attenuate and rather asymmetrical, the margins entire, the apex acuminate or acute; petioles 0.25–0.8 cm long, sparsely pubescent; blades of minor leaves 2.5–3.2 cm long, 0.65–1.6 cm wide, ovate, the major veins 2–3 on each side of mid-vein, the base short-attenuate, the margins entire, the apex acute; petioles 0.2–0.3 cm long, sparsely pubescent. Inflorescences axillary, 4–5-flowers per axil; flowering pedicels 1–17 (–25) mm long, terete, slightly striate, erect, geniculate at anthesis, green, sparsely pubescent, the eglandular trichomes short, antrorse; pedicels scars inconspicuous. Buds ovoid, greenish-yellow. Flowers 5-merous. Calyx 1.75–2 mm long, 2.3–2.5 mm wide, cup-shaped, thick, green, sparsely pubescent with the same eglandular trichomes as those of stems, the calyx appendages five (rarely absent), 1–1.5 mm long, equal or subequal, spreading to reflexed, erect, usually cylindrical. Corolla 6.5–8 (8.5–) mm long, 8.5–9 mm in diameter, thin, yellow outside, pale or dull yellow slightly spotted with yellowish-green within, stellate with interpetalar membrane, lobed less than halfway to the base, pubescent adaxially with sparse glandular hairs (stalk 2–3-celled; head peltate, unicellular) in the throat and the lobes; the tube 3.5–5 mm, with sparse short eglandular trichomes abaxially, the lobes 3–3.5 mm long, 1.8–2 mm wide, triangular, with sparse eglandular trichomes abaxially and in the margins, the margins involute, the tips cucullate, papillate. Stamens five, equal; filaments 2–2.5 mm long, white, inserted on the corolla ca. 1.2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.6–2 mm long, ellipsoid, yellow, not connivent at anthesis. Gynoecium with ovary ca. 1.2 mm long, 1.5 mm in diameter, dark green, subglobose; ovules more than two per locule; nectary ca. 0.2–0.3 mm tall; styles homomorphic, 3–3.5 mm, exserted ca. 1 mm beyond the anthers, white, clavate; stigma ca. 0.2 mm long, 0.5 mm wide, discoid, light green. Berry 7–10 mm in diameter, globose, green when immature, bright red at maturity, deciduous, pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 1.8–2.3 cm long, erect, faintly angled, widened distally, green; fruiting calyx 2.5–3 mm in diameter, persistent, not accrescent, green, discoid, with a strong annular constriction at junction with the pedicel, the appendages up to 1.4 mm long, spreading or reflexed. Seeds 5–19 per fruit, 4–4.5 mm long, 3–3.5 mm wide, C-shaped, ellipsoid or subglobose, dark brown, the seed coat diffusely reticulate and slightly tuberculate at margins (SM), reticulate or reticulate-cerebelloid (SEM), the cells irregular in seed body and polygonal at margins, the lateral walls sinuate in the seed body and straight and wavy at margins; embryo imbricate.
Distribution
Capsicum minutiflorum is endemic to central Bolivia (Cochabamba, La Paz, Santa Cruz Departments) (Fig.
Ecology
Capsicum minutiflorum is an uncommon plant that grows in the subtropical semi-deciduous forest (Chiquitano Forest) and transitional forests to the Yungas on slopes or along streams and occasionally on road-sides, between 350 and 1,500 (–2,800) m elevation.
Phenology
Flowering from October to May; fruiting from November to July.
Chromosome number
Not known.
Common names
None recorded.
Uses
None recorded, but it is likely that the fruits are used as spices due to their similarities in colour and pungency with C. baccatum var. baccatum.
Preliminary conservation assessment
EOO (101,632.300 km2); AOO (108 km2). Capsicum minutiflorum has a relatively large extent of occurrence growing in more than ten localities; it can be assigned the preliminary Least Concern (LC) category. However, this species is of some conservation concern because of its relatively small AOO and the small number of records in protected areas (Amboro National Park, Santa Cruz).
Discussion
Capsicum minutiflorum is a member of the Bolivian clade (
Capsicum minutiflorum is often confused with the sympatric C. baccatum var. baccatum and C. neei. All these three taxa share the few-flowered inflorescences and the red pungent fruits. Capsicum minutiflorum and C. neei can be distinguished by its yellow corollas with greenish-yellow spots within (vs. white corollas with greenish-yellow spots within in C. baccatum var. baccatum), this character not being evident in herbarium specimens. In addition, C. minutiflorum (Fig.
Specimens examined
See Suppl. material
Capsicum mirabile
Capsicum mirabile var. grandiflorum Sendtn., Fl. Bras. (Martius) 10(6): 144. 1846. Type. [Brazil]. Brasilia, F. Sellow 209 (possible original material B, destroyed [F neg. 2871], no additional material found).
Capsicum buforum
Hunz., Kurtziana 5: 394. 1969. Type. Brazil. São Paulo: Eugênio Lefévre, a unos 26 km de Campos do Jordão, ca. 1400 m elev., 5 Dec 1967, A.T.
Type
Brazil. “Habitat in sylvis fere ubique per Provinciam Sebastianopol. et Paulinam”, Dec., C.F.P. von Martius s.n. (lectotype, designated by
Description
Erect shrubs or subshrubs (0.70–) 1.2–2.5 (–3) m tall or rarely small trees, with the main stem thick 2.5–4 cm in diameter at base, much branched above, the branches dichotomously spreading in a typical “zig-zag” appearance. Young stems angled, fragile, green, glabrous or glabrescent, with antrorse, curved, simple, uniseriate, 3–4-celled, eglandular trichomes 0.1–0.3 mm long, new growth with sparse whitish pubescence; nodes purple, slightly purple or green; bark of older stems dark brown, angled, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous to coriaceous, slightly discolorous to discolorous, green above, light green beneath, glabrous adaxially, glabrescent abaxially and margins, especially on the veins, with sparse, 2–3-(–6)-celled, eglandular trichomes 0.2–0.5 mm long; blades of major leaves 5–13.5 cm long, (1–) 1.3–3 (–5.5) cm wide, elliptic or narrowly elliptic to ovate, the major veins 4–6 (–7) on each side of mid-vein, the base asymmetric and attenuate, the margins entire, the apex acuminate to long-acuminate; petioles 0.7–2 (–2.5) cm long, glabrous; the blades of minor leaves 2–3.3 (–4) cm long, 0.9–1.7 cm wide, elliptic or ovate, the major veins 3–4 on each side of mid-vein, the base attenuate, the margins entire, the apex acute; petioles 0.2–0.4 cm long, glabrous. Inflorescences axillary, 2–5 (–7) flowers per axil, rarely flowers solitary; flowering pedicels (13–) 16–25 mm long, terete or slightly angled, erect or slightly spreading, geniculate at anthesis, green or greenish-purple, glabrous or glabrescent, the eglandular trichomes short, antrorse; pedicels scars inconspicuous. Buds globose to ovoid, inflated, greenish-purple or dark purple or clearer near anthesis. Flowers 5-merous. Calyx 1.5–2.2 mm long, ca. 2–2.5 mm wide, cup-shaped, thin, yellowish-green or green, glabrous or glabrescent, the calyx appendages five, (0.4–) 0.5–1.5 (–3) mm long, subequal, thick, green, erect or spreading, cylindrical, inserted very close to the margin. Corolla (6–) 7.5–12 mm long, (9–) 10–13 mm in diameter, thick, purple with white margin and yellowish-green at the base outside, similar colour within, but sometimes the purple or reddish-brown spots diffuse up to absent, stellate with interpetalar membrane, lobed halfway or less of the way to the base, the tube 3.5–5 mm long, with a continuous ring of glandular trichomes (stalk long, 1–3-celled; head globose, peltate, unicellular) adaxially, glabrous abaxially, the lobes 3–5 mm long, 3.2–4 mm wide, broadly triangular, spreading, glabrous abaxially and adaxially, the margins papillate, the tips cucullate, papillate. Stamens five, equal; filaments (2.8–) 3–3.8 (–4) mm long, equal, light green or greenish-white, inserted on the corolla 1–1.8 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.5–2 mm long, ellipsoid, yellow when young and reddish-brown or grey-purple post-dehiscent, not connivent at anthesis. Gynoecium with ovary 1.1–1.5 mm long, 1–1.2 mm in diameter, green, globose to ovoid; ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, 3.5–4.7 mm long, at the same level or barely exserted beyond the anthers, yellowish-white or light green, clavate; stigma 0.2 mm long, 0.6–0.8 mm wide, discoid or globose, pale green. Berry 7–9 mm in diameter, globose, green when immature, greenish-golden yellow, deciduous, pungent, the pericarp thin, translucent, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 20–32 mm long, pendent and curved, angled, widened and with a slight constriction distally, green; fruiting calyx 3–3.5 mm in diameter, persistent, not accrescent, green, discoid, the appendages 0.7–3.5 mm long, 0.3–0.4 mm wide, spreading. Seeds (2–) 3–8 (–10) per fruit, 2.5–3.2 mm long, 2–2.5 mm wide, C-shaped, brownish-black to black, the seed coat reticulate and tuberculate at margins (SM), reticulate with pillar-like outgrowths at margins (SEM), the cells polygonal in shape, the lateral walls straight; embryo coiled.
Distribution
This species is endemic to east and south-eastern Brazil (Bahia, Espírito Santo, Minas Gerais, Rio de Janeiro and São Paulo States) (Fig.
Ecology
Capsicum mirabile is found in the understorey, edges and interior of the wet Atlantic Forest (Mata Atlântica), primarily in the Floresta Ombrófila Densa Montana, at 800–1,900 m elevation.
Phenology
Flowering from November to April and fruiting from December to May.
Chromosome number
n = 13 (
Common name
Brazil: Pimenta do sapo (São Paulo,
Uses
None recorded.
Preliminary conservation assessment
EOO (190,248.624 km2); AOO (224 km2). Based on the large extent of occurrence, as well the many collections made in several protected areas (Parque Nacional Serra das Lontras, PN do Caparaó, Parque Estadual Serra do Brigadeiro, PN Itatiaia, Parque Estadual Pico do Itacolomi, Reserva Florestal Uaimi, Parque Estadual Três Picos, PN da Serra dos Órgãos, Parque Estadual Maciço da Pedra Branca, Estação Biológica de Boracéia and PN Serra do Bocaina), we assign Capsicum mirabile the Least Concern (LC) category. Subpopulations are not rare in the interior of forests, but this species could be at risk due to the fragmentation and loss of its primary forest habitat outside the legal conservation units.
Discussion
Capsicum mirabile is an enigmatic and morphologically variable species of the Brazilian Atlantic Forest clade (
Capsicum mirabile is characterised by shrubby habit with dichotomous branching, glabrous or glabrescent pubescence, short petioles, elliptic to ovate leaves, few flowers (2–7) per node, five short to long calyx appendages, purple corolla with white margin and yellowish-green or yellowish-white centre and greenish-golden yellow fruits (Table
Capsicum mirabile A plant B flower buds on geniculate pedicels C flower, in pre-anthesis D flower, seen from behind E, H flowers, in front view, showing corollas with different colouration F flower, in full anthesis G flower, seen from behind I immature fruits J mature fruit A, B, D–J from Barboza & Deanna 5024 C from Bianchetti & Bustamante 1518 A, B, D–H photos by G.E. Barboza C photo by L. Bianchetti I, J photos by R. Deanna.
The distinctive corolla spots of this species were not mentioned in the protologue (Martius 1846), although the other diagnostic characters were clearly stated; this may have introduced confusion into the understanding of this species. Variations in some of the morphological features have been observed in the field, such as the length of the calyx appendages, which can range from very short (< 1 mm long, Rio de Janeiro, Espírito Santo and Minas Gerais States) to longer (up to 3 mm, São Paulo and Minas Gerais States) and the shape of the leaves (usually elliptic, but also ovate). The most variable trait is the extent and intensity of tones of the reddish-brown spots in the corolla (Fig.
Capsicum mirabile is sympatric with C. friburgense near the top of Cerro Calêdonia (Nova Friburgo, Rio de Janeiro) where the latter is endemic. Both species share geniculate pedicels, a calyx with five appendages, greenish-golden yellow fruits and blackish-brown or black seeds. They are easily told apart by the shape and colour of the corolla (stellate and multi-coloured in C. mirabile vs. campanulate-urceolate and entirely lilac or pink in C. friburgense) and the leaf shape (elliptic to ovate in C. mirabile vs. ovate in C. friburgense).
The two species similar to C. mirabile are C. villosum and C. muticum. The main difference amongst the three species is the pubescence, since C. mirabile is almost completely glabrous (or sparsely pubescent) and the other two species are densely pubescent on stems, leaves, pedicels and calyx. Capsicum villosum has five calyx appendages (as C. mirabile), but the corolla is smaller and the lobes are more white than purple and the greenish-yellow centre is more extensive in comparison with C. mirabile. Capsicum muticum lacks calyx appendages and the purple pigmentation is very scarce, as lines or small spots bordering the greenish-yellow centre of the corolla.
When
Specimens examined
See Suppl. material
Capsicum mirum , sp. nov.
Diagnosis
Capsicum mirum is morphologically most similar to C. cornutum (Hiern) Hunz., but differs in having antrorse pubescence on stems and leaves, longer petioles, a 2–3-flowered inflorescence, shorter pedicels, purple buds, subequal calyx appendages, smaller corollas almost entirely purplish outside and within and longer filaments.
Type
Brazil. São Paulo: Mun. Bananal, a unos 17 km al sur de Bananal, por ruta SP 247, rumbo a Sertão do Bocaina, 22°45'31"S, 44°23'09"W, 1150 m elev., 26 Feb 2006, G.E. Barboza, E.M. Filippa, A. Gutierrez & G. Bertone 1648 (fl, fr) (holotype: CORD [CORD 00101760], isotypes: BHCB [BHCB 195615], CORD [CORD00101760]).
Description
Erect to compact shrubs 1.5–2 (–3) m tall, much branched above, the branches dichotomous, much spreading horizontally and leafy. Young stems angled, fragile, green, densely pubescent with flexuous, antrorse, curved, simple, uniseriate, 4–7-celled, eglandular trichomes 0.3–0.8 mm long; nodes solid, purple; bark of older stems brown, moderately pubescent; lenticels absent. Sympodial units difoliate, the leaves geminate, the leaf pair unequal in size and similar or dissimilar in shape. Leaves membranous, slightly discolorous, green adaxially, pale green with the mid-vein and the secondary veins prominent abaxially, moderately pubescent on both surfaces and margins, abaxial veins densely pubescent with antrorse, curved, 3–6-celled, eglandular trichomes; blades of major leaves 7–10.5 cm long, 2.5–3.5 cm wide, elliptic or ovate, the major veins 5–6 on each side of mid-vein, the base unequal and attenuate, the margins entire, the apex acute to acuminate; petioles 1.4–2.5 cm long, green adaxially and abaxially, moderately pubescent, the trichomes antrorse; blades of minor leaves 4.5–5.7 cm long, 2.5–2.7 cm wide, ovate, the major veins 3–4 on each side of mid-vein, the base attenuate or rounded, the margins entire, the apex acute; petioles 0.8–1 cm long, green, moderately pubescent, the trichomes antrorse. Inflorescences axillary, 2–3 flowers per axil or flowers solitary; flowering pedicels 12–17 mm long, thin, angled, erect to slightly spreading, geniculate at anthesis, green or with purple lines, the youngest purple, densely pubescent, the eglandular trichomes long and spreading, the glandular trichomes sparse, small; pedicels scars inconspicuous. Buds globose to ovoid, purple to strongly purple and green at the base. Flowers 5-merous. Calyx 1.8–2 mm long, ca. 2.5 mm wide, cup-shaped, circular in outline, fleshy, green, strongly 10-nerved, the veins purple, densely pubescent with short antrorse eglandular trichomes, the calyx appendages 10, (1.7–) 2–3.2 mm long, subequal, thin, spreading, cylindrical, green, inserted very close to the margin, densely pubescent with spreading, long, eglandular trichomes. Corolla 6–8 mm long, 11–14 mm in diameter, almost entirely purple, with a thin white margin and yellowish-green centre outside and within, stellate with thin interpetalar membrane, lobed halfway to the base, pubescent adaxially with glandular trichomes (stalk long, 2–3-celled; head globose, peltate, unicellular) in the throat and base of the lobes, glabrous abaxially, the tube 2–3 mm long, the lobes 4–5 mm long, 3.5–4 mm wide, triangular, the margins with short eglandular trichomes, the tips papillate or with short eglandular trichomes. Stamens five, equal; filaments 3–3.2 mm long, white, inserted on the corolla ca. 1 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.3–1.6 mm long, ellipsoid, lilac or pale blue, with a broad cream connective before opening, not connivent at anthesis. Gynoecium with ovary ca. 1.2 mm in diameter, light green, globose; ovules more than two per locule; nectary ca. 0.5 mm tall, paler than the ovary; styles homomorphic, 3.5–5 mm long, barely exserted beyond the anthers, cream, clavate; stigma 0.2–0.3 mm long, ca. 0.8–0.9 mm wide, discoid, cream. Berry globose, green when immature (mature berries not seen), pungent, the pericarp with giant cells (endocarp alveolate), stone cells absent; fruiting pedicels 20–30 mm long, pendent and curved, angled, slightly widened at the apex, green; fruiting calyx 3–4 mm in diameter, persistent, not accrescent, discoid, green or purple, the appendages 2.5–3.5 mm long, spreading, straight or slightly curved. Seeds 9–12 per fruit, 2.4–3.2 mm long, 1.8–2.3 mm wide, C-shaped, brownish-black to black, the seed coat reticulate and tuberculate at margins (SM), reticulate with pillar-like outgrowths at margins (SEM); the cells irregular in shape, the lateral walls sinuate, polygonal at margins; embryo imbricate.
Capsicum mirum A plant B flowering branch C bud on geniculate pedicel D flower in pre-anthesis E flower, lateral view F flower, in front view G flower, seen from behind H fruiting calyx I immature fruits. No specimen vouchers. Photos taken in situ by J. Lackey (Associazione PepperFriends).
Distribution
Ecology
Capsicum mirum occurs in small populations in the coastal Atlantic Forests (Mata Atlântica), at the margins of the forest usually in the sun, between 1,100 and 1,300 m elevation.
Phenology
The species has been collected in flower and in immature fruits (but mature seeds) in February and April.
Chromosome number
Not known.
Etymology
The specific epithet comes from the Latin mirus (wonderful, extraordinary), referring to the remarkable beauty of this species given by the colourful corolla and the showy calyx.
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (1.205 km2); AOO (8 km2). Capsicum mirum is the species with the narrowest extent of occurrence in the genus and with only three collections probably belonging to a single population. The species has been collected in an unprotected clearing of the Atlantic Forest where a continuing decline of the mature individuals has been observed. According to IUCN criteria, C. mirum is proposed as a Critically Endangered species (CR; B1ab; C2a(i)).
Discussion
Capsicum mirum belongs to the Atlantic Forest clade (
Capsicum mirum is morphologically similar to C. cornutum, C. mirabile and C. carassense with which it shares geniculate flowering pedicels at anthesis, stellate corollas, pungent fruits, brownish-black to black seeds with seed coat reticulate and tuberculate at margins and similar habitat. Differences between these species are provided in Table
Capsicum mirum (as C. hunzikerianum) resolved as sister to C. schottianum (
Paratypes
Brazil. São Paulo: Mun. Bananal, a unos 17 km al sur de Bananal, por ruta SP 247, rumbo a Sertão do Bocaina, 22°45'50"S, 44°23'35"W, 1150 m elev., 26 Feb 2006 (fl, fr), G.E. Barboza et al. 1649 (CORD); do cruzamento de Bananal rumo à Estacão Ecológica de Bananal, 22°46'55.8"S, 44°22'41.1"W, 1333 m elev., 5 Apr 2018 (fl, fr), J.R. Stehmann et al. 6474 (= G.E. Barboza & R. Deanna 5028) (BHCB).
Capsicum muticum , comb. nov.
Capsicum villosum var. muticum
Sendtn., Fl. Bras. (Martius) 10(6): 144. 1846. Type. [Brazil. Rio de Janeiro]: “Serra d’Estrella,” [no date], H.W. Schott 5416 (lectotype designated by
Bassovia leptopoda Dunal, Prodr. [A. P. de Candolle] 13(1): 411. 1852. Type. [Brazil. Rio de Janeiro]: Rio de Janeiro, 1819, F. Sellow s.n. (holotype: BM [BM000798808]; isotypes: G-DC [G00131651], MPU [MPU023056]).
Capsicum leptopodum (Dunal) Kuntze, Revis. Gen. Pl. 2: 449. 1891. Type. Based on Bassovia leptopoda Dunal.
Capsicum villosum forma vimineum Wawra, Itin. Princ. S. Coburgi 1: 100. 1883. Type. Brazil. Rio de Janeiro: “Petropolis; an gerodeten Stellen (Benod). Coll II 10”, 1879 [coll. Princes August and Ferdinand of Saxe-Coburg] (no material found).
Type
Based on Capsicum villosum var. muticum Sendtn.
Description
Erect shrubs or subshrubs (0.80–) 1–2.5 m tall, with the main stem ca. 1.5 cm in diameter at base, much branched above, the branches dichotomously spreading in a typical “zig-zag” appearance. Young stems angled, fragile, green, densely pubescent with spreading, flexuous to rigid, white or slightly ferruginous (dried specimens), simple, uniseriate, (3–) 4–8-celled, eglandular trichomes 0.5–2 mm long; nodes solid, green or light purple; bark of older stems brown or dark brown, with ferruginous pubescence; lenticels few. Sympodial units difoliate, the leaves geminate; leaf pair markedly unequal in size, similar or dissimilar in shape. Leaves membranous, slightly discolorous, dark green above, light green beneath, moderately pubescent adaxially, with appressed-antrorse trichomes similar to those of the stems, densely pubescent abaxially and margins, with appressed (on the lamina) or spreading (on the veins), flexuous, 4–6-celled, eglandular trichomes 0.5–1.7 mm long; blades of major leaves 4.5–11 cm long, (1.5–) 2–3.6 cm wide, elliptic or narrowly elliptic, the major veins 5–8 on each side of mid-vein, the base attenuate, slightly unequal, the margins entire, the apex acuminate; petioles 0.5–1.5 (–2) cm long, densely pubescent; blades of minor leaves 2–4.3 cm long, 1–1.4 cm wide, elliptic, the major veins 3–4 on each side of mid-vein, the base attenuate, the margins entire, the apex acute; petioles 0.2–0.5 (–1.5) cm long, densely pubescent. Inflorescences axillary, 2–5 flowers per axil, rarely flowers solitary; flowering pedicels 1–17 mm long, angled, erect, geniculate at anthesis, green, densely pubescent, with long spreading eglandular trichomes and small sparse glandular trichomes (stalk unicellular; head dark multicellular); pedicels scars conspicuous. Buds globose, inflated, yellowish-green. Flowers 5-merous. Calyx ca. 2 mm long, 2 mm wide, cup-shaped, pentagonal in outline, thick, green, strongly 5-nerved, densely pubescent with the same trichomes as the pedicels, calyx appendages five, up to 0.2 mm long, erect, green. Corolla 6–9 mm long, 12–14 mm in diameter, white with wide greenish-yellow pigmentation outside, white with predominance of greenish-yellow spots, sparse and diffuse purple or brown spots and a cream centre within, stellate with interpetalar membrane, lobed halfway or less of the way to the base, pubescent adaxially with a continuous ring of glandular trichomes (stalk long, 2–3-celled; head globose, peltate, unicellular) in the throat and base of the lobes, the tube 3–5 mm long, glabrous abaxially, the lobes 3–4 mm long, 3–4.3 mm wide, widely triangular, spreading, with eglandular trichomes especially on the veins abaxially, the margins papillate, the tips cucullate, papillate. Stamens five, equal; filaments 2.5–3.2 mm long, white, inserted on the corolla ca. 1.2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1–1.5 mm long, ellipsoid, cream when young, purplish post-dehiscent, not connivent at anthesis. Gynoecium with ovary ca. 1.2 mm long, 1 mm in diameter, light green, subglobose; ovules more than two per locule; nectary ca. 0.4 mm tall, pale yellow; styles homomorphic, 4–4.5 mm long, exserted 0.5–0.7 mm beyond the anthers, white, clavate; stigma 0.1 mm long, 0.5 mm wide, discoid, pale green. Berry 7–8 mm in diameter, globose, green when immature and pungent, mature fruit colour not seen; fruiting pedicels ca. 20 mm long, pendent and curved, angled, widened distally, green; fruiting calyx 4–4.5 mm in diameter, persistent, not accrescent, discoid, green. Seeds (4–) 8–11 per fruit, 3.25–3.75 mm long, 2.5–2.75 mm wide, C-shaped, brownish-black to black, the seed coat reticulate and tuberculate at margins (SM), reticulate with pillar-like outgrowths at margins (SEM), the cells rectangular to polygonal in shape, the lateral walls straight; embryo imbricate.
Distribution
Capsicum muticum is confined to central-eastern Rio de Janeiro State (Brazil) (Fig.
Ecology
Capsicum muticum is a component of the montane forests in the Atlantic Forest (Mata Atlântica); it was found growing in the Serra dos Órgãos mountain range (Serra d’Estrella, Serra dos Órgãos, Alto da Serra), mainly in the Dense Ombrophilous Forest (Floresta Ombrófila Densa) in full sun or semi-shade, between 1,000 and 1,300 m elevation.
Phenology
Flowering from December to April. Fruiting from February to May.
Chromosome number
Not known.
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (15.520 km2); AOO (16 km2). Capsicum muticum is one of the rarest species. The few collections are mostly historical, date from the early 1800s to 1900s and have imprecise locations for the Serra dos Órgãos (Rio de Janeiro). The species was rediscovered in 1986 in Alto da Serra (Petropolis) and gathered several times at the same location. In this site, Capsicum muticum is severely threatened since a progressive decline of the habitat has been observed due to anthropogenic disturbance and serious deforestation of the area. Based on the extent of occurrence, the advanced fragmentation, loss of habitat and the few known records for C. muticum, we assign this species a threat status of Critically Endangered (CR; B1ab(iii,iv)).
Discussion
Capsicum muticum is a member of the Atlantic Forest clade (
Capsicum villosum and C. muticum are densely pubescent shrubs with long, spreading, flexuous, eglandular trichomes, geniculate flowering pedicels and stellate corollas (Fig.
Specimens examined
See Suppl. material
Capsicum neei
Type
Bolivia. Chuquisaca: Prov. Hernando Siles, a 4.1 km del puente nuevo de Monteagudo viniendo desde Monteagudo, sobre mano derecha, -19.804617°S, -64.019923°W, 16 Dec 2017, G.E. Barboza 4927 (holotype: LPB [LPB0003513]; isotypes: CORD [CORD00006935, CORD00006956], NY [04206098]).
Description
Low erect shrubs 0.70–2 (–3) m tall, with the main stem 1–1.5 cm in diameter at base, laxly branched above, the branches thin. Young stems slightly angled, fragile, green, glabrescent; bark of older stems light brown, glabrous; lenticels few. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous, concolorous, glabrous or glabrescent on both surfaces and margins, with antrorse, simple, 4–7-celled, eglandular trichomes 0.2–0.5 mm long; blades of major leaves (5.5–) 6.7–11 cm long, 2.1–4 (–4.5) cm wide, elliptic or ovate, major veins 3–5 on each side of mid-vein, the base attenuate, the margins entire, the apex acute; petioles 0.3–0.8 (–1.5) cm long, moderately pubescent; blades of minor leaves 2.7–4.6 (–6) cm long, 1.2–1.8 (–2.3) cm wide, elliptic or ovate, the major veins 3–4 on each side of mid-vein, the base short-attenuate, the margins entire, the apex obtuse or acute; petioles 0.2–0.5 (–0.8) cm long, with similar pubescence as in major leaves. Inflorescences axillary, 2–4-flowers per axil, rarely flowers solitary; flowering pedicels (6.5–) 8–15 mm long, filiform, angled, pendent, slightly curved, non-geniculate at anthesis, green, with sparse antrorse eglandular trichomes and small dark glandular trichomes (stalk unicellular; head multicellular); pedicels scars inconspicuous. Buds ovoid, greenish-yellow. Flowers 5-merous. Calyx 1.7–2.5 mm long, 2–3 mm wide, cup-shaped, with 10 veins clearly evident, green, moderately pubescent with eglandular trichomes, the calyx appendages 10, unequal, the five main appendages longer (0.7–) 0.9–1.75 (–2) mm long, emerging very close from the margin, the five secondary appendages shorter 0.2–0.8 (–1.2) mm long, emerging 0.8–1 mm below the margin, linear, green, with the same non-glandular trichomes of the calyx tube. Corolla (6–) 8–10 mm long, 6–8 (–12) mm in diameter, entirely yellow or with small greenish spots in the base of the lobes and tube outside and within, stellate with a thin interpetalar membrane, lobed nearly halfway to the base, the tube 3–4.5 mm long, pubescent adaxially with small glandular trichomes (head and stalk one-celled each), glabrescent abaxially, the lobes 3.5–5.5 mm long, ca. 2 mm wide, ovate or triangular, spreading, glabrous adaxially and with sparse eglandular trichomes abaxially, the margins finely ciliate, the tips cucullate and papillate. Stamens five, slightly subequal; filaments 1.4–1.75 mm long, cream, inserted on the corolla ca. 1.2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers (1.5–) 1.8–2 mm long, ellipsoid, light yellow, not connivent at anthesis. Gynoecium with ovary ca. 1.2 mm long, 1.3 mm in diameter, light green, ovoid or subglobose; ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, 3.75 mm long, 0.8–1 mm beyond the anthers, cream, clavate; stigma ca. 0.2 mm long, 0.3 mm wide, somewhat bilobed, light green. Berry 4–9 mm in diameter, globose, slightly apiculate, green when immature turning to orange-coloured or red at maturity, pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels (13–) 18–34 mm long, pendent, angled or terete, widened distally, green; the fruiting calyx ca. 4 mm in diameter, persistent, not accrescent, discoid, green, with an annular constriction at junction with the pedicel, the appendages 1–2 mm long, spreading or reflexed. Seeds 5–10 per fruit, 4–5 mm long, 3–4.25 mm wide, subglobose, pale yellow to white, the seed coat smooth and reticulate at margins (SM), reticulate-cerebelloid (SEM), the cells polygonal in shape, the lateral walls straight to wavy or sinuate; embryo not seen.
Distribution
Capsicum neei is endemic to central and southern Bolivia (Chuquisaca and Santa Cruz Departments) (Fig.
Ecology
Capsicum neei is most commonly collected in the Boliviano-Tucumano Forest from the understorey of deciduous cloud forests, between 1,100 and 1,750 m elevation.
Phenology
Flowering and fruiting from October to May.
Chromosome number
Not known.
Preliminary conservation assessment
EOO (13,880.224 km2); AOO (48 km2). Capsicum neei has been collected in more than 10 localities and many times in the last 23 years, in a recently Protected Area (National Park and Integrated Management Natural Area “Serranía Inao”) and in nearby areas, which suggests that both the geographic range (EOO and AOO) and the population size are likely not to be significantly affected in the forthcoming years. Due to this, C. neei can be assessed as Least Concern (LC).
Discussion
In phylogenetic analyses, Capsicum neei was recovered within the Bolivian clade, resolved as sister to C. caballeroi (
Capsicum neei is morphologically most similar to the Bolivian C. minutiflorum in having stellate, yellow corollas and red fruits at maturity (Fig.
Capsicum neei A flowering branch B inflorescence C flower bud D flower E glandular trichome of the pedicels F calyx G eglandular trichome of the outer surface of the calyx H glandular trichome of the inner surface of the calyx I opened corolla J glandular trichome of the inner surface of the corolla K eglandular trichome of the inner surface of the corolla lobes L gynoecium M fruit. From Barboza 4927. Drawn by S. Montecchiesi. Published in
Capsicum neei A plant B leaves, abaxial surface C flower bud D flower, in lateral view E flower, in front view F flower, seen from behind G immature fruit H, I mature fruits J fruit, longitudinal section, showing the seeds A–G from Barboza et al. 4927 H–J from Barboza & Reyes 5040. Photos by G.E. Barboza. Modified from
Capsicum neei is sympatric with C. baccatum var. baccatum, which has geniculate pedicels, a calyx with five subequal appendages, white corollas with greenish-yellow spots within and globose to ellipsoid upright red fruits.
Specimens examined
See Suppl. material
Capsicum parvifolium
Fregirardia leptoclada Dunal, Prodr. [A. P. de Candolle] 13(1): 505. 1852. Type. Brazil. Bahia: “le bas des montagnes. Certam du Rio Fco [Rio San Francisco]”, 1838, J.S. Blanchet 2823 (lectotype, designate here, G-DC [G00200561]; isolectotypes: BM [BM001016675, BM000617948], CORD [CORD00087945, CORD00087948], F [v0043297F, acc. # 646814], G [G00390274], K [K000585899, K000585900], LE, MPU [MPU026995 fragment ex G-DC, MPU023035 fragment ex G, MPU023036 fragment ex G], P [P00410185], W [acc. # 0001348, acc. # 1889-0118245]).
Capsicum leptocladum (Dunal) Kuntze, Revis. Gen. Pl. 2: 450.1891. Type. Based on Fregirardia leptoclada Dunal.
Bassovia ciliata
J.R.Johnst., Proc. Amer. Acad. Arts 40: 694. 1905. Type. Venezuela. Nueva Esparta: Isla Margarita El Valle, 30 Aug 1903, J.R. Johnston 75 (lectotype, designated by
Type
[Brazil]. Bahia: Serra Açuruá, Rio San Francisco, 1838, J.S. Blanchet 2823 (lectotype, designated by
Description
Erect shrubs 1–4 (–5) m tall, with an almost fastigiate habit, much branched above. Young stems angled, slender, fragile, greyish-brown, moderately pubescent with antrorse, simple, uniseriate, 4–6-celled, eglandular trichomes 0.3–1.1 mm long; nodes light brown; bark of old stems grey or brown, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous, slightly discolorous, dark green above, paler beneath, sparsely to moderately pubescent on both surfaces, the trichomes similar to those of the stems, rarely few dendritic trichomes on the veins; blades of major leaves 3–8 (–9) cm long, 1–4.2 cm wide, ovate, the major veins 5–7 on each side of mid-vein, the base short-attenuate and unequal, the margins entire, the apex acuminate; petioles 0.5–2.4 cm, moderately to densely pubescent; blades of minor leaves 1.5–2.5 cm long, 0.8–1.5 cm wide, ovate, the major veins 3–4 on each side of mid-vein, the base short-attenuate, the margins entire, the apex acute; petioles 0.3–0.5 cm, moderately to densely pubescent. Inflorescences axillary, 3–6 (–8) flowers per axil; flowering pedicels 9–18 (–20) mm long, angled, pendent, non-geniculate at anthesis, green, densely pubescent, with short antrorse eglandular trichomes; pedicels scars conspicuous, corky. Buds subglobose, pale violet at the apex and cream near the base. Flowers 5-merous. Calyx (1.2–) 1.5–2.4 mm long, 2–3 mm wide, cup-shaped, thick, strongly 5-nerved, green, densely pubescent like the pedicels, the calyx appendages five or, exceptionally, up to seven, 0.7–2 (–2.2) mm long, subequal, erect or spreading, green. Corolla 4.5–7.3 (–8) mm long, 1–2 cm in diameter, purple with a narrow white margin and a yellowish-green centre outside and within, stellate with interpetalar membrane, lobed nearly halfway to the base, pubescent adaxially with small glandular trichomes (stalk 2-celled; head unicellular) in the throat and base of the lobes, glabrous abaxially, the tube (2.4–) 3–4 (–4.5) mm long, the lobes (2.1–) 2.9–3.5 mm long, 2–3.5 mm wide, broadly triangular, spreading, the margins involute, papillate or with long simple trichomes, the tips cucullate, densely papillate. Stamens five, equal; filaments 1.5–2.5 mm long, greenish-white, inserted on the corolla 1.3–1.5 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.7–2.3 mm long, ellipsoid, yellow or violet, not connivent at anthesis. Gynoecium with ovary 1–1.4 mm long, 1 mm in diameter, greenish-white, ovoid; ovules more than two per locule; nectary 0.3–0.4 mm tall; styles homomorphic, 3.5–5.1 mm long, exserted ca. 1.5 mm beyond the anthers, whitish-green, clavate; stigma ca. 0.3 mm long, ca. 1–1.1 mm wide, somewhat discoid, green. Berry 8.5–9.5 mm in diameter, globose, slightly flattened at the apex, dark green when immature, greenish-golden yellow at maturity, deciduous, pungent, the pericarp thin, translucent, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 10–18 mm long, angled, pendent, curved, widened distally, green; fruiting calyx 4–6 mm in diameter, persistent, not accrescent, discoid, strongly 5(–10)-nerved, green, the appendages spreading or reflexed. Seeds (2–) 4–13 per fruit, 3–3.8 mm long, 2.7–3 mm wide, C-shaped, brownish-black, the seed coat reticulate and slightly tuberculate at margins (SM), reticulate-cerebelloid (SEM), the cells irregular in shape, the lateral walls sinuate in the seed body, straight and wavy at margins; embryo imbricate.
Distribution
Capsicum parvifolium has a disjunct distribution in South America (Fig.
Ecology
Capsicum parvifolium is a common element in seasonally dry tropical forest, mainly in the outcrops (‘inselbergs’) of the arborescent-shrubby Caatinga, at 500–1,200 m. In Venezuela and Colombia, it is found in coastal areas with higher humidity at lower elevations (0–350 m).
Phenology
Flowering from August to April and fruiting from February to June in the Caatinga; flowering from April to October and fruiting from May to October in Venezuela and Colombia.
Chromosome number
2n = 2x = 24 (
Common names
Brazil: Alecrim-quebrado (Piauí, Mendes et al. 509), Jiriquiti (Piauí, Mendes et al. 539), Murta (Sergipe, Oliveira et al. 552), Pimentinha (Pernambuco, Lucena 91).
Uses
None recorded.
Preliminary conservation assessment
EOO (4,493,957.435 km2); AOO (228 km2). Capsicum parvifolium is under threat in its fragmented habitat. Although in Brazil it is common, its habitat is restricted to the Caatinga, the least protected of all the major ecoregions in the country (
Discussion
Capsicum parvifolium belongs to the Caatinga clade (
Capsicum parvifolium A fruiting branch B branched trichome of the leaf C flower D calyx E opened corolla F, G anthers, dorsal and ventral views, respectively H gynoecium I fruits A, C, E–I from Agra & Barboza 7075 B, D from Pickersgill 72–366. Drawn by L. Ribulgo. Published in
Capsicum parvifolium A habitat (Caatinga) B plant C reproductive branch D flower bud and flower on pendent pedicels E flower, in front view F fruiting calyces and immature fruit G mature fruits H fruiting calyx, inside view. From Agra & Barboza 7075. Photos by G.E. Barboza. Modified from
Specimens examined
See Suppl. material
Capsicum pereirae
Type
Brazil. Espírito Santo: Mun. Castelo, Castelo-Forno Grande, 6 Dec 1956, E. Pereira 2245 (holotype: CORD (CORD00006630); isotypes: CORD [CORD00006631, CORD00006632], HB [acc. # 7060], RB [RB00722054, acc. # 96194]).
Description
Erect shrubs (0.5–) 0.8–3 (–8?) m tall, the branches slender and horizontal in a typical “zig-zag” appearance. Young stems slightly angled, fragile, green, white mottled, glabrous; nodes solid, purple; bark of older stems brown, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves coriaceous, discolorous, bright dark green above, opaque light green beneath, glabrous on both surfaces, with the abaxial nerves white mottled; blades of major leaves (6.1–) 9–15 (–20) cm long, 2–4 (–7) cm wide, elliptic to narrowly elliptic, the major veins (7–) 8–12 on each side of mid-vein, the base asymmetric, short-attenuate or attenuate, the margin entire, the apex acute to acuminate; petioles 0.5–1 (–1.5) cm, glabrous; blades of minor leaves 2.5–3 (–5.4) cm long, 0.75–1.5 cm wide, elliptic to narrowly elliptic, the major veins 4–7 on each side of mid-vein, the base short-attenuate, the margin entire, the apex acute; petioles 0.3–0.5 cm, glabrous. Inflorescences axillary, 2–6 flowers per axil, rarely flowers solitary; flowering pedicels 15–30 mm long, slightly striate, pendent, sometimes curved, non-geniculate at anthesis, green or green-purple, glabrous; pedicels scars inconspicuous. Buds subglobose, white with green or greenish-yellow spots at the base. Flowers 5-merous. Calyx 1.5–2 (–3) mm, (2.5–) 3–3.2 mm wide, cup-shaped, circular or pentagonal in outline, thin and translucent, green or light green, glabrescent, with short eglandular trichomes on the margin, the calyx appendages absent or five, minute, less than 0.5 mm. Corolla 9–10 mm long, 8–19 mm in diameter, entirely white or white with greenish-yellow spots outside, white with purple and greenish-yellow pigmentation in the lobes and throat and a cream centre within, stellate with interpetalar membrane, lobed nearly halfway to the base, the tube ca. 4 mm long, with a continuous ring of small glandular trichomes (stalk long, 1–2-celled; head globose, peltate unicellular) adaxially, glabrous abaxially, the lobes 5–6 mm long, 4.5–5 (–6) mm wide, broadly triangular, spreading, glabrous adaxially and abaxially, the margins involute and papillate, the tips strongly cucullate, densely papillate. Stamens five, equal; filaments 3–4 (–5) mm long, equal, cream, inserted on the corolla ca. 1 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.8–2.1 (–3) mm, ellipsoid, dark green or greyish, not connivent at anthesis. Gynoecium with ovary ca. 1.5 mm long, ca. 1.2 mm in diameter, light green, subconic; ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, (3.7–) 4–6 (–7) mm long, barely exserted beyond the anthers, cream, clavate, slightly curved; stigma 0.2–0.3 mm long, ca. 0.7 mm wide, discoid, somewhat bilobulate, light green. Berry (6–) 8–10 mm in diameter, globose, hardly depressed, green when immature, greenish-golden yellow at maturity, deciduous, pungent, the pericarp thin, translucent, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels (20–) 23–28 (–35) mm, slightly angled, pendent, slightly widened distally, green; fruiting calyx 5–6 mm in diameter, persistent, not accrescent, discoid, green. Seeds (3–) 5–20 per fruit, 3–3.7 mm long, 2.5–3.4 mm wide, subglobose or teardrop-shaped, brownish-black to black, the seed coat reticulate and tuberculate at margins (SM), reticulate with pillar-like outgrowths at margins (SEM), the cells irregular in shape, the lateral walls sinuate, straight to the hilum; embryo imbricate.
Capsicum pereirae A flowering branch B leaf C flower bud D flower E calyx F eglandular trichome of the calyx G opened corolla H gynoecium I fruiting branch J fruit, in cross section K anatomical detail of the pericarp (note the giant cells in the mesocarp) L seed M seed, in cross section N embryo A–J, L–N from Pereira 2245 K from Hunziker 25248. Drawn by N. de Flury. Published in
Capsicum pereirae A flowering branch B young branch showing lanceolate leaves and buds on pendent pedicels C flower showing mutic calyx D, E flowers, in front view, with different patterns of purple colouration F flower without purple spots A, B, E from Bianchetti et al. 1558 F from Bianchetti et al. 1567, photos by L. Bianchetti C, D no specimen vouchers, photos taken in situ by C. dal Zovo (Associazione PepperFriends). Modified from
Distribution
Ecology
Capsicum pereirae inhabits the Atlantic Forest (Mata Atlântica), mainly in the Dense Ombrophilous Forest (Floresta Ombrófila Densa), between 500 and 1,650 m elevation. It is found in marginal or interior forests or in aquatic depressions usually in shady places. In the Parque Estadual do Ibitipoca (Minas Gerais), the species is found close to wet caves in the Nanofloresta Nebular (Mata de Grota) formation.
Phenology
Flowering from late October to March. Fruiting from February to May.
Chromosome number
n = 13 (
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (271,639.782 km2); AOO (92 km2). Capsicum pereirae has a relatively large extent of occurrence and is found in more than 10 localities, the majority of them in protected areas (Bahia: RPPN Palmeira, PN de Boa Nova; Espírito Santo: Estação Biológica de Santa Lúcia; Mina Gerais: APA Felício, Parque Estadual do Ibitipoca and RPPN Loredano Aleixo). For these reasons, we assign the preliminary LC category. However, subpopulations outside conservation areas within the Atlantic Forest may be threatened.
Discussion
Capsicum pereirae belongs to the Atlantic Forest clade (
Capsicum pereirae also shares with C. schottianum the absence of evident calyx appendages, but the two species can be distinguished by general pubescence (plants glabrous in C. pereirae vs. plants glabrescent to pubescent in C. schottianum), consistency of the leaves (coriaceous vs. membranous) and geniculation of the flowering pedicels (non-geniculate vs. geniculate). Stems and leaves of C. pereirae are glabrous like C. hunzikerianum from which it can be differentiated by the lack of appendages in the calyx, the smaller flowers and the shorter fruiting pedicels. The presence of membranous leaves and calyx with five conspicuous appendages distinguish C. mirabile (Figs
Specimens examined
See Suppl. material
Capsicum piuranum
Type
Peru. Piura: Prov. Huancabamba, borde de carretera y riachuelo, 5°22'46"S, 79°33'47"W, 2311–2459 m elev., 22 Mar 2011, T. Mione 812 (holotype: CORD [CORD00006936]; isotype: NY [03231447]).
Description
Erect shrubs 2–2.20 (–3) m tall, densely branched above, the branches scandent. Young stems angled, fragile, flexuous, green, glabrous and shiny; bark of older stems green to dark brown, angled, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair markedly unequal in size and similar or dissimilar in shape. Leaves membranous, discolorous, dark green and shiny adaxially, light green and opaque abaxially, the mid-vein and primary veins raised abaxially, glabrous or both surfaces with sparse antrorse, simple, 4–5-celled, eglandular trichomes 0.5–1.2 mm long, occasionally trichomes more abundant on main veins and margins; blades of major leaves (8–) 12–17.7 cm long, (2–) 2.5–4.5 cm wide, elliptic, the major veins 7–9 on each side of mid-vein, the base asymmetric and attenuate, the margins entire, the apex long-acuminate; petioles 0.7–1.4 (–1.7) cm long, slightly winged from the decurrent leaf bases, glabrous or glabrescent; blades of minor leaves 2.5–4.5 cm long, 1.5–2.6 cm wide, ovate or elliptic, the major veins 3–4 on each side of mid-vein, the base short-attenuate or rounded and asymmetric, the margins entire, the apex acute or slightly rounded; petioles 0.2–0.5 cm long, glabrescent or moderately pubescent. Inflorescences axillary, 1–3-flowered; flowering pedicels 19–26 mm long, filiform, terete, pendent, slightly curved, non-geniculate at anthesis, usually green, sometimes with purple spots, glabrous or glabrescent, the eglandular trichomes short, antrorse; pedicels scars inconspicuous. Buds ovoid, yellow or pale yellow. Flowers 5-merous. Calyx 1.5–2.6 (–3) mm long, 3–4 mm wide, cup-shaped, thick, purple or greenish-purple, glabrescent to moderately pubescent, the calyx appendages five, (0.9–) 2.5–3 mm long, 0.5–0.8 mm wide, equal, thick, erect, subulate, inserted close to the margin, glabrous or glabrescent with the same trichomes as pedicels and calyx tube. Corolla 14.5–17 mm long, 12–17 mm in diameter, thick, entirely yellow outside and within, tubular-campanulate with interpetalar membrane, lobed less than 1/3 of the way to the base, glabrous adaxially and abaxially, the tube 11–12 mm long, ca. 6 mm in diameter, the lobes 3.5–5 mm long, 4.5–5 mm wide, broadly ovate, erect, the margins finely ciliate, the tips papillate. Stamens five, subequal; filaments 3–5 mm long, greenish-white, inserted on the corolla 3–4 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 2–2.5 (–2.8) mm long, ellipsoid, yellowish-white, slightly connivent at anthesis. Gynoecium with ovary 1.25–1.5 mm long, 1.5 mm in diameter, white, subglobose; ovules more than two per locule; nectary ca. 0.5 mm tall; styles homomorphic, 7.5–8 mm long, barely exserted beyond the anthers, white, clavate; stigma 0.5 mm long, 0.8–1 mm wide, somewhat bilobed, green. Berry 9–12 mm in diameter, globose, slightly flattened at the apex, green or white when immature, orange to red at maturity, non-pungent, the pericarp thick, opaque, lacking giant cells (endocarp smooth); stone cells two, polyhedral, yellowish-white; fruiting pedicels 28–36 mm long, pendent, slightly angled, widened distally, green; fruiting calyx ca. 4 mm in diameter, persistent, not accrescent, discoid, green-purple or green, the appendages 5–6.1 mm long, 0.8–1 mm wide at base, reflexed. Seeds ca. 50–80 per fruit, ca. 2.5 mm long, 2–2.2 mm wide, subglobose or teardrop-shaped, dark brown to black, the seed coat reticulate (SM), reticulate-cerebelloid (SEM), the cells polygonal or irregular in shape, the lateral walls straight or slightly sinuate; embryo imbricate.
Distribution
Capsicum piuranum is endemic to a restricted area in northern Peru (Piura Department) (Fig.
Ecology
Capsicum piuranum grows in misty rain montane forests, in margins or near streams, between 2,300 and 2,860 m elevation.
Phenology
Flowering from November to May, with a peak of fruiting in March–May.
Chromosome number
2n = 2x = 26 (
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (10.195 km2); AOO (8 km2). Capsicum piuranum has a very restricted distribution (EOO and AOO) and a small population size (< 250 individuals). This species is proposed as Critically Endangered (CR; C2a(i)) because of the inferred continuing decline in the number of mature individuals in each subpopulation (< 50).
Capsicum piuranum A reproductive branch B flower C calyx D eglandular trichome of the outer surface of the calyx E opened corolla F, G, H anthers, ventral, dorsal and lateral views, respectively I gynoecium J fruit K seed. From G.E. Barboza & S. Leiva González 4841. Drawn by S. Leiva González. Published in
Discussion
Capsicum piuranum belongs to the Andean clade (
Capsicum piuranum A plant B leaves, abaxial surface C Fruiting branch with leaf pair dissimilar in shape and size D flower bud E flower and immature fruit on pendent pedicels F mature fruit G fruit, in cross section, showing placenta and seeds H fruit, in cross section, showing a stone cell at the apex (arrow). From Barboza & Leiva González 4841. Photos by S. Leiva González and G.E. Barboza. Published in
Capsicum piuranum is sympatric with other two Andean species, C. geminifolium and C. rhomboideum, both of which also have yellow corollas and non-pungent fruits, but a moderate to dense pubescence on stems and leaves. Capsicum geminifolium differs in having longer calyx appendages (3–6.5 mm long) compared to C. piuranum (2.5–3 mm long) and campanulate generally purple spotted yellow corollas (tubular-campanulate and entirely pure yellow in C. piuranum, Fig.
Specimens examined
See Suppl. material
Capsicum pubescens
Capsicum violaceum Kunth, Nov. Gen. Sp. [H.B.K.] 3: 49. 1818. Type. Ecuador. Pichincha: Quito, [no date], F.W.H.A. von Humboldt & A.J.A. Bonpland 3027 (holotype: P [P00670654]).
Capsicum quitense Willd. ex Roem. & Schult., Syst. Veg., ed. 15 bis [Roemer & Schultes] 4: 809. 1819. Type. Ecuador. Pichincha: In Quito (holotype: B [B-W04433-01-0]).
Brachistus ? lanceifolius Miers, Ann. Mag. Nat. Hist. ser. 2, 3(16): 267. 1849, as “lanceaefolius”. Type. Ecuador. Loja: Loja, Aug 1847, B.C. Seemann 879 (lectotype, designated here: K [K000585923]; isolectotype: BM [BM000992131].
Capsicum maximowiczii Regel & Rach, Index Seminum [St. Petersburg (Petropolitanus)]: 40. 1859. Type. Cultivated in St. Petersburg, Russia [protologue “Cultum in hortis circa Valparaiso sub nomine “Agi dulce”. Semina misit Maximowicz. (Rch.)”] “Ex horto bot. Petropolitano”, 27 May 1858, L.T. Rach (no specimens cited; lectotype, designated here: LE).
Capsicum pubescens var. oviforme Hassk., Bonplandia (Hanover) 8(6): 95. 1860. Type. “Ab incolis Peruviae” (no specimens cited; no original material found).
Capsicum lanceifolium (Miers) Kuntze, Revis. Gen. Pl. 2: 449. 1891, as “lanceaefolium”. Type. Based on Brachistus? lanceifolius Miers.
Capsicum annuum var. violaceum (Kunth) Alef., Landw. Fl.: 134. 1866. Type. Based on Capsicum violaceum Kunth.
Type
Peru. Pasco: “Ex Pozuzo” [protologue - “Habitat affatim in Peruviae cultis, praesertim ad Panatahuarum Provinciam et in Andium nemoribus”], H. Ruiz & J. Pavón s.n. (lectotype, designated here: MA [MA-815154]; isolectotypes: CORD [CORD 00101751, fragment from G], G, MA [MA-815153, MA-815155]).
Description
Erect and scandent shrubs or perennial herbs, 1–3 (–4) m tall, with the main stem ca. 3 cm in diameter at base, much branched above, the branches in a typical “zig-zag” appearance. Young stems angled, fragile, green or green with purple spots and dark brown ridges, glabrescent to densely pubescent with a soft whitish pubescence of long, spreading, simple, uniseriate, 4–11-celled, eglandular trichomes (some forked) 0.5–1.5 mm long; nodes frequently dark purple; bark of older stems dark brown, smooth or striate, glabrescent to densely pubescent; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair markedly unequal in size, similar or dissimilar in shape. Leaves membranous, concolorous or discolorous, green above, light green beneath, rugose (the youngest leaves) or somewhat smooth with the mid-vein and primary veins raised abaxially, glabrescent to densely pubescent on both surfaces and margins, with similar trichomes like those on stems; blades of major leaves 6–12 (–16) cm long, 2.4–4 (–7) cm wide, ovate or more rarely elliptic, the major veins 4–6 on each side of mid-vein, the base asymmetric and attenuate or cuneate, the margins entire, the apex acuminate; petioles 1.5–2 cm long, densely pubescent to glabrescent; blades of minor leaves (2.5–) 3.5–5 (–6) cm long, 1.8–2.4 cm wide, ovate, the major veins 3–4 on each side of mid-vein, the base rounded, the margins entire, the apex acute or acuminate; petioles 0.5–0.7 cm long, densely pubescent to glabrescent. Inflorescences axillary, 1–2 flowers per axil, rarely up to four flowers; flowering pedicels 15–25 mm long, angled, erect, geniculate at anthesis, green or purple-ribbed, moderately to densely pubescent, the eglandular trichomes long, spreading; pedicels scars conspicuous. Buds globose or ovoid, dark purple. Flowers 4–8-merous. Calyx 2–3 mm long, ca. 4–4.3 mm wide, cup-shaped, thick, green, moderately to densely pubescent with the same trichomes as pedicels, sometimes sparse forked trichomes, the calyx appendages 4–8, (0.3–) 0.5–1.5 (–1.7) mm long, subequal or somewhat unequal, thin, erect, cylindrical, green, inserted close to the margin, pubescent with the same trichomes as calyx tube. Corolla 10–15 mm long, 15–22 (–25) mm in diameter, thick, dark purple or violet with a white centre outside and within (sometimes with a weak yellowish-green centre within), rotate to stellate, with thin interpetalar membrane, lobed 1/3 or a little more of the way to the base, pubescent adaxially with short glandular trichomes (stalk 1–2-celled; head globose, unicellular) in the throat and base of the lobes, the tube 5–8 mm long, glabrous abaxially, the lobes 3.5–6.5 (–7.2) mm long, 4.7–7.4 (–8.5) mm wide, broadly triangular, spreading, with eglandular trichomes abaxially especially on the veins, the margins pubescent with very short purple eglandular trichomes, the tips acute or obtuse, cucullate or not, sometimes papillate. Stamens 4–8, equal; filaments 2–3.3 (–4.25) mm long, purple or lilac, inserted on the corolla 1.4–1.6 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 2–2.8 mm long, ellipsoid or ovoid, purple with a wide cream connective, not connivent at anthesis. Gynoecium with ovary 2–3-carpelar, 2–2.5 mm long, ca. 2.5 mm in diameter, light green, ovoid or pear-shaped; ovules more than two per locule; nectary ca. 1.2 mm tall; styles heteromorphic, short, 3–3.5 mm, not exceeding the anthers, medium near the same length as the anthers or long 4.5–5.2 mm, exserted 1–1.5 mm beyond the anthers, lilac or purple, clavate; stigma 0.3 mm long, 0.7 mm wide, discoid or slightly globose, light green. Berry 20–40 mm long, 17–25 mm in diameter (semi-domesticated plants) or larger up to 50 mm long, 55 mm in diameter (cultivated plants), round, blocky or elongate-curved or not, the base obtuse, truncate or lobate, sometimes narrowed forming a neck-like, the apex blunt or sunken, rarely pointed, green when immature, brightly coloured at maturity (from red to light yellow or blackish), persistent, very pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 35–50 (–55) mm long, pendent, stout, curved or not, strongly angled, widened distally, usually green; fruiting calyx 8–13 mm in diameter, persistent, slightly accrescent, discoid, green, the appendages 0.8–2.1 mm long, ca. 0.3–0.4 mm wide, spreading. Seeds 15–45 per fruit, 5.5–7 mm long, 4.8–6 mm wide, C-shaped, subglobose or irregular, brownish-black to black, the seed coat reticulate (SM), reticulate-cerebelloid (SEM), the cells polygonal or irregular in shape, the lateral walls wavy to sinuate in the seed body, straight at margins; embryo imbricate.
Capsicum pubescens A reproductive branch B trichome of the leaf C calyx D section of the calyx showing the venation E flower, upper view F sector of opened corolla G, H anthers, dorsal and ventral views, respectively I, J, K gynoecium with short, medium and long style, respectively L anatomical detail of the pericarp (note the giant cell in the mesocarp) M seed N seed, in cross section O structure of seed coat at the seed margin P structure of seed coat at the seed body Q embryo. From Hunziker 25484. Drawn by J. de Ugarte.
Capsicum pubescens A plant B reproductive nodes C young leaves, adaxial surface D flower bud on geniculate pedicel E–H flowers, in front view, showing 4-7-merous corollas I, J flowers, seen from behind K flower, in lateral view L–O mature fruits from different provenance (L, N from Bolivia, M from Peru, O from Mexico) P fruit, in longitudinal section, showing the black seeds A–D, G Barboza et al. 4889 E, P from Barboza et al. 4890 F from Palombo 21 H from Barboza et al. 1847 I from Palombo 22 J from Palombo 23 K, L no specimen vouchers (cult. Córdoba, Argentina) M no specimen voucher (cult. Huancayo-Peru) N from Carrizo García et al. 35 O no voucher specimen (bought in Mexico) A–D, G, H, L, M, O, P photos by G.E. Barboza E, F, I–K, N photos by N. Palombo.
Distribution
Capsicum pubescens is native to Bolivia and Peru (Fig.
Ecology
Capsicum pubescens is frequently found in the Andean mid-elevations to highlands from (800–) 1,200–3,500 m and rarely below 500 m elevation. In Indonesia, the cultivation of C. pubescens occurs also in highlands, over 1,400 m elevation (
Phenology
Probably flowering and fruiting all year, depending on the cultivation area.
Chromosome number
2n = 2x = 24 (
Common names
Bolivia: Locato (La Paz, Heiser C272), Locoto (La Paz, Lewis 88629; Santa Cruz, Saldías P. 563), Locote (La Paz, Duke & Winters 17330), Locotito (Santa Cruz, Vargas C. 932); Colombia: Ají (Nariño, López Jurado & Riascos 613; Putumayo, Bristol 1115), Ají rocoto (Huila, Romero Castañeda 6674); Ecuador: Ají (Chimborazo, Moina Z 23; Loja, Ellemann 66689; Tungurahua, Cascante 6), Ají rocoto (Azuay, Steyermark 52690; Pichincha, Mejía 002); Guatemala: Siete caldos, Caballo (
Indigenous names
Bolivia: Uchu rocoto (= ají globoso) (Rentzell s.n.); Colombia: Totsha (Kamsá, Putumayo, Bristol 1115); Ecuador: Huchu (Quechua, Loja, Ellemann 66689).
Uses
The fruits of Capsicum pubescens are one of the most appreciated in the Andean cuisine for their unique aroma, flavour, meatiness, juiciness and pungency (
Preliminary conservation assessment
EOO (7,150,643 km2); AOO (536 km2). Capsicum pubescens is a widespread cultivated species across the Americas and can be assigned the Least Concern (LC) status.
Discussion
Capsicum pubescens belongs to the Pubescens clade (
The fruit is the most variable character in this species and its different common names refer to this (Heiser and Smith 1948;
In the protologue of C. pubescens,
The protologue of C. maximowiczii (
Specimens examined
See Suppl. material
Capsicum rabenii
Capsicum microcarpum var. tomentosum
Chodat & Hassl., Bull. Herb. Boissier 2,4: 80. 1903. Type. Paraguay. Paraguarí: In regione collium, Cerros de Paraguay, Dec 1900, É. Hassler 6498 (lectotype, designated by
Capsicum praetermissum Heiser & P.G.Sm., Brittonia 10(4): 198. 1958. Type. Brazil. Minas Gerais: Viçosa, road to São Miguel, near km 4, 700 m elev., 3 Jan 1930, Y. Mexia 4205 (holotype: F [F-0093991F]; isotypes: BM [BM000992134], CAS [CAS0002376], CORD [CORD00086136], GH [GH00077006], K [K001073038], NY [00138603], MICH [1109872], MO [MO-503526, acc. # 1164250], U [U.1736360], UC [UC509516], S [S-04-2815], US [US00027398], Z [Z000028484]).
Capsicum baccatum var. praetermissum (Heiser & P.G.Sm.) Hunz., Kurtziana 6: 242. 1971. Type. Based on Capsicum praetermissum Heiser & P.G.Smith
Type
Brazil. Rio de Janeiro: “In prov. Sebastianopolitana: Com. de [F. C.] Raben (n. 301)” (lectotype, designated here: BR [BR 0000005529261].
Description
Erect shrubs or scandent subshrubs (0.50–) 0.70–2.5 (–3) m tall, with the main stem 2–2.5 cm in diameter at base, rarely perennial herbs, much branched from near the base and above. Young stems angled, somewhat rigid, green, pubescent with appressed-antrorse or spreading, simple, uniseriate, 4–8-celled, eglandular trichomes 0.3–1.5 mm long, the new growth with a dense whitish pubescence; nodes green or purple; bark of older stems striate, dark brown, glabrescent; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair subequal in size and similar or dissimilar in shape. Leaves membranous, slightly discolorous, green above, light green and with the primary veins raised beneath, glabrescent to moderately pubescent, with appressed-antrorse trichomes like those on stems on adaxial surface and margins, sparsely pubescent on the lamina, but with tufts of eglandular trichomes in the vein axils and with long spreading trichomes along the veins giving a woolly appearance abaxially; blades of major leaves 5.3–10 cm long, 2.4–6.5 cm wide, ovate, the major veins 4–6 (–7) on each side of mid-vein, the base asymmetric and attenuate, the margins entire, the apex acuminate or long-acuminate; petioles (1.5–) 2.5–3.5 cm long, winged distally for the decurrent base of the lamina, moderately to densely pubescent; blades of minor leaves 3–4 cm long, 1.4–2 cm wide, ovate or elliptic, the major veins 2–3 on each side of mid-vein, the base rounded or truncate, the margins entire, the apex acute or acuminate; petioles 0.8–1.2 cm long, moderately to densely pubescent. Inflorescences axillary, 2–4 flowers per axil; flowering pedicels (8–) 12–18 (–25) mm long, angled, erect, geniculate at anthesis, moderately to densely pubescent, the eglandular trichomes spreading or antrorse; pedicels scars inconspicuous. Buds globose, whitish-green or with green spots at the base. Flowers 5-merous, rarely 4-merous. Calyx 1.5–2 mm long, ca. 2–2.5 mm wide, cup-shaped, thick, green, pubescent with the same trichomes as pedicels, the calyx appendages 5, 0.8–1.1 mm long, 0.3 mm wide, subequal, thick, erect, cylindrical, inserted close to the margin, moderately pubescent with the same trichomes as calyx tube. Corolla 6–7 mm long, (9–) 12–15 mm in diameter, thick, white or lilac and greenish-yellow outside, mostly purple or lilac with greenish-yellow or green spots and cream centre within, rotate to stellate with interpetalar membrane, lobed less than 1/3 to nearly halfway to the base, pubescent adaxially with short glandular trichomes (stalk 1–3-celled; head globose, peltate, unicellular), glabrous abaxially, the tube 4–4.5 mm long, the lobes 2–2.5 mm long, 2.2–4 mm wide, broadly triangular, spreading, the margins with very short eglandular trichomes, the tips acute, papillate. Stamens five, rarely four, equal; filaments (0.8–) 1.2–1.5 (–2) mm long, purple or lilac, inserted on the corolla 0.8 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.2–1.5 (–1.8) mm long, ellipsoid, white, cream or yellow, not connivent at anthesis. Gynoecium with ovary 1.3–1.5 mm long, 1.4–1.5 mm in diameter, green, ovoid; carpels two, ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, 2.6–2.8 mm long, exserted 0.8–1.2 mm beyond the anthers, yellowish-white, cylindrical; stigma 0.5 mm long, 0.7–0.75 mm wide, discoid, pale green. Berry globose or subglobose, 5–7.5 mm in diameter or ellipsoid, 7–11 mm long, (4–) 6–8 mm in diameter, green when immature, orange-coloured or bright red at maturity, deciduous, pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels (14–) 20–28 mm long, erect, strongly angled, widened distally, green; fruiting calyx 4–4.5 mm in diameter, persistent, not accrescent, cup-shaped or discoid, green, the appendages 1–2 mm long, ca. 0.3–0.4 mm wide, erect or spreading. Seeds (5–) 7–15 per fruit, 3–4.2 mm long, 2.5–2.8 mm wide, C-shaped or subglobose, pale yellow or yellow, the seed coat smooth (SM), reticulate-cerebelloid (SEM), the cells irregular in shape, the lateral walls sinuate in seed body, straight at margins; embryo imbricate.
Distribution
Capsicum rabenii is native to eastern and central Brazil, occupying a large range in Brazil from Bahia to Rio Grande do Sul States and into central Paraguay (Fig.
Ecology
Capsicum rabenii occurs in a wide variety of vegetation types, from the Atlantic Forest (Semi-deciduous Seasonal Forest, Ombrophilous Forest) to xerophytic forests in the Cerrado and Caatinga; it can be found in disturbed gallery forest, calcareous outcrops vegetation, creek and river margins and as ruderal around anthropogenic and cultivated areas, between (35–) 100 and 1,300 m elevation. It is also frequent as an escape from cultivation or in cultivation around houses and growing commercially in small farms.
Phenology
Flowering from November to May. Fruiting from December to May and June extending to September when in cultivation.
Chromosome number
n = 12 (Heiser and Smith 1958, as C. praetermissum;
Common names
Brazil: Comarim (Minas Gerais, Krieger 5212), Pimenta (Mina Gerais, Mexia 4205; São Paulo, Briske s.n.), Pimentinha (Santa Catarina, Reitz 4749), Pimenta brava (Minas Gerais, Anonymous s.n.), Pimenta comary (São Paulo, Handro 860), Pimenta camari (São Paulo, Coelho s.n.), Pimenta cumarí (Goiás, Vieira 700; Minas Gerais, Krieger 5212; São Paulo, Hoehne & Gehrt s.n.), Pimenta cumarina (Minas Gerais, Tavares et al. 17), Pimenta-cumari-verdadeira (Santa Catarina, Schwirkowski 885), Pimenta Jorobinha (Rio de Janeiro, Glaziou 8841), Pimenta Passarinho (Southeast Brazil,
Uses
In Brazil, fresh fruits are very appreciated in markets (
Preliminary conservation assessment
EOO (2,746,764 km2); AOO (404 km2). Capsicum rabenii is well-adapted to different environments including highly disturbed anthropogenic areas and is easily cultivated in farms, which suggest that it is not under threat. This species is proposed as Least Concern (LC).
Discussion
Capsicum rabenii is a member of the Baccatum clade (
Flowering and fruiting specimens of C. rabenii are very similar to C. baccatum var. baccatum on herbarium specimens, it sometimes being impossible to distinguish one from another if there are no annotations of corolla colour or if specimens are only in fruit. Both species have long petiolate leaves, rotate or rotate-stellate corollas, orange to red globose or elliptic upright fruits and pale yellow seeds. Capsicum rabenii is distinguished, however, not only for its purple-margined corollas (Fig.
Capsicum rabenii A plant B leaf, abaxial surface showing pubescence on the veins C reproductive nodes D bud on geniculate pedicel E flower, lateral view F rotate corolla G stellate corolla H flower showing the exserted style I immature fruits J globose mature fruits K ellipsoid mature fruit and fruiting calyx A–I, K from Barboza & Deanna 5003 J from Barboza et al. 1646. Photos by G.E. Barboza.
Capsicum rabenii differs from C. chacoense, with which is sympatric in Paraguay, in its rotate, coloured corollas and the characteristic pubescence of the leaves beneath, while C. chacoense has stellate, entirely white corollas and a variable pubescence, but is never woolly (Fig.
It is possible that C. rabenii hybridises naturally with C. baccatum and C. frutescens in Brazil as has already been shown through reciprocal crosses that produced some fruits with viable seeds (Heiser and Smith 1958), but intermediates amongst these species have not been observed in field. Our observations indicate that the three species are usually distinguishable using the corolla colour, the pubescence (see above) and calyx features.
When Heiser and Smith (1958) described C. praetermissum, they stated that none of the species of “Flora Brasiliensis” (
Specimens examined
See Suppl. material
Capsicum recurvatum
Type
Brazil. São Paulo: Apiahy, Nov 1888, [G.L.T. Puiggari] 3577 (lectotype, designated here: WU [acc. # 0037948]; isolectotype: CORD [CORD00006951 fragment from WU]).
Description
Erect shrubs (1–) 1.5–4 m high, with the main stem thick, 2–2.5 cm in diameter at base, much branched above, the branches dichotomously spreading in a typical “zig-zag” appearance. Young stems angled, fragile, green or light green, glabrescent to moderately pubescent, with antrorse, curved, simple, uniseriate, 3–6 (–8)-celled, eglandular trichomes 0.3–0.9 mm long; nodes solid, purple or green; bark of older stems dark brown, striate, glabrous; lenticels few. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar or dissimilar in shape. Leaves membranous, discolorous, dark green above, light green beneath, glabrous to moderately or densely pubescent on both surfaces and margins, especially on the veins beneath, with uniseriate, 2–5-celled, eglandular trichomes 0.9–1.2 mm long; blades of major leaves 5–16 (–20) cm long, 1.4–4.8 cm wide, elliptic or narrowly elliptic to ovate, the major veins 4–6 (–7) on each side of mid-vein, the base asymmetric and attenuate, the margins entire, the apex acute or acuminate; petioles 0.3–1.5 (–2) cm long, moderately pubescent; blades of minor leaves 3.2–4.7 cm long, 1.4–2.2 cm wide, elliptic or ovate, the major veins 3–4 on each side of mid-vein, the base attenuate, the margins entire, the apex acute or obtuse; petioles 0.2–0.4 cm long, glabrous or moderately pubescent. Inflorescences axillary, 2–4 flowers per axil, rarely flowers solitary; flowering pedicels 9–20 mm long, terete, sometimes slightly striate, erect, geniculate at anthesis, green, with sparse antrorse eglandular trichomes; pedicels scars inconspicuous. Buds globose, inflated, white with greenish-yellow spots. Flowers 5-merous. Calyx 1.5–2 mm long, ca. 2–2.7 mm wide, cup-shaped, thick, green, glabrous to moderately pubescent, with 2–4-celled eglandular trichomes and small glandular trichomes (stalk one-celled; head dark, multicellular), the calyx appendages 5–9 (–10), the five main appendages longer and subequal 1–2.5 mm long, 0.2–0.5 mm wide, the 1–4 (–5) secondary appendages shorter and unequal 0.1–0.7 mm long, 0.2–0.5 mm wide, spreading or recurved, cylindrical or more commonly triangular, laterally compressed, green, inserted 0.1–0.3 mm below the margin leaving a rudimentary membranous hyaline sleeve portion. Corolla 6–7 mm long, ca.11 mm in diameter, thick, white with green spots outside, white with different patterns of greenish-yellow spots in the base of the lobes and in the throat and a white centre within, stellate without interpetalar membrane, lobed more than 1/3 to nearly halfway to the base, the tube 3–3.5 mm long, pubescent adaxially with a continuous ring of glandular trichomes (stalk long, 1–2-celled; head globose peltate, unicellular), glabrous abaxially, the lobes 3–3.7 mm long, 3.5–4 mm wide, triangular, spreading, glabrous adaxially and abaxially, the margins papillate, the tips cucullate, papillate. Stamens five, equal; filaments 2–2.5 mm long, white, inserted on the corolla 1–1.2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1–1.8 (–1.9) mm long, ellipsoid, yellow, light green or grey, brownish post-dehiscent, not connivent at anthesis. Gynoecium with ovary 1–1.8 mm long, 1–1.3 mm in diameter, green, subglobose; ovules more than two per locule; nectary 0.3–0.5 mm tall; styles homomorphic, 3.2–3.5 mm long, exserted 0.8–1 mm beyond the anthers, white, clavate; stigma 0.2 mm long, 0.5–0.9 mm wide, discoid, pale green. Berry 7–9 mm in diameter, globose, green when immature, greenish-golden yellow at maturity, deciduous, very pungent when immature and rather sweet at maturity, the pericarp thin, translucent, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 18–25 mm long, pendent, angled, widened distally, green; fruiting calyx (3–) 4–4.5 mm in diameter, persistent, not accrescent, discoid, green, the appendages 0.4–2.6 mm long, 0.3–0.5 mm wide, strongly recurved and compressed. Seeds (4–) 8–25 per fruit, 2.5–2.8 mm long, 2–2.5 mm wide, C-shaped, reniform or subglobose, dark brown or black, the seed coat reticulate-tuberculate (SM), reticulate with pillar-like outgrowths (SEM), the cells irregular or polygonal in shape, the lateral walls wavy to sinuate in the seed body and straight at margins; embryo imbricate.
Capsicum recurvatum A reproductive branch B flower, seen from behind C eglandular trichome of the outer surface of the calyx D glandular trichome of the inner surface of the calyx E sector of opened corolla F gynoecium G fruit H anatomical detail of the pericarp (note the giant cells in the mesocarp) I seed J seed, cross section K structure of seed coat at the seed margin L structure of seed coat at the seed body M embryo A, C, D, G, H from Hunziker 20775 B, E, F from Hunziker 19546 I–M from Barboza et al. 2023. Drawn by L. Ochoa.
Distribution
Ecology
Capsicum recurvatum is quite common along the Serra do Mar, in different formations of the coastal Atlantic Forest (Floresta Ombrófila Densa Montana, Floresta Ombrófila Densa Submontana, Floresta Estacional Semidecídual, Floresta Ombrófila Mista, Floresta Tropical Pluvial Atlântica de Encosta and Floresta Tropical de Encosta). It is found growing on moist ground mainly in semi-shade in primary or secondary forests, between 50 and1,800 m elevation.
Phenology
Flowering from late October to April; in fruit from January to June.
Chromosome number
n = 13 (
Common names
Brazil: Pimenta silvestre (São Paulo, Hoehne s.n.), Pimenta de bugre (Santa Catarina, Reitz C 924), Pimenta-do-mato (Santa Catarina, Schwirkowski 139), Pimenta do pasarinho (São Paulo, Barboza et al. 2023).
Uses
None recorded.
Preliminary conservation assessment
EOO (288,689.164 km2); AOO (380 km2). Capsicum recurvatum occupies a large range along the Serra do Mar and is very frequent in many conservation units. Based on these criteria and the number of locations, we consider this species in the Least Concern (LC) category.
Discussion
Capsicum recurvatum belongs to the Atlantic Forest clade (
In the protologue of C. recurvatum,
Capsicum regale
Type
Colombia. Caquetá: Mun. Florencia, Corregimiento El Caraño, Finca de don Isauro, camino al río, en interior de bosque fuertemente inclinado, 01°44'10.6"N, 75°40'78.3"W, 1004 m elev., 22 Aug 2019, A. Orejuela, L. Bohs, G.E. Barboza, P. González, R. Deanna, J. Urdampilleta, J. Valencia & G. Sierra 3034 (holotype: COL; isotypes: COAH, CORD, HUAZ [to be distributed]).
Description
Erect slender shrubs (1–) 1.8–2.5 (–3) m tall, with the main stem somewhat thick, ca. 0.8 cm in diameter at base, sparsely branched towards apex, the branches dichotomous, weak, spreading horizontally. Young stems angled, fragile, glossy, pale green; nodes green; bark of older stems dark brown, glabrous; lenticels present. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size and shape. Leaves membranous, slightly discolorous, green adaxially, pale green with the mid-vein prominent and purple and the secondary veins lilac or green abaxially, glabrous on both surfaces; blades of major leaves 17–20 (–24) cm long, 4.7–8 (–9.2) cm wide, elliptic, the major veins 6–8 on each side of mid-vein, the base unequal and attenuate, the margins entire, the apex acuminate to long-acuminate; petioles (0.8–) 1.5–2.3 cm long, green adaxially and purple abaxially, glabrous; blades of minor leaves 2–5 cm long, 1–3 cm wide, ovate, the major veins 3–5 on each side of mid-vein, the base unequal, the margins entire, the apex obtuse; petioles 0–0.4 cm long, green, glabrous. Inflorescences axillary, ca. 10 mm long, unbranched or rarely forked, with 5–13 flowers; rachis elongate, 4.5–6 mm long; peduncle 0–5.5 mm; flowering pedicels 12–14 mm long, thin, angled, erect to spreading, non-geniculate at anthesis, purple to green, glabrous; pedicels scars conspicuous, corky. Buds ellipsoid, green. Flowers 5-merous. Calyx 2–3 mm long, ca. 2 mm wide, cup-shaped, circular in outline, fleshy, green or greenish-purple, glabrous, the calyx appendages absent or 4–5, 1–1.8 mm long, 0.8–1.1 mm wide, subequal, thick, purple, triangular-compressed, wings-like, reflexed, inserted very close to the margin. Corolla 7–8 mm long, ca. 10 mm in diameter, thick, pure yellow or yellow with maroon pigmentation outside and greenish-yellow with lobes marginally maroon inside, deeply stellate with narrow interpetalar tissue, lobed 2/3 of the way to the base, glabrous adaxially and abaxially, the tube 2–2.5 mm long, the lobes 5–5.5 mm long, ca. 2 mm wide, triangular, the margins with short eglandular trichomes, the tips papillate. Stamens five, subequal; one filament longer than the others, long filament 3.5–4.3 mm long, shorter filaments (2–) 3–3.2 mm long, white, inserted on the corolla ca. 1 mm from the base, with auricles fused to the corolla at the point of insertion; anthers ca. 2 mm long, ellipsoid, lilac or pale bluish, not connivent at anthesis. Gynoecium with ovary ca. 1.3 mm long, ca. 1 mm in diameter, light green, ovoid; ovules more than two per locule; nectary ca. 0.4 mm tall; styles homomorphic, 4.3–4.5 mm long, barely exserted beyond the anthers, white, clavate; stigma ca. 0.1 mm long, ca. 0.8 mm wide, globose or somewhat discoid, light green. Berry 6–9 mm in diameter, globose, green when immature, becoming nearly white and translucent and then dark blue to purple at maturity, non-pungent, the pericarp thick, opaque, without giant cells (endocarp smooth); stone cells absent; fruiting pedicels ca. 18 mm long, 1.8–2 mm in diameter proximally, 2.5–2.6 mm in diameter distally, erect, fleshy, slightly angled and strongly thickened distally, brilliant dark purple; fruiting calyx 3.75–4.25 mm in diameter, persistent, not accrescent, discoid, brilliant purple, with a conspicuous annular constriction at the junction with the swollen pedicel, the appendages reflexed, brilliant purple, fleshy and laterally compressed. Seeds 7–17 per fruit, 2.75–3.40 mm long, 2.25–2.70 mm wide, C-shaped, black, the seed coat smooth, tuberculate at margins (SM), cerebelloid with pillar-like outgrowths (SEM), the cells irregular in shape to polygonal at seed margins, the lateral walls sinuate to straight; embryo annular.
Distribution
Capsicum regale is endemic to southern Colombia (Caquetá Department), eastern Ecuador (Morona-Santiago, Napo and Sucumbío Provinces) and northern Peru (Loreto Department), known mainly on the eastern slopes of the Andes (the Andean-Amazonian Piedmont) (Fig.
Ecology
Capsicum regale occurs in small populations in the understorey of the premontane or montane humid tropical forests of the Amazonian slopes of the Andes, between 700 and 1,900 m elevation.
Phenology
Flowering and fruiting in April and from August to December.
Chromosome number
2n = 2x = 26 (
Common names
None recorded.
Uses
None recorded.
Preliminary conservation assessment
EOO (47,806.378 km2); AOO (32 km2). Based on the EOO and AOO, C. regale is considered Endangered (EN B2a,b iii). Although this species has been collected in some protected areas, the habitat quality outside of these reserves is susceptible to human disturbance, such as crop planting and high levels of deforestation (
Discussion
Capsicum regale belongs to the Andean clade (
Capsicum regale. A habitat B apical branch, showing leaf pairs dissimilar in shape and size C abaxial surface of leaf with purple main vein D forked inflorescence; note the scars of the deciduous flowers E flower, in lateral view, on an unbranched elongate inflorescence F, G flowers with and without pigmentation, respectively H–K various stages of fruit maturity K mature fruit showing the constriction between the pedicel and the berry (arrow) A–F, H–K from Orejuela R. et al. 3034, photos by A. Orejuela, P. Gonzáles and G.E Barboza G from Hoyos et al. 127, photo by L. Coca. Published in
Specimens examined
See Suppl. material
Capsicum rhomboideum
Witheringia rhomboidea Dunal, Solan. Syn. 1. 1816. Type. Colombia. Quindio: “Mont Quindiu”, F.W.H.A. von Humboldt & A. Bonpland s.n. (holotype: P-Bonpl. [P00670657]).
Witheringia dumetorum Dunal, Solan. Syn. 1. 1816. Type. Ecuador. Pasto: Pasto, F.W.H.A. von Humboldt & A. Bonpland s.n. (holotype: P-Bonpl. [P00670658]).
Witheringia ciliata Kunth, Nov. Gen. Sp. [H.B.K.] (quarto ed.) 3: 11. 1818. Type. Ecuador. Pasto: “Crescit in Andibus frigidis Pastoensium prope Tulcan, alt. 1580 hex.” [474 m], F.W.H.A. von Humboldt & A. Bonpland s.n. (holotype: P [not found]; isotype: B destroyed, photo F neg. 2875]).
Witheringia mollis Kunth, Nov. Gen. Sp. [H.B.K.] (quarto ed.) 3: 12. 1818. Type. Peru. Cajamarca: “Crescit in Regno Peruviano prope urbem Caxamarca, alt. 1500 hex” [450 m], F.W.H.A. von Humboldt & A. Bonpland s.n. (lectotype, designated here: P-Bonpl. [P00670656]; isotype: B [destroyed, photo F neg. 2877]).
Capsicum aggregatum Willd. ex Roem. & Schult., Syst. Veg., ed. 15 bis [Roemer & Schultes] 4: 809. 1819. Type. No locality information given “Habitat …”, “Capsicum aggregatum, herb. Willd.)” (holotype: B [B-W04434-01-0]).
Witheringia diversifolia Klotzsch ex Walp., Repert. Bot. Syst. (Walpers) 3: 29. 1844. Type. Cultivated in Berlin Botanical Garden, Germany “In Mexico- (Floruit in horto Berolinensi botanico mense Aprili anni 1841)” (no specimens cited; no original material found).
Brachistus ciliatus (Kunth) Miers, Ann. Mag. Nat. Hist. ser. 2, 3(16): 263. 1849. Type. Based on Witheringia ciliata Kunth.
Brachistus rhomboideus (Dunal) Miers, Ann. Mag. Nat. Hist. ser. 2, 3(16): 264. 1849. Type. Based on Witheringia rhomboidea Dunal.
Brachistus mollis (Kunth) Miers, Ann. Mag. Nat. Hist. ser. 2, 3(16): 264. 1849. Type. Based on Witheringia mollis Kunth.
Brachistus dumetorum (Dunal) Miers, Ann. Mag. Nat. Hist. ser. 2, 3(16): 265. 1849. Type. Based on Witheringia dumetorum Dunal.
Brachistus diversifolius (Klotzsch ex Walp.) Miers, Ann. Mag. Nat. Hist. ser. 2, 3(16): 268. 1849. Type. Based on Witheringia diversifolia Klotzsch ex Walp.
Fregirardia luteiflora
Dunal ex Delile, Ind. Sem. Hort. Monsp.: 7. 1849. Type. Cultivated in Montpellier, France. “Hort. Montpellier”, 17 Feb 1849, M.F. Dunal s.n. (lectotype, designated by
Fregirardia rhomboidea (Dunal) Dunal, Prodr. [A. P. de Candolle] 13(1): 504. 1852. Type. Based on Witheringia rhomboidea Dunal.
Fregirardia dumetorum (Dunal) Dunal, Prodr. [A. P. de Candolle] 13(1): 504. 1852. Type. Based on Witheringia dumetorum Dunal.
Fregirardia mollis (Kunth) Dunal, Prodr. [A. P. de Candolle] 13(1): 505. 1852. Type. Based on Witheringia mollis Kunth.
Fregirardia vargasii Dunal, Prodr. [A. P. de Candolle] 13(1): 505. 1852. Type. Venezuela. Distrito Federal: Circa Caracas, 1830, J.M. Vargas 249 (holotype: G-DC [G00200549; isotype: MPU [MPU023030]).
Solanum mendax
Van Heurck & Müll.Arg., Observ. Bot. (Van Heurck): 61. 1870. Type. Ecuador. Tungurahua: Baños, Aug 1857, R. Spruce 5050 (lectotype, designated by
Brachistus pringlei S.Watson, Proc. Amer. Acad. Arts 25: 159. 1890. Type. Mexico. Nuevo León: in the Sierra de la Silla near Monterrey, 28 May 1889, C. G. Pringle 2544 (holotype: GH [00936714]; isotypes: AC [AC00320467], BKL [BKL00004329], BR [BR0000005530854, BR0000005531189], CM [CM1923, acc. # 260433], COLO [00352195], E [E00570143], EAP [EAP71917], F [v0072759F], G [G00342807], GOET [GOET003410], K [K000585889], KFTA [KFTA0002452, KFTA0002453], M [M-0171536], MEL [MEL2442144], MEXU [00028827], MO [MO-022273, acc. # 3727961; MO-153287, acc. # 1768532], NA [NA-0026220], NDG [NDG44985], NY [00138549, 00138550], P [P00410033, P00410034], PH [PH00008049], PUL [PUL00000045], RSA [RSA0006263, acc. # 102428], S [acc. # S-G-1004], UC [UC548235], US [00027409, acc. # 48601], VT [UVMVT026402], W [acc. # 1890-0000820], WU [acc. # 0033958]).
Capsicum ciliatum (Kunth) Kuntze, Revis. Gen. Pl. 2: 450. 1891. Type. Based on Witheringia ciliata Kunth.
Capsicum diversifolium (Klotzsch ex Walp.) Kuntze, Revis. Gen. Pl. 2: 450. 1891. Type. Based on Witheringia diversifolia Klotzsch ex Walp.
Capsicum dumetorum (Dunal) Kuntze, Revis. Gen. Pl. 2: 450. 1891. Type. Based on Witheringia dumetorum Dunal.
Capsicum molle (Kunth) Kuntze, Revis. Gen. Pl. 2: 450. 1891. Type. Based on Witheringia mollis Kunth.
Capsicum vargasii (Dunal) Kuntze, Revis. Gen. Pl. 2: 450.1891. Type. Based on Fregirardia vargasii Dunal.
Capsicum mendax (Van Heurck & Müll.Arg.) J.F.Macbr., Candollea 5: 402. 1934. Type. Based on Solanum mendax Van Heurck & Müll. Arg.
Brachistus haughtii Svenson, Amer. J. Bot. 33: 481, t. 19, f. 2. 1946. Type. Peru. Piura: summit of Cerro Prieto, 4°45'S, 81°15'W, 2500 ft elev., 28/30 Mar 1941, O. Haught & H.K. Svenson 11621 (holotype: BKL [BKL00004328]).
Capsicum pringlei (S.Watson) J.F.Macbr. & Standl., Publ. Field Mus. Nat. Hist., Bot. Ser. 11: 173. 1936. Type. Based on Brachistus pringlei S.Watson.
Brachistus vargasii (Dunal) Pittier, Cat. Fl. Venez. [Pittier] 2: 358. 1947. Type. Based on Fregirardia vargasii Dunal.
Capsicum haughtii (Svenson) J.F.Macbr., Publ. Field Mus. Nat. Hist., Bot. Ser. 13 (V-B, 1): 72. 1962. Type. Based on Brachistus haughtii Svenson.
Type
Based on Witheringia rhomboidea Dunal.
Description
Erect, slender and sprawling shrubs or rarely trees (0.5–) 2–3 (–5) m tall, with the main stem 1.8–2.5 cm in diameter at base, profusely branched above, the branches erect or decumbent. Young stems terete or slightly angled, somewhat rigid, glabrous or sparsely to densely pubescent with white or rarely ochraceous, antrorse or spreading, simple, uniseriate, eglandular trichomes 0.3–0.9 mm long or furcate to 3–5-branched (or more) trichomes 0.2–0.8 (–1.5) mm long; nodes green; bark of older stems longitudinally ridged, grey, dark brown or brownish-purple; lenticels abundant, white or light brown. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size and similar or dissimilar in shape. Leaves membranous, discolorous, dark green, slightly glossy above, dull light green, with the primary vein raised beneath, moderately pubescent to glabrous adaxially, moderately to densely pubescent abaxially, with similar antrorse trichomes like those of the stems, sometimes a tuft of trichomes in the vein axils beneath; blades of major leaves (4–) 4.8–12 cm long, 2–5 cm wide, ovate, elliptic or rhomboid-ovate, the major veins 4–6 on each side of mid-vein, the base attenuate or truncate, sometimes asymmetric, the margins entire, the apex acute or obtuse-acuminate; petioles 0.5–1.5 (–1.8) cm, with moderate to dense pubescence of spreading trichomes; blades of minor leaves 2–3 cm long, 1.5–2.5 cm wide, ovate or rhomboid-ovate, the major veins 3–4 on each side of mid-vein, the base short-attenuate, the margins entire, the apex acute or obtuse; petioles 0.3–0.8 cm with similar pubescence as major leaves. Inflorescences axillary, 3–8 (–13)-flowers per axil, rarely solitary; flowering pedicels (8–) 11–28 mm, terete or slightly striate, pendent, non-geniculate at anthesis, green, glabrescent to densely pubescent, the eglandular trichomes short or long, spreading or antrorse; pedicels scars conspicuous, corky. Buds globose, yellow or green. Flowers 5-merous. Calyx 2.7–3.8 mm long, 5–6 mm wide, cup-shaped, green, glabrescent to densely pubescent with antrorse or spreading simple, furcate or dendritic trichomes 0.3–0.8 mm long, the calyx appendages usually five (rare 3–4), 0.9–3 mm long, subequal, erect or spreading, linear-subulate, 0.0–0.3 mm below the margin, with the same pubescence as the calyx tube. Corolla (5–) 6–10 mm long, (8–) 10–12 mm in diameter, yellow, sometimes tinged greenish outside and within, campanulate or campanulate-rotate with a wide interpetalar membrane connecting the lobes up to the distal end, hardly lobed, glabrous adaxially and abaxially, the tube (4.4–) 5.4–9 mm long, the lobes 0.6–1 mm long, 1.6–1.9 mm wide, broadly ovate, the margins finely ciliate, the tips cucullate, papillate. Stamens five, equal; filaments 1.2–2.3 mm long, pale yellow or light green, inserted on the corolla 1–1.3 mm from the base, with auricles fused to the corolla at the point of insertion; anthers (1.4–) 1.6–2.3 mm long, ellipsoid, pale yellow or yellow, not connivent at anthesis. Gynoecium with ovary 1.3–1.6 mm long, 1.2–1.3 mm in diameter, greenish-white, ovoid; ovules more than two per locule; nectary 0.5–0.6 mm tall; styles homomorphic, 3.9–5.8 mm long, barely exserted beyond the anthers, pale yellow, clavate; stigma 0.25–0.5 mm long, 0.4–0.6 mm wide, usually bilobed, light green. Berry 5–9 mm in diameter, globose or globose-depressed, green when immature, bright red turning dark burgundy at maturity, non-pungent, the pericarp thick, opaque, lacking giant cells (endocarp smooth); stone cells absent; fruiting pedicels 15–35 mm long, pendent, terete or angled, widened distally, green; fruiting calyx 4–5 mm in diameter, persistent, not accrescent, discoid, green, the appendages 3–6 mm long, spreading or reflexed. Seeds 24–60 per fruit, 2.4–2.8 mm long, 1.8–2.2 mm wide, C-shaped, reniform, rarely teardrop-shaped, dark brown, rarely light brown, the seed coat reticulate (SM), reticulate-cerebelloid (SEM), the cells irregular in shape, the lateral walls mostly sinuate, wavy at margins; embryo annular or imbricate.
Capsicum rhomboideum A reproductive branch B flower C calyx D eglandular trichome of the calyx E, F branched trichomes of the calyx G glandular trichome of the abaxial surface of the calyx H sector of opened corolla I eglandular trichome of the corolla lobes J gynoecium K fruit L fruit, in cross section M anatomical detail of the pericarp (note the absence of giant cells in the mesocarp) N seed O seed, in cross section P structure of seed coat at the seed margin Q structure of seed coat at the seed body R embryo A from Parra V. 089 B–D, G–J from Moore 3831 E, F from Haught & Svenson 11621 K–R from Acosta Solís 14234. Drawn by N. de Flury. Published in
Capsicum rhomboideum A plant B main stem with lenticels flower buds C branch showing leaf pair dissimilar in shape and size D reproductive branch E flower bud F flower, in lateral view G flower, seen from behind H, J flower, in front view, showing connivent and not connivent anthers, respectively I node with a pendent flower and pedicels scars K mature fruits A, J from Barboza & Leiva González 4854 B, D–F from Leiva González 6585 C, G from Barboza et al. 5050 H, I, K from M. Scaldaferro 73. Photos by G.E. Barboza.
Distribution
Ecology
Capsicum rhomboideum is ecologically adapted to different vegetation formations, but grows mostly in xerophytic thickets and dry forests or in tropical deciduous forests, at 250–2,500 (–3,000) m elevation.
Phenology
Flowering and fruiting all year.
Chromosome number
2n = 2x = 26 (
Common names
Colombia: Tinto (Norte de Santander, Garganta 857); Ecuador: Arrayán (Chimborazo, Cerón 15714), Motupe (Imbabura, Vivar C. s.n.), Caspi morocho (Chimborazo, Scolnik 1594), Hierva dura (Carchi, Cerón 11092), Hierba mora (Pichincha, Cerón 6953), 7 varas (Pichincha, Cerón 13171); El Salvador: Mora silvestre (San Miguel, Villacorta et al. 2819), Veranera silvestre (Ahuachapán, Sandoval 308), Arito de niña (Ahuachapán, Sandoval 550); Venezuela: Cachimbito (Distrito Federal, Buschel s.n.),
Indigenous name
Mexico. Tumaltez (Tzeltal, Chiapas, Alonso Méndez 7709).
Uses
Ecuador. Long stems are used to make drum hoops (Cerón 13171). Used in medicine (see Table
Preliminary conservation assessment
EOO (7,747,997.619 km2); AOO (1,964 km2). Capsicum rhomboideum is widespread from Mexico to Peru; we assign the Least Concern (LC) status.
Discussion
Capsicum rhomboideum belongs to the Andean clade (
Amongst the various names coined by
We found in the P-Bonpland Herbarium a sheet (P00670656) that seems to be original material of Witheringia mollis and this is selected as the lectotype.
Specimens examined
See Suppl. material
Capsicum schottianum
Type
[Brazil. Rio de Janeiro]: Serra d’Estrella, [no date], H.W. Schott [5425] (lectotype, designated here: W [acc. # 0074666]; isolectotypes: CORD [CORD00006649], F [v0072906F, acc. # 874709], W [acc. # 0074665, acc. # 0074667]).
Description
Erect shrubs 1–2.5 m tall or small trees 3–5 m tall, with the main stem (1.5–) 2–3 (–6) cm in diameter at base, much branched above, the branches dichotomously spreading in a typical “zig-zag” appearance. Young stems 3–4-angled, rigid, green or light purple, moderately pubescent, with antrorse, curved, simple, uniseriate, 2–6-celled, eglandular trichomes 0.25–0.5 mm long; nodes solid, swollen, dark green almost black or lilac; bark of older stems dark brown, glabrous; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar in shape. Leaves membranous, slightly discolorous to discolorous, opaque, green above, light green beneath, glabrous or sparsely pubescent, with similar trichomes as in stems on both surfaces; blades of major leaves (4–) 9.5–16.5 (–25.5) cm long, 2–4 (–6) cm wide, elliptic to ovate, the major veins 6–8 on each side of mid-vein, the base attenuate or cuneate, unequal, the margins entire, the apex acuminate; petioles 0.8–2.5 cm long, glabrescent to moderately pubescent; blades of minor leaves 3.4–4.2 cm long, 1.5–1.7 cm wide, elliptic or ovate, the major veins 3–4 on each side of mid-vein, the base attenuate, the margins entire, the apex acute or obtuse; petioles 0.1–0.4 cm long, with similar pubescence as the major leaves. Inflorescences axillary, 2–5 (–7) flowers per axil, very rarely flowers solitary; flowering pedicels 8–25 mm long, thin, angled, erect or slightly spreading, geniculate at anthesis, light green, glabrescent, the eglandular trichomes short, antrorse; pedicel scars conspicuous, corky. Buds globose-ovoid, green or greenish-yellow, rarely purple. Flowers 5-merous. Calyx (1.5–) 2–2.4 mm long, 2.25–2.5 mm wide, cup-shaped, green, circular or pentagonal in outline, sometimes the primary veins conspicuously marked on the surface, glabrescent, with sparse small glandular trichomes (stalk one-celled; head dark, multicellular) and 2–4-celled eglandular trichomes, the calyx appendages absent or with five minute appendages ca. 0.35 mm long. Corolla 7–8 (–10) mm long, 8–12 (–14) mm in diameter, mostly white with green or greenish-yellow and pale purple spots outside, white with discontinuous purple or brownish spots, greenish-yellow spots and white centre within (in some populations purple pigmentation absent); stellate without or with a thin interpetalar membrane, lobed more than 1/3 to halfway to the base, pubescent adaxially with a continuous ring of glandular trichomes (stalk long, 2-celled; head globose, peltate, unicellular) in the throat and base of the lobes, glabrous abaxially, the tube 2.4–4.2 mm long, the lobes 2–3.5 (–5) mm long, 2–4 mm wide, triangular or ovate, spreading, the margins finely ciliate or papillate, the tips acute and cucullate, papillate. Stamens five, equal; filaments (2.4–) 3–4 mm, white or cream, inserted on the corolla 1.5–2 mm from the base, with auricles fused to the corolla at the point of insertion; anthers (1.1–) 1.3–1.8 mm, ellipsoid, yellow, light green, grey or light purple, not connivent at anthesis. Gynoecium with ovary 1.25–1.7 mm long, 1.35–1.5 mm in diameter, light green, globose-ovoid; ovules more than two per locule; nectary ca. 0.5 mm tall; styles homomorphic, 3–4.5 mm long, barely exserted beyond the anthers, white, clavate, sometimes slightly curved; stigma 0.12–0.25 mm long, 0.75–0.85 mm wide, discoid or globose, pale green. Berry 7–9 mm in diameter, globose or subglobose, green when immature, greenish-golden yellow at maturity, deciduous, pungent when immature and less pungent when mature, the pericarp thin, translucent, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 17–27 mm long, pendent, terete or angled, widened distally, green; fruiting calyx 3–4.5 mm in diameter, thin, persistent, not accrescent, discoid, light green, with a thin constriction at the junction with the calyx. Seeds (6–) 7–19 (–23) per fruit, 2.6–3.5 mm long, 2–3 mm wide, C-shaped, ellipsoid or teardrop-shaped, brownish-black to black, the seed coat reticulate and tuberculate at margins (SM), reticulate with pillar-like outgrowths (SEM), the cells irregular polygonal to irregular in shape, the lateral walls straight to sinuate; embryo imbricate.
Distribution
Capsicum schottianum is endemic to south-eastern Brazil (Minas Gerais, Rio de Janeiro and São Paulo States) (Fig.
Ecology
Capsicum schottianum grows from the coast to the interior of the Atlantic Forest (Mata Atlântica). It is quite common, often forming large populations in the Floresta Ombrófila Densa and Floresta Estacional Semidecidual and can be found in margins and interior of primary and disturbed forests, near the watercourses, in sun or in semi-shade, between 250 and 1,700 m elevation.
Phenology
Flowering from September to April; fruiting from December to June.
Chromosome number
n = 13 (
Common names
Brazil. Pimenta bentiví (Minas Gerais, Hunziker 25145).
Uses
None recorded.
Preliminary conservation assessment
EOO (148,467.320 km2); AOO (216 km2). Capsicum schottianum is distributed mainly along the Serra do Mar system (Serra da Carioca, Serra da Bocaina, Serra do Paranapiacaba, Serra dos Órgãos and others) and is very frequent in many conservation units. Considering the large extent of occurrence, the high number of locations in Natural Reserves and the large population size with many highly reproductive individuals, we suggest a Least Concern (LC) category for this species.
Capsicum schottianum A reproductive branch B flower C section of the calyx showing the venation D eglandular trichome of the calyx E sector of opened corolla F gynoecium G fruit H fruit (one carpel), in cross section I anatomical detail of the pericarp (note the giant cells in the mesocarp) J seed K seed, cross section L structure of seed coat at the seed margin A–L from Hunziker 19577 L from Macedo 3079. Drawn by L. Sánchez.
Discussion
Capsicum schottianum is a member of the Atlantic Forest clade (
Capsicum schottianum A plant B flower buds on geniculate pedicel C flower, in pre-anthesis D–J flowers, in front view, showing variations in the purple and greenish pigmentation of the corolla K, L flowers, seen from behind M flower in lateral view, showing not connivent anthers and style slightly exserted N immature fruits O mature fruit A, C Barboza et al. 1638 B, J, K, M from Barboza 5043 D, G, L from Barboza & Deanna 5019 E from Barboza & Deanna 5017 F from Barboza & Deanna 5029 H from Barboza & Deanna 5018 I, O from Barboza & Deanna 5014 N from Barboza & Deanna 5006. Photos by G.E. Barboza and R. Deanna.
Capsicum schottianum is extremely difficult to distinguish from C. campylopodium in herbaria when the specimens have only immature fruits and there is no any indication of the corolla colour in the labels. The calyx of C. schottianum is generally pentagonal in outline and measures 1.5–2.4 mm long, corollas reach 7–10 mm long and 8–14 mm in diameter and have generally purple and greenish-yellow colouration within, the filaments are equal and the berry is globose to subglobose with 6–23 seeds. In contrast, the calyx of C. campylopodium is always circular in outline and measures (1–) 1.2–1.6 mm long, corollas reach 4.5–6.5 (–8) mm long, (6–) 6.4–7.5 (–11) mm in diameter and has golden yellow or ochraceous spots within, the filaments are unequal (3 + 2) and the berry is globose-depressed with only four (very rare six) seeds.
Capsicum schottianum can also be confused with C. pereirae which has a similar pattern of colouration in the corolla and calyx shape, but differs in the geniculation of the pedicels (geniculate in C. schottianum vs. non-geniculate in C. pereirae) and leaf morphology (membranous and opaque in C. schottianum vs. coriaceous and shiny in C. pereirae).
In describing C. schottianum,
Capsicum tovarii
Type
Peru. Huancavelica: Prov. Tayacaja: Andaimarca, entre Colcabamba y Surcubamba, valle del Mantaro, 2000 m elev., 14 Apr 1954, O. Tovar 1867 (holotype: USM [USM000895]; isotypes: MU (n.v.), US [00027402, acc. # 3048334], USM [USM248309]).
Description
Erect subshrubs or shrubs, 0.5–1.5 (–2.5) m tall, with the main stem thick, 1.5–3.5 cm in diameter at base, much branched from the base, the branches fragile and scandent. Young stems 2–3-angled, green, rigid, glabrescent to moderately pubescent with appressed-antrorse or spreading, simple, uniseriate, 2–6-celled, eglandular trichomes 0.08–0.9 (–1.2) mm long and sparse small, simple, glandular trichomes (stalk short, unicellular; head dark, oblong, multicellular); nodes green, bark of older stems greenish-brown, fissured, glabrescent; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair subequal in size and shape. Leaves membranous, discolorous, dark green above, greenish-grey below, moderately to densely pubescent, with simple eglandular trichomes and glandular trichomes similar to those of the stems, these latter especially abundant on the veins abaxially; blades of all leaves (2.5–) 4–15.5 cm long, (1–) 1.5–5.7 cm wide, ovate or lanceolate, the major veins 3–5 on each side of mid-vein, the base short-attenuate and asymmetric, the margins entire, the apex acuminate; petioles 1–3 cm long, densely pubescent. Inflorescences axillary, (2–) 4–8 flowers; flowering pedicels 3–10 mm long, short, angled or terete, spreading to pendent, non-geniculate at anthesis, green, moderately pubescent, the eglandular trichomes long, spreading; pedicel scars inconspicuous. Buds globose to ovoid, yellowish-green. Flowers 5-merous. Calyx 1.5–2 mm long, 1.8–2.5 mm wide, cup-shaped, pentagonal in outline, thin, light green with dark green veins, moderately pubescent with the same eglandular trichomes as stems and leaves, sometimes sparse glandular trichomes, the calyx appendages absent or five, equal, 0.5 mm long, inserted very close to the margin, sparsely pubescent. Corolla (4.5–) 6–8 mm long, 8–10 mm in diameter, yellowish-green with lilac interpetalar membrane outside, purple with greenish-yellow spots in the lobes and greenish-yellow centre within or lobes mostly yellowish-green and diffuse lilac pigmentation within, campanulate or campanulate-stellate, lobed less than 1/3 of the way to the base, the tube 3.8–4.62 mm long, with sparse small glandular trichomes (stalk unicellular; head globose, unicellular) adaxially, glabrous abaxially, the lobes 2.5–3.2 mm long, 2.5–3.5 mm wide, ovate, erect, glabrous adaxially and abaxially, the margins papillate, the tips acute, slightly papillate. Stamens five, equal; filaments 1.6–2.5 mm long, lilac, inserted on the corolla 0.8–1.3 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.5–1.8 (–2) mm long, ellipsoid, purple or lilac, somewhat connivent or not connivent at anthesis. Gynoecium with ovary 1.2–2 mm long, 1–1.5 mm in diameter, 2 (–4)-carpellate, light green, ovoid or subglobose; ovules more than two per locule; nectary ca. 0.4 mm tall; styles dimorphic, short style 1–1.6 mm, not exceeding the anthers, long style 4.2–7 mm long, exserted ca. 1 mm beyond the anthers, cream with lilac pigmentation, clavate; stigma 0.2–0.37 mm long, ca. 0.75 mm wide, capitate or lobed, pale yellow. Berry 5–7.2 mm in diameter, globose, green turning nearly black when immature, bright red at maturity, deciduous, pungent, the pericarp thick, opaque, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels 7–12 (–15) mm long, erect, strongly angled, widened distally, green; fruiting calyx 4–6 mm in diameter, persistent, not accrescent, discoid, the appendages 0.0–0.6 mm long, appressed to the berry. Seeds 4–12 per fruit, 3.7–4.8 mm long, 2–3 mm wide, C-shaped or subglobose, yellow to brownish-yellow, the seed coat reticulate (SM), reticulate-cerebelloid (SEM), the cells irregular in shape, the lateral walls mostly sinuate, wavy at margins; embryo imbricate or coiled.
Capsicum tovarii A flowering branch B, C eglandular trichomes of the leaf D, E glandular trichomes of the leaf F flower G calyx showing the venation H sector of opened corolla I glandular trichome of the abaxial surface of the corolla J gynoecium with long style K gynoecium with short style L fruit M seed N seed, cross section O structure of seed coat at the seed margin P structure of seed coat at the seed body Q embryo. From Eshbaugh E 1137. Drawn by N. de Flury.
Capsicum tovarii A plant B leaf, adaxial surface C leaf, abaxial surface D node with flower buds on pendent pedicels E flower, in lateral view F, G flower in front view, showing different pattern of purple colouration H immature fruits I mature fruit. From Barboza 5044. Photos by G.E. Barboza.
Distribution
Ecology
Capsicum tovarii grows in xerophytic to mesophytic habitats with abundant columnar cacti, Bombax ruizii K.Schum. (
Phenology
The collections seen are from February to May, all in flower and fruit.
Chromosome number
n = 12 (
Common names
Peru. Ají silvestre (Huancavelica, Tovar 1867), Mucuru (Huancavelica, Tovar 1867; Junín, Barboza 5044), Mucuru-uchu (Huancavelica, Tovar 1867).
Uses
The fruits are very pungent and eaten by local people (Tovar 1867, Barboza 5044), used as a spice (
Preliminary conservation assessment
EOO (1,422.442 km2); AOO (28 km2). Capsicum tovarii occupies a very narrow geographic range in Central Peru. Based on its EOO, AOO and the few nearby locations where it was found, none of them in protected areas and considering that the quality of the habitat will decline by the increased agricultural development, we assign the Endangered (EN; B1+B2ab(iii)) status to this species.
Discussion
Capsicum tovarii appears as an isolated branch in the phylogeny of the genus (
This species has been rarely collected (< 10 collections) in Central Peru and the majority of the collections have been made by the late Peruvian botanist Oscar Tovar (USM). The species deserves more field observations to better understand the morphological variation in trichome type, corolla colour, heteromorphism of styles, number of carpels, breeding system and area of distribution. The pubescence varies from glabrescent with a predominance of small glandular trichomes along leaf veins, petioles and pedicels to moderately pubescent with predominance of antrorse eglandular trichomes hiding the glandular ones (but these were somewhat visible by virtue of their dark head). The corolla colour has been annotated as purple or purple-blue in Tovar´s labels (e.g. Tovar 1867, 5012 & 5363), but corollas that are entirely cream or cream with two green spots within are also reported in the protologue (Eshbaugh et al. 2013). Similar cream or pale yellow corollas have been observed in plants obtained from seeds from Pariahuanca-Huancayo (sent by O. Tovar in 1999 to A. T. Hunziker, CORD), in which corollas were pale yellow with two green spots at the base of each lobes and throat, while others had purple tones in the interpetalar membrane (Hunziker 25654). In a different plant from the same seed accession (Hunziker 25655), corollas displayed an evident purple pigmentation in the lobes and adjacent zone of the throat, as was seen by Barboza in another population from Potrero, Huancayo (Barboza 5044, Fig.
Capsicum villosum
Capsicum villosum var. latifolium Sendtn., Fl. Bras. (Martius) 10(6): 144. 1846. Type. Brazil, Rio de Janeiro: “Habitat in udis umbrosis sylvarum primaevarum, Provinciae Sebastianopolitanae, Dec.” [no year], C.F.P. von Martius s.n. (lectotype, designated here: M [M-0171535]; isotype: CORD [CORD00006950, fragment from M]).
Type
Brazil, Minas Gerais: “Villa Riccam” [Ouro Preto], [no date], J.B.E. Pohl [3674] (lectotype, designated here: W [acc. # 0074662]; isolectotypes: CORD [CORD00006949], F [acc. # 874864], W [acc. # 0074663, acc. # 0074664).
Description
Erect shrubs or subshrubs, (0.80–) 1–3 (–5) m tall, with the main stem thick, ca. 1.5 cm in diameter at base and sprouts from adventitious roots, much branched above, the branches dichotomously spread in a typical “zig-zag” appearance. Young stems 3–angled, somewhat rigid, green, densely pubescent, with spreading, rigid or flexuous, white or slightly ferruginous (dried specimens), simple, uniseriate, 5–8-celled, eglandular trichomes 0.5–1.8 mm long; nodes solid, green; bark of older stems striate, brown or dark brown, glabrescent or sparsely pubescent; lenticels absent. Sympodial units difoliate, the leaves geminate; leaf pair unequal in size, similar or dissimilar in shape. Leaves membranous, discolorous, dark green above, light green beneath, moderately pubescent with appressed-antrorse trichomes similar to those of the stems on adaxial surface, densely pubescent, with long spreading 5–9-celled, eglandular trichomes 0.3–2 mm long on abaxial surface and margins; blades of major leaves 6–17 (–24.5) cm long, (2–) 2.4–7.5 cm wide, ovate, elliptic or narrowly elliptic, the major veins 5–7 on each side of mid-vein, the base attenuate, the margins entire, the apex long-acuminate; petioles 0.4–2.3 (–3.8) cm long, densely pubescent; blades of minor leaves 2.2–2.8 (–5) cm long, 1–1.4 cm wide, ovate or elliptic, the major veins 3–4 on each side of mid-vein, the base attenuate, the margins entire, the apex acute; petioles 0.2–0.3 cm long, densely pubescent. Inflorescences axillary, 3–4 flowers per axil, rarely flowers solitary; flowering pedicels 12–17 (–20) mm long, angled, erect, geniculate at anthesis, green, sparsely to moderately pubescent, the eglandular trichomes long, spreading; pedicels scars inconspicuous. Buds ovoid or globose, inflated, yellowish-green with purple spots or almost entirely purple. Flowers 5-merous. Calyx 1.75–2 mm long, 2–2.5 mm wide, cup-shaped, thick, green, densely pubescent, the calyx appendages 5, (0.5–) 1–2 (–2.5) mm long, 0.2–0.3 mm wide, subequal, thin, erect, cylindrical or subulate, inserted very close to the margin. Corolla 7–9 mm long, 14–14.5 mm in diameter, white with yellowish-green spots and pale purple pigmentation outside, white with intense purple spots, greenish-yellow star and a white centre within, stellate without interpetalar membrane, lobed halfway or less of the way to the base, pubescent adaxially with a continuous ring of glandular trichomes (stalk long, 2–3-celled; head globose, peltate, unicellular) in the throat and base of the lobes, glabrous abaxially, the tube 3.5–4.5 mm long, the lobes (3.5–) 4.2–4.5 mm long, (3.7–) 4–4.75 mm wide, triangular or ovate, spreading, the margins finely ciliate, the tips acute or cucullate, papillate. Stamens five, equal; filaments 2.4–3.6 mm long, white or cream, inserted on the corolla ca. 1.75 mm from the base, with auricles fused to the corolla at the point of insertion; anthers 1.5–2 mm long, ellipsoid, yellow or cream, not connivent at anthesis. Gynoecium with ovary 1.3–1.5 mm in diameter, light green, subglobose; ovules more than two per locule; nectary ca. 0.3 mm tall; styles homomorphic, 3–4.5 mm long, at the same level or barely exserted beyond the anthers, clavate, cream; stigma 0.3 mm long, 0.75–0.9 mm wide, discoid, pale green. Berry 7–9 mm in diameter, globose or globose-depressed, green when immature, greenish-golden yellow at maturity, deciduous, pungent, the pericarp thin, translucent, with giant cells (endocarp alveolate); stone cells absent; fruiting pedicels (13–) 15–24 mm long, pendent, angled, widened distally, green; fruiting calyx 4–5 mm in diameter, persistent, not accrescent, discoid, green, the appendages 0.7–3 mm long, spreading, green. Seeds 8–15 per fruit, (2–) 2.4–2.8 mm long, 2.2–2.5 mm wide, C-shaped or ellipsoid, brownish-black to black, the seed coat reticulate in seed body and tuberculate at margins (SM), reticulate with pillar-like outgrowths at margins (SEM); the cells polygonal in shape, the lateral walls wavy, straight at margins; embryo imbricate.
Distribution
Capsicum villosum is endemic to south-eastern Brazil, most commonly in Rio de Janeiro, Minas Gerais and São Paulo States and only one collection from the State of Espírito Santo (Fig.
Ecology
Capsicum villosum inhabits montane rain forests of the coastal and interior Atlantic Forest (Mata Atlântica), highly adapted to different formations (Floresta Ombrófila Densa, Floresta Ombrófila Mista Aluvial, Campo Rupestre). It forms small colonies along paths near rivers, streams or waterfalls and in open clearings exposed to sun or under the shade, between 500 and 1,900 m elevation.
Phenology
Flowering from October to April. Fruiting from late December to July.
Chromosome number
n = 13 (
Common names
Brazil. Pimentinha (São Paulo, Kirizawa 3288).
Uses
None recorded.
Preliminary conservation assessment
EOO (216,703.381 km2); AOO (172 km2). Capsicum villosum is relatively widespread in the Brazilian Atlantic Forest and occurs in several reserves or in restored and recuperating forests of this system. Although C. villosum has a large EOO and occurs in a number of locations, we assign the Near Threatened (NT) category, since the pressure on this ecosystem continues due to the illegal extraction of the forests and its replacement by other land uses which may adversely affect some subpopulations.
Discussion
Capsicum villosum is a hairy species of the Atlantic Forest clade (
Capsicum villosum A flowering branch B flower C section of the calyx showing the venation D eglandular and glandular trichomes of the calyx E sector of opened corolla F gynoecium G fruit H anatomical detail of the pericarp (note the giant cells in the mesocarp) I seed J structure of seed coat at the seed margin A, B, D–F from
Capsicum villosum A plant B flower bud on a geniculate pedicel C flower, seen from behind D flower before full anthesis, front view E flower at full anthesis, front view F fruiting branch, with mature and mature fruits A, B, E, F from Barboza et al. 1655, photos by G.E. Barboza C, D from Barboza & Deanna 5026 bis, photos by R. Deanna.
The density and type of pubescence on C. villosum is highlighted in the specific epithet. Capsicum cornutum, C. muticum and C. rabenii are also variable in pubescence density. Capsicum villosum can be distinguished for these other pubescent species mainly by corolla colour or size and calyx appendages. Capsicum villosum and C. rabenii have five subequal calyx appendages and similar corolla size, but the corolla of C. villosum has intense purple spots inside, while in C. rabenii, the corolla is only purple or lilac on the margins (Fig.
Capsicum villosum is related to C. mirabile (
In describing C. villosum,
Doubtful names
Capsicum annuum var. fingerhuthi Alef., Landw. Fl.: 133. 1866.
Type. No locality cited (no specimens cited, no original material located). This variety was compared with C. annuum var. cordiforme (Mill.) Alef. but with scarlet fruit = C. annuum var. annuum or C. chinense Jacq.
Capsicum annuum var. leptoceras Alef., Landw. Fl.: 132. 1866.
Type. “So erhielt ich Exempl. aus Mexico” (no specimens cited, no original material located). The short German description for this variety refers to the size, shape and colour of the fruit and the calyx appendages shape which can apply to both Capsicum annuum var. annuum or C. baccatum var. pendulum (Willd.) Eshbaugh
Capsicum annuum var. varians Voss in Vilm., Vilm. Blumengärtn., ed. 3. 1: 723. 1894.
Type. No locality cited (no specimens cited, no original material located).
Capsicum annuum var. weinmanni Alef., Landw. Fl.: 133. 1866.
Type. No locality cited (no specimens cited, no original material located).
Capsicum bauhini Dunal, Prodr. [A. P. de Candolle] 13(1): 428. 1852.
Type. No locality cited.
Capsicum cerasiflorum Link, Enum. Hort. Berol. Alt. 1: 190. 1821.
Type. “Habitat....”. (no specimens cited; original material in B, destroyed). As Link’s description is very brief and the characters mentioned are common to many species (“Petiolis junioribus ciliatis baccis erectis globosis”), its identity is uncertain and could apply to any of these domesticated species: Capsicum annuum L., C. chinense Jacq., or C. baccatum L.
Capsicum cerasiforme var. cerasiflorum (Link) Dunal, Prodr. [A. P. de Candolle] 13 (1): 422. 1852.
Type: Based on Capsicum cerasiflorum Link (a doubtful name, see above).
Capsicum cerasiforme var. minus Fingerh., Monogr. Capsic.: 20. 1832.
Type. “Patria America australis et India? orientalis” (no specimens cited; no original material located).
Capsicum chamaecerasus Nees, Trans. Linn. Soc. London 17: 65. 1834. nom. illeg. superfl.
Type. Based on Capsicum cerasiforme Poir., Enc. Meth. 5 [in error Suppl. v.] p. 325. (nec Willd.), 1804 and Capsicum purpureum Roxb. (ad partem) (both are cited in synonymy). As there is no accurate evidence of Nees concept for C. cerasiforme (in this treatment C. cerasiforme Lam. and C. cerasiforme Willd. are synonyms of C. chinense) and because C. purpureum Roxb. is considered here synonymy of C. annuum var. annuum, we cannot properly assign identity of C. chamaecerasus.
Capsicum conicum Hornem., Hort. Bot. Hafn. Suppl.: 27. 1819, nom. illeg., not C. conicum G. Mey. (1794).
Type. “Hab. - - C. [Planta Caldarii] intr. 1813. Missum a celb. Sohradero sub hoc nomime” (no original material located).
Capsicum cordiforme var. cerasicarpon Dunal, Prodr. [A. P. de Candolle] 13(1): 428. 1852.
Type. “v.v. in hort. Monsp. s. in h. meo.” (no original material located).
Capsicum hornemanii Dunal, Prodr. [A. P. de Candolle] 13(1): 429. 1852.
Type. Replacement name for C. conicum Hornem. (see above).
Capsicum indicum Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 22. 1829.
Type. “Capsicum indicum Lobelii”;
Capsicum indicum Dierb. [unranked category] 1) macrocarpon, Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 22. 1829.
Capsicum indicum Dierb. [unranked category] 2) pachycarpon, Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 25. 1829.
Capsicum indicum Dierb. [unranked category] 3) cerasocarpon, Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 26. 1829.
Capsicum indicum Dierb. [unranked category] 4) elaeocarpon, Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 28. 1829.
Capsicum indicum Dierb. [unranked category] 5) microcarpon, Dierb., Arch. Apotheker-Vereins Nordl. Teutschl. 30: 29. 1829.
Capsicum micranthum Link, Enum. Hort. Berol. Alt. 1: 190. 1821.
Type. “Hab. in Brasilia” (no specimens cited; no original material located);
Capsicum pyramidale var. torulosum (Hort.Matr. ex Hornem.) Fingerh., Monogr. Capsic.: 15. 1832.
Type. Based on Capsicum torulosum Hort.Matr. ex Hornem. (a doubtful name itself).
Capsicum torulosum Hort.Matr. ex Hornem., Hort. Bot. Hafn. Suppl.: 27. 1819.
Type. “Hab.- - C. [Planta Caldarii] intr. 1815”.
Names (designations) not validly published
Designations are listed in alphabetic order; ICN Articles refer to the Shenzhen Code (
Capsicum section Eucapsicum Kuntze, Revis. Gen. Pl. 2: 448. 1891, not validly published, the name of the subdivision formed from the name of the genus by adding the prefix Eu- (Art. 21.3).
Capsicum section Eucapsicum Wettst., Nat. Pflanzenfam. 4(3b): 20. 1891, not validly published, the name of the subdivision formed from the name of the genus by adding the prefix Eu- (Art. 21.3).
Capsicum annuum forma chlorocarpum Kuntze, Revis. Gen. Pl. 2: 449. 1891, nomen subnudum;
Capsicum annuum forma leucocarpum Kuntze, Revis. Gen. Pl. 2: 449. 1891, certainly not intended as a new name, citation of “albidi (Mill. em.)” = C. annuum var. annuum
Capsicum annuum forma luteum Kuntze, Revis. Gen. Pl. 2: 449. 1891, certainly not intended as a new name, citation of “fructus aurantiaci vel rubri vel flavi (W. em.)” = C. annuum var. annuum
Capsicum annuum forma violaceum Kuntze, Revis. Gen. Pl. 2: 449. 1891, certainly not intended as a new name, citation of “fructus violacei, atropurpurei (Brouss. em.)” = C. annuum var. annuum
Capsicum annuum var. leucocarpon Voss in Vilm., Vilm. Blumengärtn., ed. 3. 1: 723.1894, nom. subnudum; the very brief German description does not provide a character to distinguish this variety from any other and it is not validly published.
Capsicum baccatum Buch.-Ham. ex Wall., Numer. List [Wallich] n. 2644. 1831, nomen nudum; a manuscript name for a specimen from Jolpighurri (India) held at K [K001116731] = C. frutescens L.
Capsicum baccatum hort.Genev. ex Dunal, Prodr. [A. P. de Candolle] 13(1): 420. 1852, pro syn. Capsicum angustifolium Dunal = C. annuum var. annuum
Capsicum caerulescens Besser, Cat. Hort. Crem.: 29. 1816, not validly published; name in a list with no diagnosis or description (Art. 38.1) and referred to other nomen nudum Capsicum luteum H.V. [(Schott) H. Vindebonensis] cited in
Capsicum cerasiforme hort. ex Dunal, Prodr. [A. P. de Candolle] 13(1): 420. 1852, pro syn. C. baccatum L.; based on a manuscript name “Capsicum baccatum hort.”, at G-DC [G0000131879] = Capsicum baccatum L.
Capsicum cydoniforme hort. ex Roem. & Schult., Syst. Veg., ed. 15 bis [Roemer & Schultes] 4: 561. 1819; pro syn. C. tetragonum Mill. = C. annuum var. annuum.
Capsicum longum Bouton ex Dunal, Prodr. [A. P. de Candolle] 13(1): 414. 1852, pro syn. Capsicum conoide Mill.; based on a manuscript name “Capsicum longum, Isle Mauricie, 1839, L. Boutoun” at G-DC [G0000131840] = Capsicum frutescens L.
Capsicum minimum Blanco, Fl. Filip. [F.M. Blanco]: 133. 1837, not intended as a new name, citation of Capsicum minimum Roxb. in text (
Capsicum narunca Dunal, Prodr. [A. P. de Candolle] 13(1): 414. 1852, pro syn. Capsicum bicolor Jacq.; based on a manuscript name “Capsicum naruna” at G-DC [G00131853], = Capsicum annuum var. annuum.
Capsicum odoratum Steud., Nomencl. Bot. [Steudel], ed. 2. 1: 279. 1840, not validly published; no diagnosis or description (Art. 38.1), name in a list with only “Arrab. [Anton da Arrabida], Brasil” as reference = identity uncertain.
Capsicum pubescens Dunal, Prodr. [A. P. de Candolle] 13(1): 415. 1852, pro syn. C. chlorocladum Dunal; based on a manuscript name “Capsicum pubescens Dun. 2 Feb 1844”, at G-DC [G00131884] = C. annuum var. glabriusculum (Dunal) Heiser & Pickersgill.
Capsicum tournefortii Besser, Cat. Hort. Crem.: 30. 1816, not validly published; no diagnosis or description (Art. 38.1), name in a list referred to other nomen nudum Capsicum “(Tourneforti) pomiferum L.” (
Capsicum violaceum Desf., Tabl. École Bot. 70. 1804; Desv. in Ham. Prod. 25. 1804, not validly published; no diagnosis or description (Art. 38.1) = probably C. annuum var. annuum
Capsicum violaceum H.R.M.Brouss., Elench. Monsp.: 12. 1805, not validly published; no diagnosis or description (Art. 38.1), name in a list as “C. violaceum H.R.M. [Horto Regis Matritensis] = C. annuum var. annuum
Solanum brachypodum Dunal, Prodr. [A. P. de Candolle] 13(1): 411. 1852, pro syn. Bassovia brachypoda Dunal; based on a manuscript name at G; the Pavon specimen at G is annotated “Solanum brachypodum Dun.” = Capsicum hookerianum (Miers) Kuntze
Solanum divergens Dunal, Prodr. [A. P. de Candolle] 13(1): 411. 1852, pro syn. Bassovia leptopoda Dunal; based on a manuscript name at H. Banks (BM000798808) where it is ink-annotated “S. divergens Dun. ined.” and with a pencil annotation on the same label “n. 131” = Capsicum muticum Barboza
Solanum leptopodum Dunal, Prodr. [A. P. de Candolle] 13(1): 411. 1852, pro syn. Bassovia leptopoda Dunal; based on handwritten names at MPU (MPU023056) and G-DC (G00131651) = Capsicum muticum Barboza
Excluded names
Capsicum anomalum Franch. & Sav., Enum. Pl. Jap. 2: 452. 1878 = Tubocapsicum anomalum (Franch. & Sav.) Makino (
Capsicum asterotrichum Standl., Publ. Field Mus. Nat. Hist., Bot. Ser. 4: 259. 1929 = Witheringia asterotricha (Standl.) Hunz. (
Capsicum boninense Koidz., Fl. Symb. Orient.-Asiat.: 31. 1930 = Tubocapsicum anomalum (Franch. & Sav.) Makino (
Capsicum costaricense Standl. & C.V.Morton, Publ. Field Mus. Nat. Hist., Bot. Ser. 18: 1040. 1938 = Witheringia asterotricha (Standl.) Hunz. (
Capsicum dunalii Kuntze, Revis. Gen. Pl. 2: 450. 1891, nom. nov. for Fregirardia angustifolia Dunal = Lycianthes lycioides (L.) Hassl. (
Capsicum glandulosum Dunal, Prodr. [A. P. de Candolle] 13(1): 417.1852 = Vassobia breviflora (Sendtn.) Hunz. (
Capsicum grandiflorum Kuntze, Revis. Gen. Pl. 3[3]: 218. 1898 = Vassobia fasciculata (Miers) Hunz. (
Capsicum inaequale Vell., Fl. Flumin.: 61. 1829 (“1825”) = Solanum didymum Dunal (
Capsicum isothrix Standl., Publ. Field Mus. Nat. Hist., Bot. Ser. 18: 1567. 1938 = Witheringia solanacea L’Her. (
Capsicum lundellii C.V.Morton, Contr. Univ. Michigan Herb. 4: 25. 1940 = Witheringia affinis (C.V. Morton) Hunz. (Gentry & Standley 1974).
Capsicum macranthum Standl. & C.V.Morton, Publ. Field Mus. Nat. Hist., Bot. Ser. 18: 1042.1938 = Witheringia macrantha (Standl. & C.V.Morton) Hunz. (
Capsicum maculatum Standl. & C.V.Morton, Publ. Field Mus. Nat. Hist., Bot. Ser. 18: 1043. 1938 = Witheringia maculata (Standl. & C.V.Morton) Hunz. (
Capsicum malacophyllum Standl., Publ. Field Mus. Nat. Hist., Bot. Ser. 4: 260. 1929 = Witheringia stramoniifolia Kunth (
Capsicum multiflorum Standl. & C.V.Morton, Publ. Field Mus. Nat. Hist., Bot. Ser. 18: 1043. 1938 = Witheringia asterotricha (Standl.) Hunz. (
Capsicum silvigaudens Standl. & L.O.Williams, Ceiba 3: 57. 1952 = Witheringia meiantha (Donn. Sm.) Hunz. (
Capsicum solanaceum var. glabrescens Kuntze, Revis. Gen. Pl. 2: 450. 1891 = Witheringia solanacea L’Her. (
Capsicum solanaceum var. pubescens Kuntze, Revis. Gen. Pl. 2: 450. 1891 = Witheringia solanacea L’Her. (
Capsicum stenophyllum C.V.Morton & Standl., Publ. Field Mus. Nat. Hist., Bot. Ser. 18: 1044. 1938 = Witheringia meiantha (Donn. Sm.) Hunz. (
Capsicum subulatum Standl. & C.V.Morton, Publ. Field Mus. Nat. Hist., Bot. Ser. 18: 1044. 1938 = Witheringia stramonifolia Kunth (
Capsicum tetramerum Standl. & C.V.Morton, Publ. Field Mus. Nat. Hist., Bot. Ser. 18: 1045. 1938 = Witheringia solanacea L’Her. (
Capsicum torulosum Vell., Fl. Flumin.: 60. 1829 (“1825”) = Pombalia atropurpureum (St.Hil.) Paula-Souza (Violaceae) (
Capsicum velutinum Kuntze, Revis. Gen. Pl. 2: 448. 1891 = Athenaea velutina (Sendtn.) D’Arcy (
Capsicum viscidum Standl., Publ. Carnegie Inst. Wash. 461: 84. 1935 = Witheringia nelsonii (Fernald) Hunz. (
Acknowledgements
Firstly, we are very indebted to all colleagues and friends for their support during these years. We are also grateful to directors, curators and assistants’ curator of all herbaria cited in the text, for their assistance during our visits to the herbaria, loan of specimens or for provision of digital images and barcodes of type specimens not available in databases. Many thanks to S. Knapp for the constructive discussions about the taxonomy and nomenclature of some taxa. Special thanks to L. Ariza Espinar (CORD), C. Bräuchler (W), C. Deori (ASSAM), R. Garilleti (University of Valencia), S. Knapp (BM), O. Ryding (C) and J. de Vos (BAS) for sharing useful information to understand problematic issues of some type collections, to V. Palchetti for your help in assigning the conservation assessment of the species, to R. Deanna for taking photographs of specimens from several herbaria, especially from LE, to M. Carine (BM) and G. Bernardello (CORD) for the permission to reproduce the images of the types of C. annuum and C. mirum, respectively, to G. Beltrán, P. Bosland, L. Coca, C. dal Zovo, R. Deanna, P. Gonzáles, J. Lackey, S. Leiva González, A. Orejuela, N. Palombo and J.R. Stehmann for kindly permitting the reproduction of their personal photographs, to the artists N. de Flury, S. Montechiessi, L. Ochoa, P. Peralta, L. Ribulgo, L. Sánchez, J. de Ugarte and S. Leiva González for preparing line drawings, to G. Aburrá, M. Matesevach and V. Palchetti for designing black and white and colour illustrations, to M.F. Agra, G. Bertone, C. Cerón, M.T. Cosa, R. Deanna, L. Fernández, S. Leiva González, E. Marini, R. Minhot, C.I. Orozco, A. Orejuela, C. Reyes, X. Reyes, J.R. Stehmann, E. Trujillo Trujillo and field guides for their most valuable help and assistance in field explorations; to M. Gritti, V. Kapetanovic, J. Ponce, P. Hick, A. Pérez and P. Wiemer for technical assistance and to M. Cuasolo for facilitating the access of rare books and journals. We also thank the reviewers for their constructive and valuable comments and for the edition of the English which greatly improved this manuscript.
Financial assistance was provided by National Science Foundation, DEB 1457366; Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PID 11220170100147); Cooperación CONICET-FWF, Argentina; Secretaría de Ciencia y Tecnología, SECYT-UNC (CONSOLIDAR, 2018–2021), Argentina; Fondo para la Investigación Científica y Tecnológica, FONCyT, PICT 2015-3022, Argentina.
References
- Acquadro A, Barchi L, Portis E, Nourdine M, Carli C, Monge S, Valentino D, Lanteri S (2020) Whole genome resequencing of four Italian sweet pepper landraces provides insights on sequence variation in genes of agronomic value. Scientific Reports 10: e9189. https://doi.org/10.1038/s41598-020-66053-2
- Adedeji O, Akinniyi TA (2015) Pollen morphology of some species in the Family Solanaceae. Journal of Advanced Laboratory Research in Biology 6(4): 125–129.
- Adsersen H (1989) The rare plants of the Galápagos Islands and their conservation. Biological Conservation 47: 49–77. https://doi.org/10.1016/0006-3207(89)90019-0
- Aguilar-Meléndez A, Morell PL, Roose ML, Kim S-C (2009) Genetic diversity and structure in semiwild and domesticated chiles (Capsicum annuum, Solanaceae) from Mexico. American Journal of Botany 96(6): 1190–1202. https://doi.org/10.3732/ajb.0800155
- Aguilera PM, Debat HJ, Sánchez García Y, Martí DA, Grabiele M (2014) IAPT chromosome data 18. Taxon 63(6): 1387–1393[E1–E3]. https://doi.org/10.12705/636.37
- Albrecht E, Zhang D, Saftner RA, Stommel JR (2012a) Genetic diversity and population structure of Capsicum baccatum genetic resources. Genetic Resources and Crop Evolution 59: 517–538. https://doi.org/10.1007/s10722-011-9700-y
- Albrecht E, Zhang D, Mays AD, Saftner RA, Stommel JR (2012b) Genetic diversity in Capsicum baccatum is significantly influenced by its ecogeographical distribution. BMC Genetics 13: e68. https://doi.org/10.1186/1471-2156-13-68
- Alcorcés de Guerra N (2001) Estudios cromosómicos de cuatro selecciones de Capsicum chinense Jacq. Revista UDO Agrícola 1(1): 34–41.
- Alefeld F (1866) Landwirthschaftliche Flora. Wiegandt & Hempel, Berlin, 363 pp.
- Aleksic D (1960) The induction of tetraploidy in peppers. Arhiv za Poljoprivredne Nauke 13: 106–113.
- Aloni B, Peet M, Pharr M, Karni L (2001) The effect of high temperature and high atmospheric CO2 on carbohydrate changes in bell pepper (Capsicum annuum) pollen in relation to its germination. Physiologia Plantarum 112: 505–512. https://doi.org/10.1034/j.1399-3054.2001.1120407.x
- Al-Nowaihi AS, Mourad MM (1999) Morphological and anatomical characters of the spermoderm of certain taxa of the tribe Solaneae (Solanaceae). Taeckholmia 19(2): 157–181. https://doi.org/10.21608/taec.1999.12643
- Al-Snafi AE (2015) The pharmacological importance of Capsicum species (Capsicum annuum and Capsicum frutescens) growing in Iraq. Journal of Pharmaceutical Biology 5(3): 124–142.
- Alleemullah M, Haigh AM, Holford P (2000) Anthesis, anther dehiscence, pistil receptivity and fruit development in the Longum group of Capsicum annuum. Australian Journal of Experimental Agriculture 40: 755–762. https://doi.org/10.1071/EA99038
- American Journeys Collection (2003) Letters of Dr. Chanca on the Second Voyage of Columbus. Document N° AJ-065. Wisconsin Historical Society, Wisconsin, 35 pp.
- András S (1966) A paprika. Akadémiai Kiadó, Budapest, 392 pp.
- Andrews J (1984) Peppers. The domesticated Capsicums, University of Texas Press, Austin, 170 pp.
- Andrews J (1993) Diffusion of Mesoamerican food complex to south-eastern Europe. Geographical Review 83: 194–204. https://doi.org/10.2307/215257
- Antongiovanni M, Venticinque EM, Fonseca CR (2018) Fragmentation patterns of the Caatinga drylands. Landscape Ecology 33: 1353–1367. https://doi.org/10.1007/s10980-018-0672-6
- Arenas P, Scarpa GF (2007) Edible wild plants of the Chorote Indians, Gran Chaco, Argentina. Botanical Journal of the Linnean Society 153(1): 73–85. https://doi.org/10.1111/j.1095-8339.2007.00576.x
- Augustin B (1907) Historisch-kritische und anatomisch-entwicklungsgeschichtliche Untersuchungen uber den Paprika, Mg. Thesis, Németbogsán.
- Aza-González C, Núñez-Palenius HG, Ochoa-Alejo N (2011) Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant Cell Reports 30: 695–706. https://doi.org/10.1007/s00299-010-0968-8
- Baba VY, Rocha KR, Gomes GP, Fatima Ruas C, Ruas PM, Rodrigues R, Gonçalves LSA (2016) Genetic diversity of Capsicum chinense accessions based on fruit morphological characterization and AFLP markers. Genetic Resources and Crop Evolution 63: 1371–1381. https://doi.org/10.1007/s10722-015-0325-4
- Bachman S, Moat J, Hill AW, de Torre J, Scott B (2011) Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 150: 117–126. https://doi.org/10.3897/zookeys.150.2109
- Bailey LH (1923) Capsicum. Gentes Herbarum 1: 128–129. https://doi.org/10.1136/bmj.1.3238.128-a
- Baral JB, Bosland PW (2002) Genetic diversity of a Capsicum germplasm collection from Nepal as determined by Randomly Amplified Polymorphic DNA markers. Journal of the American Society for Horticultural Science 127(3): 316–324. https://doi.org/10.21273/JASHS.127.3.318
- Baral JB, Bosland PW (2004) Unraveling the species dilemma in Capsicum frutescens and C. chinense (Solanaceae): A multiple evidence approach using morphology, molecular analysis, and sexual compatibility. Journal of the American Society for Horticultural Science 129(6): 826–832. https://doi.org/10.21273/JASHS.129.6.0826
- Barbosa RI, Luz FJF, Nascimento-Filho HR, Maduro CB (2002) Pimentas do gênero Capsicum cultivadas em Roraima, Amazônia Brasileira. I. Espécies domesticadas. Acta Amazonica 32(2): 177–192. https://doi.org/10.1590/1809-43922002322192
- Barbosa RI, Luz FJF, Nascimento-Filho HR, Maduro CB (2006) Pimentas de Roraima (Catálogo de Referência). EDUA/Editora INPA (Série Biblioteca Científica da Amazônia), Manaus, 93 pp.
- Barbosa RI, Luz FJF, Nascimento-Filho HR, Maduro CB (2008) Peppers (Solanaceae: Capsicum) of Roraima, Brazilian Amazonia. INPA/Embrapa/MIRR, Manaus. http://agroeco.inpa.gov.br/reinaldo/RIBarbosa_ProdCient_Usu_Visitantes/2008Banco%20de%20Imagens_Pimentas-Roraima.pdf [accessed 25.09.2020]
- Barbosa RI, Mourão Júnior M, Luz FJF (2010) Morphometric patterns and preferential uses of Capsicum peppers in the State of Roraima, Brazilian Amazonia. Horticultura Brasileira 28: 477–482. https://doi.org/10.1590/S0102-05362010000400017
- Barboza GE (2011) Lectotypifications, synonymy, and a new name in Capsicum (Solanoideae, Solanaceae). PhytoKeys 2: 23–38. https://doi.org/10.3897/phytokeys.2.730
- Barboza GE (2013) Capsicum. In: Barboza GE (Coord.) Solanaceae. Flora Argentina. Vol. 13. IBODA-IMBIV, CONICET, Buenos Aires, 21–25.
- Barboza GE (2016) Capsicum. In: Bernal R, Gradstein SR, Celis M (Eds) Catálogo de Plantas y Líquenes de Colombia, Vol. 2. Universidad Nacional de Colombia-Sede Bogotá, 2404–2405.
- Barboza GE, Bianchetti L (2005) Three new species of Capsicum (Solanaceae) and a key to the wild species from Brazil. Systematic Botany 30(4): 863–871. https://doi.org/10.1600/036364405775097905
- Barboza GE, Hunziker AT (1992) Estudios sobre Solanaceae. XXXIII. El género Lycianthes en la Argentina. Darwiniana 31(1–4): 17–34.
- Barboza GE, Agra MF, Romero MV, Scaldaferro MA, Moscone EA (2011) New endemic species of Capsicum (Solanaceae) from the Brazilian caatinga: comparison with the re-circumscribed C. parvifolium. Systematic Botany 36(3): 768–781. https://doi.org/10.1600/036364411X583718
- Barboza GE, Carrizo García C, Leiva González S, Scaldaferro M, Reyes X (2019) Four new species of Capsicum (Solanaceae) from the tropical Andes and an update on the phylogeny of the genus. PLoS ONE 14(1): e0209792. https://doi.org/10.1371/journal.pone.0209792
- Barboza GE, Bianchetii L, Stehmann JR (2020a) Capsicum carassense (Solanaceae), a new species from the Brazilian Atlantic Forest. PhytoKeys 140: 125–138. https://doi.org/10.3897/phytokeys.140.47071
- Barboza GE, Carrizo García C, Scaldaferro M, Bohs L (2020b) An amazing new Capsicum (Solanaceae) species from the Andean-Amazonian Piedmont. PhytoKeys 167: 13–29. https://doi.org/10.3897/phytokeys.167.57751
- Barth OM, Duarte SG (2008) Morfologia polínica de espécies arbóreas de Solanaceae do Estado de Santa Catarina, Brasil. Hoehnea 35: 379–386. https://doi.org/10.1590/S2236-89062008000300005
- Basu SK, De AK (2003) Capsicum: historical and botanical perspectives. In: De AK (Ed.) Capsicum, the genus Capsicum. Taylor & Francis, London and New York, 1–15.
- Bauhin C (1623) Pinax theatri botanici. Sumptibus & Ludovici Regis, Basilea, 522 pp.
- Bauhin J, Cherler JH, Dominique C (1651) Historia plantarum universalis, nova, et absolutissima, cum consensu et dissensu circa eas. Ebroduni, Yverdon, 1074 pp. https://doi.org/10.5962/bhl.title.146639
- Belletti P, Marzachi C, Lanteri S (1998) Flow cytometric measurement of nuclear DNA content in Capsicum (Solanaceae). Plant Systematics and Evolution 209: 85–91. https://doi.org/10.1007/BF00991526
- Bernardello LM (1983) Estudios en Lycium (Solanaceae). III. Estructura y desarrollo de fruto y semilla en Lycium y Grabowskia. Boletín de la Sociedad Argentina de Botánica 22(1–4): 147–176.
- Bertoloni A (1838) Horti Botanici Bononiensis Plantae Nova vel minus cognitae. Emygdii ab Ulmo et J. Tiocchi, Bologna, 14 pp. [+ 5 Tab.] https://doi.org/10.5962/bhl.title.139032
- Besser WS (1811) Catalogue des plantes du Jardin botanique du Gymnase de Volhynie à Krzemieniec. Imprimerie du Gymnase, Krzemieniec [Kremenets], 117 pp.
- Besser WS (1815) Catalogue des plantes du Jardin botanique du Gymnase de Volhynie à Krzemieniec. Supplementum IV. Imprimerie du Gymnase, Krzemieniec [Kremenets], 30 pp.
- Bessis J, Guyot M (1979) An attempt to use stomatal characters in systematic and phylogenetic studies of the Solanaceae. In: Hawkes JG, Lester RN, Skelding AD (Eds) The Biology and Taxonomy of the Solanaceae. Academic Press, New York, 321–326.
- Bharath SM, Cilas C, Umaharan P (2013) Fruit trait variation in a Caribbean germplasm collection of aromatic hot peppers (Capsicum chinense Jacq.). HortScience 48: 531–538. https://doi.org/10.21273/HORTSCI.48.5.531
- Bitter G (1919) Die Gattung Lycianthes. Abhandlungen herausgegeben vom Naturwissenschaftlichen Vereins zu Bremen [preprint] 24: 292–520.
- Bitter G (1921) Aufteilung der Gattung Bassovia (im Dunalschen Sinne) zwischen Solanum, Capsicum und Lycianthes. Repertorium Specierum Novarum Regni Vegetabilis 17(492–503): 328–335. https://doi.org/10.1002/fedr.19210171913
- Bitter G (1922) Ein neues Capsicum aus der Sektion Decameris. Repertorium Specierum Novarum Regni Vegetabilis 18: 126–127. https://doi.org/10.1002/fedr.19220180413
- Bitter G (1924) Capsicum guatemalense Bitt. nov. spec. Repertorium Specierum Novarum Regni Vegetabilis 20: 377–378. https://doi.org/10.1002/fedr.19240202209
- Biurrun E, Galetto L, Anton AM, Biurrun F (2007) Plantas silvestres comestibles utilizadas en poblaciones rurales de la Provincia de La Rioja (Argentina). Kurtziana 33: 121–140.
- Blanco FM (1837) Flora de Filipinas, D. Candido Lopez, Manila, 887 pp.
- Blume CL (1826) Solanaceae. In: Bijdragen tot de Flora van Nederlandsch Indië 13: 694–708. https://doi.org/10.5962/bhl.title.115427
- Bo ML, Carrizo García C (2015) Pollen phenotyping and performance in rocoto chili (Capsicum pubescens Ruiz et Pav., Solanaceae). Grana 54(1): 37–44. https://doi.org/10.1080/00173134.2014.985606
- Bohs LA (2015) Solanaceae. In: Grayum MH, Herrera C, Zamora N (Eds) Manual de Plantas de Costa Rica. Vol. 8. Monograph in Systematic Botany of the Missouri Botanical Garden 131: 205–336.
- Bosland PW, Baral JB (2007) ‘Bhut Jolokia’-The world’s hottest known chile pepper is a putative naturally occurring interspecific hybrid. HortScience 42: 222–224. https://doi.org/10.21273/HORTSCI.42.2.222
- Bosland PW, Coon D (2015) ‘NuMex Trick-or-Treat’, a No-heat Habanero Pepper. HortScience 50(11): 1739–1740. https://doi.org/10.21273/HORTSCI.50.11.1739
- Bosland PW, Gonzalez MM (2000) The rediscovery of Capsicum lanceolatum (Solanaceae), and the importance of nature reserves in preserving cryptic biodiversity. Biodiversity and Conservation 9(10): 1391–1397. https://doi.org/10.1023/A:1008930931976
- Bosland PW, Votava EJ (2000) Peppers: Vegetable and Spice Capsicums. CABI Publishing, Wallingford, 204 pp.
- Bosland PW, Iglesias J, Bailey A (1988) Capsicum Pepper Varieties and Classification. Cooperative Extension Service-Circular 530. New Mexico State University, Las Cruces, 13 pp.
- Bosland PW, Coon D, Reeves G (2012) Trinidad Moruga Scorpion pepper is the world’s hottest measured chile pepper at more than 2 million Scoville Heat Units. HortTechnology 22: 534–538. https://doi.org/10.21273/HORTTECH.22.4.534
- Bosland PW, Coon D, Cooke PH (2015) Novel formation of ectopic (nonplacental) capsaicinoid secreting vesicles on fruit walls explains the morphological mechanism for super-hot chile peppers. Journal of the American Society of Horticultural Sciences 140: 253–256. https://doi.org/10.21273/JASHS.140.3.253
- Bostock J, Riley HT (transl) (1856) The Natural History of Pliny, vol. 4, book 20. J. Billing, London.
- Brilhante BDG, Santos T de O, Santos PHAD, Kamphorst SH, Neto JDS, Rangel LH, Valadares FV, de Almeida RN, Rodrigues R, Júnior ACS, Moulin MM (2021) Phenotypic and molecular characterization of Brazilian Capsicum germplasm. Agronomy 11(5): e854. https://doi.org/10.3390/agronomy11050854
- Britton NL (1918) Flora of Bermuda. Charles Scribner’s Sons, New York, 585 pp. https://doi.org/10.5962/bhl.title.23085
- Brown CH, Clement CR, Epps P, Luedeling E, Wichmann S (2013) The paleobiolinguistics of domesticated chili pepper (Capsicum spp.). Ethnobiology Letters 4: 1–11. https://doi.org/10.14237/ebl.4.2013.2
- Busso GS, Amaral ZP, Bianchetti L, Machado FR, Ferreira ME (2003) Genetic variability and phylogenetic analysis of Brazilian species of Capsicum. Capsicum and Eggplant Newsletter 22: 13–16.
- Cárdenas M (1969) Manual de plantas económicas de Bolivia. Imprenta Icthus, Cochabamba, 421 pp.
- Carluccio F, Saccardo F (1977) Karyotype studies in Capsicum. In: Pochard E (Ed.) Capsicum 77. Avignon-Montfavet, France, 39–50.
- Carvalho S, Bianchetti L, Costa Ribeiro S da, Lopes CA (2006) Pimentas do gênero Capsicum no Brasil. Embrapa Hortaliças Documentos 94: 1–27.
- Carvalho S, Ragassi C, Bianchetti L, Reifschneider F, Buso G, Faleiro F (2014) Morphological and genetic relationships between wild and domesticated forms of peppers (Capsicum frutescens L. and C. chinense Jacquin). Genetics and Molecular Research 13: 7447–7464. https://doi.org/10.4238/2014.September.12.11
- Carvalho S, Bianchetti L, Ragassi C, Ribeiro C, Reifschneider F, Buso G, Faleiro F (2017) Genetic variability of a Brazilian Capsicum frutescens germplasm collection using morphological characteristics and SSR markers. Genetics and Molecular Research 16(3): gmr16039689. [18 pp] https://doi.org/10.4238/gmr16039689
- Carrizo García C, Sterpetti M, Volpi P, Ummarino M, Saccardo F (2013) Wild Capsicums: identification and in situ analysis of Brazilian species. In: Lanteri S, Rotino GL (Eds) Proceedings of the XV Eucarpia Meeting on Genetics and Breeding of Capsicum and Eggplant. EUCARPIA, Torino, 205–213.
- Carrizo García C, Barfuss MHJ, Sehr EM, Barboza GE, Samuel R, Moscone EA, Ehrendorfer F (2016) Phylogenetic relationships, diversification and expansion of chili peppers (Capsicum, Solanaceae). Annals of Botany 118: 35–51. https://doi.org/10.1093/aob/mcw079
- Carrizo García C, Palombo N, Weiss-Schneeweiss H (2019) Tracing the origin of the locoto chile (Capsicum pubescens): insights through RAD-seq data. Plants, People, Planet Symposium, London.
- Carrizo García C, Fernández L, Kapetanovic V, Reyes X (2020) Rare Bolivian wild chile Capsicum eshbaughii (Solanaceae) located again: Open ending on its identity and conservation. Plant Systematics and Evolution 306: e85. https://doi.org/10.1007/s00606-020-01712-5
- Cauich O, Quezada Euán JJG, Meléndez Ramírez V, Valdovinos-Nuñez GR, Moo-Valle H (2006) Pollination of habanero pepper (Capsicum chinense) and production in enclosures using the stingless bee Nannotrigona perilampoides. Journal of Apicultural Research 45: 125–130. https://doi.org/10.1080/00218839.2006.11101330
- Cavanilles AJ (1802) Descripción de las Plantas. Imprenta Real, Madrid, 625 pp.
- Chennaveeraiah MS (1947) A preliminary note on the karyotype in Capsicum fastigiatum Bl. Current Science 16: 384–385.
- Chennaveeraiah MS, Habib AF (1966) Recent advances in the cytogenetics of Capsicums. In: Proceedings of the Autumn School in Botany, Mahabaleshwar, 69–90.
- Chiou KL, Hastorf CA (2014) A systematic approach to species–level identification of chile pepper (Capsicum spp.) seeds: Establishing the groundwork for tracking the domestication and movement of chile peppers through the Americas and beyond. Economic Botany 68(3): 316–336. https://doi.org/10.1007/s12231-014-9279-2
- Chodat R (1902) Plantae Hasslerianae I. Bulletin de l’Herbier Boissier, sér. 2, 2(9): 811–815.
- Chodat R, Hassler E (1903) Plantae Hasslerianae II. Bulletin de l’Herbier Boissier, sér. 2, 4(1): 77–92.
- Clement CR, de Cristo-Araújo M, Coppens d’Eeckenbrugge G, Alves Pereira A, Picanço-Rodrigues D (2010) Origin and Domestication of Native Amazonian Crops. Diversity 2: 72–106. https://doi.org/10.3390/d2010072
- Clusius C (1611) Curae posteriores post mortem. Antwerp.
- Cocucci AA (1999) Evolutionary radiation in Neotropical Solanaceae. In: Nee M, Symon DE, Lester RN, Jessop JP (Eds) Solanaceae IV. Royal Botanic Garden, Kew, 9–22.
- Cochran HL (1938) A morphological study of flower and seed development in pepper. Journal of Agricultural Research 56: 395–419.
- Costa G do N, Silva BMP da, Lopes ÂC de A, Carvalho LCB, Gomes RLF (2019) Selection of pepper accessions with ornamental potential. Revista Caatinga 32(2): 566–574. https://doi.org/10.1590/1983-21252019v32n230rc
- Crispim JG, Rêgo ER, Rêgo MM, Nascimento NFF, Barroso PA (2017) Stigma receptivity and anther dehiscence in ornamental pepper. Horticultura Brasileira 35: 609–612. https://doi.org/10.1590/s0102-053620170421
- Cruz DO, Oliveira de Campos LA de (2007) Biologia floral e polinização de pimenta malagueta (Capsicum frutescens L., Solanaceae): um estudo de caso. Acta Scientiarum Biological Sciences 29: 375–379. https://doi.org/10.4025/actascibiolsci.v29i4.877
- D’Agostino N, Tamburino R, Cantarella C, De Carluccio V, Sannino L, Cozzolino S, Cardi T, Scotti N (2018) The complete plastome sequences of eleven Capsicum genotypes: Insights into DNA variation and molecular evolution. Genes 9(10): e503. https://doi.org/10.3390/genes9100503
- D’Arcy WG (1970) Jacquin names, some notes on their typification. Taxon 19(4): 554–560. https://doi.org/10.2307/1218948
- D’Arcy WG (1974) [’1973’] Solanaceae, Flora of Panama. Part 9. Annals of the Missouri Botanical Garden 60: 573–780. https://doi.org/10.2307/2395139
- D’Arcy WG (1986) The calyx in Lycianthes and some other genera. Annals of the Missouri Botanical Garden 73: 117–127. https://doi.org/10.2307/2399143
- D’Arcy WG (1991) The Solanaceae since Birmingham, 1976 with a review of its biogeography. In: Hawkes JG, Lester RN, Nee M, Estrada N (Eds) Solanaceae III. Taxonomy, Chemistry, Evolution. Royal Botanic Garden, Kew, 75–137.
- D’Arcy WG, Averett JE (1996) Recognition of tribes Capsiceae and Physaleae, subfamily Solanoideae, Solanaceae. Phytologia 80(4): 273–275. https://doi.org/10.5962/bhl.part.15536
- D’Arcy WG, Eshbaugh WH (1973) The name for the common bird pepper. Phytologia 25(6): 350.
- D’Arcy WG, Eshbaugh WH (1974) New World Peppers (Capsicum-Solanaceae) North of Colombia: A Résumé. Baileya 19(3): 93–105.
- D’Arcy WG, Gentry JL, Averett JE (1981) Recognition of Brachistus (Solanaceae). Annals of the Missouri Botanical Garden 68(1): 226–227. https://doi.org/10.2307/2398829
- D’Arcy WG, Keating RC, Zhang Z-Y, Peng C-I (2001) The genus Tubocapsicum (Solanaceae). Botanical Bulletin of Academia Sinica 42: 67–84.
- Dammer KW (1905) Solanaceae I. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 36: 384–388.
- Datta PC (1968) Karyology of Indian varieties of Capsicum annuum Linn. (Solanaceae). Caryologia 21: 121–126. https://doi.org/10.1080/00087114.1968.10796291
- Dave YS (1986) Taxonomic significance and use of the pericarp structure of Capsicums (family: Solanaceae). Journal of Pant Anatomy and Morphology 3(1): 85–89.
- Dave YS, Patel ND, Rao KS (1979) Developmental and anatomical studies in the pericarp of Capsicums. Flora 168: 263–275. https://doi.org/10.1016/S0367-2530(17)31918-7
- da Silva Monteiro CE, Santana Pereira TN, Pereira de Campos K (2011) Reproductive characterization of interspecific hybrids among Capsicum species. Crop Breeding and Applied Biotechnology 11: 241–249. https://doi.org/10.1590/S1984-70332011000300006
- de Candolle AP (1813) Catalogus plantarum horti botanici monspeliensis. J. Martel, Montpellier, 155 pp.
- De Wildeman EAJ (1922) Plantae Bequaertianae. Vol. 1. Lechevalier, Paris, 593 pp.
- Dean EA (1995) Systematics and ethnobotany of Lycianthes series Meizonodontae. PhD. Thesis, University of California, Berkeley.
- Dean EA, Reyes M, Fauré R, Walden GK, Canington D, Brandon D, McNair DM (2017) Identification of the species of Lycianthes series Tricolores (Capsiceae, Solanaceae). Systematic Botany 42(1): 191–209. https://doi.org/10.1600/036364417X694566
- Dean EA, Poore J, Anguiano-Constante MC, Nee MH, Kang H, Starbuck T, Rodrígues A, Conner M (2020) The genus Lycianthes (Solanaceae, Capsiceae) in Mexico and Guatemala. PhytoKeys 168: 1–333. https://doi.org/10.3897/phytokeys.168.51904
- Debat HJ, Aguilera PM, Grabiele M (2016) IAPT chromosome data 23. Taxon 65(6): 1455–1458[E4–E5].
- Debat HJ, Aguilera PM, Grabiele M (2017) IAPT chromosome data 26. Taxon 66(6): 1487–1499[E4–E6]. https://doi.org/10.12705/666.30
- Delile R (1849) Index Seminum Horti Botanici Monspeliensis. Annales des Sciences Naturelles, Botanique, sér. 3, 12: 365–367.
- Del Vitto LA, Petenatti EM, Petenatti ME (1997) Recursos Herbolarios de San Luis (República Argentina). Primera Parte: Plantas Nativas. Multequina 6: 49–66.
- DeWitt D, Bosland PW (1997) Peppers of the World: An Identification Guide. Ten Speed Press, Portland, 256 pp.
- DeWitt D, Bosland PW (2009) The Complete Chile Pepper Book: A gardener’s guide to choosing, growing, preserving, and cooking. Timber Press, Inc., Portland, 336 pp.
- Dharamadhaj P, Prakash N (1978) Development of the anther and ovule in Capsicum L. Australian Journal of Botany 26: 433–439. https://doi.org/10.1071/BT9780433
- Dias GB, Gomes VM, Moraes TMS, Zottich UP, Rabelo GR, Carvalho AO, Moulin M, Gonçalves LSA, Rodrigues R, Da Cunha M (2013) Characterization of Capsicum species using anatomical and molecular data. Genetics and Molecular Research 12: 6488–6501. https://doi.org/10.4238/2013.February.28.29
- Dierbach JH (1829) Uebersicht der bekannten Arten des Spanischen Pfeffers. Archive des Apotheker-Vereins im Nordlichen Teutschland 30(1): 19–31. https://doi.org/10.1002/ardp.18290300103
- Dillehay TD, Goodbred S, Pino M, Vásquez Sánchez VF, Rosales Tham T, Adovasio J, Collins MB, Nehterly PJ, Hastorf CA, Chiou KL, Piperno D, Rey I, Velchoff N (2017) Simple technologies and diverse food strategies of the Late Pleistocene and Early Holocene at Huaca Prieta, Coastal Peru. Science Advances 3: e1602778. https://doi.org/10.1126/sciadv.1602778
- Dixit PD (1931) A cytological study of Capsicum annuum. Indian Journal of Agricultural Sciences 1: 419–433.
- Djian-Caporalino C, Lefebvre V, Sage-Daubèze A-M, Palloix A (2007) Capsicum. In: Singh RJ (Ed.) Genetic resources, chromosome engineering, and crop improvement. Vegetable Crops. Vol. 3. CRC Press, Taylor & Francis Group, Boca Raton, 185–235. https://doi.org/10.1201/9781420009569.ch6
- Dodoens R (1554) Cruijdeboeck. Jan van der Loe, Antwerp. http://leesmaar.nl/cruijdeboeck/index.htm [accessed 9.03.2020]
- Dodoens R (1583) Stirpium historiae pemptades sex, sive libri XXX. C. Plantini, Antwerp. https://doi.org/10.5962/bhl.title.149774
- Dorland RE, Went FW (1947) Plant growth under controlled conditions. VIII. Growth and fruiting of the Chili Pepper (Capsicum annuum). American Journal of Botany 34(8): 393–401. http://www.jstor.org/stable/2437149
- Dunal MF (1816) Solanorum generumque affinium synopsis. Renaud, Montpellier, 51 pp.
- Dunal MF (1852) Solanaceae. In: Candolle AP de (Ed. ) Prodromus systematis naturalis regni vegetabilis 13(1): 1–690.
- Egawa Y, Tanaka M (1984) Cytogenetical relationships among three species of chili peppers, Capsicum chinense, C. frutescens and C. baccatum. Japanese Journal of Breeding 34(1): 50–56. https://doi.org/10.1270/jsbbs1951.34.50
- Emboden Jr WA (1961) A preliminary study of the crossing relationships of Capsicum baccatum. Butler University Botanical Studies 14: 1–5.
- Erickson AN, Markhart AH (2002) Flower developmental stage and organ sensitivity of bell pepper (Capsicum annuum L.) to elevated temperature. Plant, Cell and Environment 25: 123–130. https://doi.org/10.1046/j.0016-8025.2001.00807.x
- Erwin AT (1929) A systematic study of the peppers Capsicum frutescens L. Proceedings of the American Society for Horticultural Science 26: 128–131.
- Eshbaugh WH (1964) A numerical taxonomic and cytogenetic study of certain species of the genus Capsicum. PhD. Thesis, Indiana University, Bloomington.
- Eshbaugh WH (1968) A nomenclatural note on the genus Capsicum. Taxon 17: 51–52. https://doi.org/10.2307/1216151
- Eshbaugh WH (1970) A biosystematics and evolutionary study of Capsicum baccatum (Solanaceae). Brittonia 22: 31–43. https://doi.org/10.2307/2805720
- Eshbaugh WH (1976) Genetic and biochemical systematic studies of chili peppers (Capsicum-Solanaceae). Bulletin of the Torrey Botanical Club 102(6): 396–403. https://doi.org/10.2307/2484766
- Eshbaugh WH (1979) Biosystematic and evolutionary study of the Capsicum pubescens complex. National Geographic Society Research Reports, 1970 Projects: 143–162.
- Eshbaugh WH (1980) The taxonomy of the genus Capsicum (Solanaceae). Phytologia 47(3): 153–166. https://doi.org/10.5962/bhl.part.4455
- Eshbaugh WH (1982) Variation and evolution in Capsicum eximium (Solanaceae). Baileya 21(4): 193–198.
- Eshbaugh WH (1983) The genus Capsicum (Solanaceae) in Africa. Bothalia 14: 845–848. https://doi.org/10.4102/abc.v14i3/4.1252
- Eshbaugh WH (1993) Peppers: History and exploitation of a Serendipitous new crop discovery. In: Janick J, Simon JE (Eds) New Crops. John Wiley and Sons, New York, 132–139.
- Eshbaugh WH (2012) The taxonomy of the genus Capsicum. In: Russo VM (Ed.) Peppers: Botany, Production and Uses. CAB International, Cambridge, 13–28. https://doi.org/10.1079/9781845937676.0014
- Eshbaugh WH, Smith PG (1971) A new variety of chili pepper, Capsicum eximium var. tomentosum (Solanaceae). Baileya 18: 13–16.
- Eshbaugh WH, Smith PG, Nickrent DL (1983) Capsicum tovarii (Solanaceae), a new species of pepper from Peru. Brittonia 35(1): 55–60. https://doi.org/10.2307/2806051
- Fay MF, Thomas VE, Knapp S (2007) 602. Mellissia begoniifolia. Curtis’s Botanical Magazine 24: 243–250. https://doi.org/10.1111/j.1467-8748.2007.00601.x
- Ferraz RM, Ragassi CF, Heinrich AG, Lima MF, PeixotoJ, Reifschneider FJB (2016) Caracterização morfoagronômica preliminar de acessos de pimentas cumari. Horticultura Brasileira 34: 498–506. https://doi.org/10.1590/s0102-053620160408
- Ferreira Pinto CMF, dos Santos IC, de Araujo FF, da Silva TP (2016) Pepper importance and growth (Capsicum spp.). In: do Rêgo ER, do Rêgo MM, Finger FL (Eds) Production and Breeding of Chilli Peppers (Capsicum spp. ). Springer International Publishing, Switzerland, 1–26. https://doi.org/10.1007/978-3-319-06532-8_1
- Filipov A (1994) Medicinal plants of the Pilagá of Central Chaco. Journal of Ethnopharmacology 44: 181–193. https://doi.org/10.1016/0378-8741(94)01185-0
- Filippa EM, Bernardello LM (1992) Estructura y desarrollo de fruto y semilla en especies de Athenaea, Aureliana y Capsicum (Solaneae, Solanaceae). Darwiniana 31(1–4): 137–150.
- Fingerhuth A (1832) Monographia generis Capsici. Arnz & Comp., Düsseldorf, 36 pp.
- Fonseca CR, Antongiovanni M, Matsumoto M, Bernard E, Venticinque EM (2017) Conservation opportunities in the Caatinga. In: Silva JMC, Leal IR, Tabarelli M (Eds) Caatinga - the largest tropical dry forest region in South America. Springer, Cham, 429–444. https://doi.org/10.1007/978-3-319-68339-3_17
- Forman LL (1997) Notes concerning the typification of names of William Roxburgh’s species of Phanerogams. Kew Bulletin 52(3): 513–534. https://www.jstor.org/stable/4110285
- Freire de Carvalho L (1985) Flora Fanerogámica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Hoehnea 12: 67–85.
- Freitas SR, Neves CL, Chernicharo P (2006) Tijuca National Park: two pioneering restorationist initiatives in Atlantic forest in southeastern Brazil. Brazilian Journal of Biology 66(4): 975–982. https://doi.org/10.1590/S1519-69842006000600004
- Fricke EC, Simon MJ, Reagan KM, Levey DJ, Riffell JA, Carlo TA, Tewksbury JT (2013) When condition trumps location: Seed consumption by fruit-eating birds removes pathogens and predator attractants. Ecology Letters 16: 1031–1036. https://doi.org/10.1111/ele.12134
- Fridvalszky L, Nagy J (1966) The differentiation, microscopic and submicroscopic structure of giant cell wall in the pericarp of Capsicum annuum L. Acta Agronomica Academiae Scientiarum Hungaricae 15: 69–78.
- Friesen Ratzlaff V (2017) Plantas medicinales del Gran Chaco. Facultad de Ciencias Agrarias, Universidad Nacional de Asunción, San Lorenzo-Paraguay, 128 pp.
- Fuchs L (1542) De Historia Stirpium Commentarii Insignes. M. Isingrin, Basel, 896 pp.
- Fujiwake H, Suzuki T, Iwai K (1980) Intracellular localization of capsaicin and its analogues in Capsicum fruit II. The vacuole as the intracellular accumulation site of capsaicinoid in the protoplast of Capsicum fruit. Plant and Cell Physiology 21(6): 1023–1030. https://doi.org/10.1093/oxfordjournals.pcp.a076070
- Fujiwake H, Suzuki T, Iwai K (1982) Intracellular distributions of enzymes and intermediates involved in biosynthesis of capsaicin and its analogues in Capsicum fruits. Agricultural and Biological Chemistry 46(11): 2685–2689. https://doi.org/10.1080/00021369.1982.10865495
- Fundación PROINPA (2007) Catálogo de ají de ecotipos conservados en campo de agricultores. ONG DEMA and Fundación PROINPA, Sucre.
- Gahungu A, Ruganintwali E, Karangwa E, Zhang X, Mukunzi D (2011) Volatile compounds and capsaicinoid content of fresh hot peppers (Capsicum chinense) Scotch Bonnet variety at red stage. Advance Journal of Food Science and Technology 3(3): 211–218.
- Garilleti R (1993) Herbarium Cavanillesianum seu enumeratio plantarum exsiccatarum aliquo modo ad novitates cavanillesianas pertinentium, qua in Horti Regii Matritensis atque Londinensis Societatis Linnaeanae herbariis asservantur. Fonlqueria 38: 6–248.
- Garruti D dos S, Pinto N de O, Alves VCC, Penha MFA, Tobaruela E de C, Araújo ÍM (2013) Volatile profile and sensory quality of new varieties of Capsicum chinense pepper. Food Science and Technology 33(Suppl. 1): 102–108. https://doi.org/10.1590/S0101-20612013000500016
- Gentry Jr JL, Standley PC (1974) Solanaceae. In: Gentry Jr JL, Standley PC (Eds) Flora of Guatemala. Fieldiana, Bot. 24(10/1–2), 1–151.
- GeoCAT (2020) Geospatial Conservation Assessment Tool. https://www.kew.org/science/projects/geocat-geospatial-conservation-assessment-tool [accessed 17.02.2021]
- Gepts P (2004) Crop domestication as a long-term selection experiment. Plant Breeding Reviews 24: 1–44. https://doi.org/10.1002/9780470650288.ch1
- Gomez Platero AM, Palma Ehrichs V (2011) Leyendas de la Amazonia brasileña. Consejería de Educación de la Embajada de España, Secretaría General Técnica, Brasilia-DF, 78 pp.
- González Estrada T, Casanova Chávez C, Gutiérrez Pacheco L, Torres Tapia L, Contreras Martín F, Peraza Sánchez S (2010) Chiles cultivados en Yucatán. In: Durán R, Méndez M (Eds) Biodiversidad y Desarrollo Humano en Yucatán. CICY CONABIO, SEDUMA, Yucatán, 342–344.
- Grabiele M, Aguilera PM, Debat HJ, Forni-Martins ER, Martí DA (2014) IAPT chromosome data 18. Taxon 63(6): 1387–1393[E6–E8]. https://doi.org/10.12705/636.37
- Greenleaf WH (1947) A spontaneous tetraploid hybrid in pepper (Capsicum frutescens). Proceedings of the American Society for Horticultural Science 49: 231–232.
- Greenman JM (1903) New and otherwise noteworthy Angiosperms from Mexico and Central America. Proceedings of the America Academy of Arts and Sciences 39(5): 69–120. https://doi.org/10.2307/20021858
- Guerra M (2012) Cytotaxonomy: the end of the childhood. Plant Biosystems 146(3): 703–710.
- Guerra-García A, Piñero D (2017) Current approaches and methods in plant domestication studies. Botanical Sciences 95(3): 345–362. https://doi.org/10.17129/botsci.1209
- Guillen NG, Tito R, Mendoza NG (2018) Capsaicinoids and pungency in Capsicum chinense and Capsicum baccatum fruits. Pesquisa Agropecuária Tropical 48(3): 237–244. https://doi.org/10.1590/1983-40632018v4852334
- Gunn CR, Gaffney FB (1974) Seed characteristics of 42 economically important species of Solanaceae in the United States. USDA Technical Bulletin 1471: 1–33.
- Guzmán Díaz FA, Dean E, Bohs L (2009) Hot and not so hot: Phylogenetic relationships in Capsicum and Lycianthes (Solanaceae). Botany 2009, Annual Meeting of ABLS, AFS, ASPT and BSA, Salt Lake City [abstract].
- Gyorffy B (1939) Tetraploid paprika. Acta Universitatis Szegediensis. Sectio scientiarum naturalium. Pars Bot. 5: 30–38.
- Halbritter H (2016a) Capsicum annuum. In: PalDat - A palynological database. https://www.paldat.org/pub/Capsicum_annuum/300445 [accessed 06.06.2020]
- Halbritter H (2016b) Capsicum pubescens. In: PalDat - A palynological database. https://www.paldat.org/pub/Capsicum_pubescens/302299 [accessed 06.06.2020]
- Halsted BD, Growth B, Robinson M, Owen E, Groth M (1912) A study of peppers. Report of the Botanical Department of the New Jersey Agricultural College Experiment Station. State Gazette Publishing Co., Trenton, New Yersey, 338–359.
- Hanausek TF (1888) Ueber die Samenhautepidermis der Capsicum-Arten. Berichte der Deutschen Botanischen Gesellschaft 6: 329–332.
- Hartwich E (1894) Über die Epidermis der Samenschale von Capsicum. Pharmacie Post Wien 27: 609, 633.
- Hazen-Hammond S (1993) Chile pepper fever. Voyageur Press, INC., Minnesota, 127 pp. https://doi.org/10.1057/9780230374706_12
- Heiser Jr CB, Smith PG (1948) Observations on another species of cultivated pepper, Capsicum pubescens R & P. Proceedings of the American Society of Horticultural Science 52: 331–335.
- Heiser Jr CB, Smith PG (1958) New species of Capsicum from South America. Brittonia 10(4): 194–201. https://doi.org/10.2307/2804950
- Heiser Jr CB, Pickersgill B (1969) Names for the cultivated Capsicum species (Solanaceae). Taxon 18: 277–283. https://doi.org/10.2307/1218828
- Heiser Jr CB, Pickersgill B (1975) Names for the bird peppers [Capsicum-Solanaceae]. Baileya 19: 151–156.
- Hiern WP (1877) Solanaceae, Acanthaceae, Gesneraceae, Verbenaceae. In: Warming E (Ed.) Symbolae ad floram Brasiliae centralis cognoscendam. Videnskabelige meddelelser fra den Naturhistoriske Forening i Kjøbenhavn 1877–1878, 37–108
- Hornemann JW (1813) Hortus Regius Botanicus Hafniensis. In Usum Tyronum et Botanophilorum, Part 1. E.A.H. Möller, Hauniae [Copenhagen], 436 pp.
- Hornemann JW (1819) Hortus Regius Botanicus Hafniensis. In Usum Tyronum et Botanophilorum, Suppl. Typis Schultzii, Hauniae [Copenhagen], 172 pp.
- Hunziker AT (1950) Estudios sobre Solanaceae. I: Sinopsis de las especies silvestres de Capsicum de Argentina y Paraguay. Darwiniana 9: 225–247.
- Hunziker AT (1956) Synopsis of the genus Capsicum. Huitième Congrès International de Botanique. Paris. Comptes Rendus des Séances, Rapports et Communications 1954, sect. 4: 73–74.
- Hunziker AT (1961) Estudios sobre Solanaceae III. Notas sobre los géneros Physalis L. y Capsicum L., con la descripción de dos nuevas especies sudamericanas. Kurtziana 1: 207–216.
- Hunziker AT (1969a) Estudios sobre Solanaceae. V. Contribución al conocimiento de Capsicum y géneros afines (Witheringia, Acnistus, Athenaea, etc.). Primera parte. Kurtziana 5: 101–179.
- Hunziker AT (1969b) Estudios sobre Solanaceae. VI. Contribución al conocimiento de Capsicum y géneros afines (Witheringia, Acnistus, Athenaea, etc.). Segunda Parte. Kurtziana 5: 393–399.
- Hunziker AT (1971) Estudios sobre Solanaceae. VII. Contribución al conocimiento de Capsicum y géneros afines (Witheringia, Acnistus, Athenaea, etc.). Tercera Parte. Kurtziana 6: 241–259.
- Hunziker AT (1979) South American Solanaceae: a synoptic survey. In: Hawkes JG, Lester RN, Skelding AD (Eds) The Biology and Taxonomy of the Solanaceae. Academic Press, New York, 49–85.
- Hunziker AT (1982) Revisión sinóptica de Acnistus. Kurtziana 15: 81–102.
- Hunziker AT (1984) Estudios sobre Solanaceae. XIX. Sinopsis de Vassobia. Kurtziana 17: 91–118.
- Hunziker AT (1987) Studies on Solanaceae XXI. A preliminary synopsis of Cuatresia. Opera Botanica 92: 73–82.
- Hunziker AT (1998) Estudios sobre Solanaceae. XLVI. Los ajíes silvestres de Argentina (Capsicum). Darwiniana 36: 201–203.
- Hunziker AT (2001) Genera Solanacearum. The genera of Solanaceae illustrated, arranged according to a new system. A. R. G. Gantner Verlag K. -G, Ruggell, 500 pp.
- Hunziker AT, Barboza GE (1990) Estudios sobre Solanaceae XXX. Revision de Aureliana. Kurtziana 30: 95–113.
- Hunziker AT, Barboza GE (1998) Estudios sobre Solanaceae XLV. Sobre la presencia de Exodeconus en Argentina y una novedad en Capsicum baccatum. Kurtziana 26: 23–31.
- Huskins CL, La-Cour L (1930) Chromosome numbers in Capsicum. The American Naturalist 64: 382–383. https://doi.org/10.1086/280324
- Ibiza VP, Blanca J, Cañizares J, Nuez F (2012) Taxonomy and genetic diversity of domesticated Capsicum species in the Andean region. Genetic Resources and Crop Evolution 59: 1077–1088. https://doi.org/10.1007/s10722-011-9744-z
- Idu M, Ogbe FM (1997) Studies on the morphology of Capsicum species. Journal of Plant Anatomy and Morphology 7(2): 114–123.
- Indira C, Susan A (1977) Morphological and cytological studies on a radiation induced polyploid in Capsicum annum Linn. Cytologia 42: 371–375. https://doi.org/10.1508/cytologia.42.371
- IPGRI AVRDC CATIE (1995) Descriptors for Capsicum (Capsicum spp.). International Plant Genetic Resources Institute, Rome, Italy. the Asian Vegetable Research and Development Center, Taipei, Taiwan, and the Centro Agronómico Tropical de Investigación y Enseñanza, Turrialba, 37 pp.
- Irish HC (1898) Revision of the genus Capsicum with special reference to garden varieties. Annual Report of the Missouri Botanical Garden 9: 53–110. https://doi.org/10.2307/2992137
- Ishikawa K (2001) Tetraploid bell pepper shows high in vitro pollen germination at 15 °C. HortScience 36(7): 1336–1336. https://doi.org/10.21273/HORTSCI.36.7.1336
- Ishikawa K, Mishiba K, Yoshida H, Nunomura O (1997) Establishment of tetraploid plants of Capsicum annuum L. by colchicine treatment with the analysis of flow cytometry. Capsicum Eggplant Newsletter 16: 44–47.
- IUCN (2022) Standards and Petitions Committee. 2022. Guidelines for Using the IUCN Red List Categories and Criteria. Version 15. Prepared by the Standards and Petitions Committee. [Downloadable from] https://www.iucnredlist.org/documents/RedListGuidelines.pdf [accessed: 1.2.2022]
- Iwai K, Suzuki T, Fujiwake H (1979) Formation and accumulation of pungent principle of hot pepper fruits, capsaicin and its analogues, in Capsicum annuun var. annuun cv. Karayatsubusa at different growth stages after flowering. Agricultural and Biological Chemistry 43(12): 2493–2498. https://doi.org/10.1271/bbb1961.43.2493
- Jacquin NJ (1776) Hortus Botanicus Vindobonensis. Typis Josephi Michaelis Gerold, Wien, 52 pp.
- Jacquin NJ (1809) Fragmenta Botanica. Typis Mathiae Andreae Schmidt, Wien, 86 pp.
- Jäger M, Jiménez A, Amaya K [Comp] (2013) Las cadenas de valor de los ajíes nativos de Bolivia. Compilación de los estudios realizados dentro del marco del proyecto “Rescate y Promoción de Ajíes Nativos en su Centro de Origen” para Bolivia. Bioversity International, Cali, Colombia, 90 pp.
- Jarret RL (2007) Morphologic variation for fruit characteristics in the USDA/ARS Capsicum baccatum L. germplasm collection. HortScience 42(5): 1303–1305. https://doi.org/10.21273/HORTSCI.42.5.1303
- Jarret RL, Dang P (2004) Revisiting the waxy locus and the Capsicum annuum L. complex. Georgia Journal of Science 62: 117–133.
- Jarret RL, Barboza GE, da Costa Batista FR, Berke T, Chou Y-Y, Hulse-Kemp A, Ochoa-Alejo N, Tripodi P, Veres A, Carrizo García C, Csillery G, Huang Y-K, Kiss E, Kovacs Z, Kondrak M, Arce-Rodriguez ML, Scaldaferro MA, Szoke A (2019) Capsicum. An Abbreviated Compendium. Journal of the American Society for Horticultural Science 144(1): 3–22. https://doi.org/10.21273/JASHS04446-18
- Jensen RJ, McLeod MJ, Eshbaugh WH, Guttman SI (1979) Numerical taxonomic analyses of allozymic variation in Capsicum (Solanaceae). Taxon 28(4): 315–327. https://doi.org/10.2307/1219739
- Jha TB, Dafadar A, Ghorai A (2012) New genetic resource in Capsicum L. from eastern Himalayas. Plant Genetic Resources 10: 141–144. https://doi.org/10.1017/S1479262112000135
- Johansen DA (1940) Plant Microtechnique. McGraw-Hill Book Co., New York, 523 pp.
- Johnston JR (1905) New plants from the Islands of Margarita and Coche, Venezuela. Proceedings of the American Academy of Arts and Sciences 40: 683–698. https://doi.org/10.2307/20022013
- Jordt S-E, Julius D (2002) Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 108(3): 421–430. https://doi.org/10.1016/S0092-8674(02)00637-2
- Kafizadeh N, Carapetian J, Manouchehri Kalantari K (2008) Effects of heat stress on pollen viability and pollen tube growth in pepper. Research Journal of Biological Sciences 3: 1159–1162.
- Kamvorn W, Techawongstien S, Techawongstien S, Theerakulpisut P (2014) Compatibility of inter-specific crosses between Capsicum chinense Jacq. and Capsicum baccatum L. at different fertilization stages. Scientia Horticulturae 179: 9–15. https://doi.org/10.1016/j.scienta.2014.09.003
- Karatela Y, Gill LS (1986) Observation on the developmental studies of stomatal differentiation in the epidermis of Solanaceae. Feddes Repertorium 97: 303–311. https://doi.org/10.1002/fedr.4910970512
- Kayani S, Hussain M, Ahmad M, Zafar M, Sultana S, Butt MA, Ali S, Shah GM, Mir S (2018) Scanning Electron Microscopy (SEM) and Light Microscopy (LM)-based palyno-morphological views of Solanaceae in Western Himalaya. Microscopy Research and Technique 2018: 1–12. https://doi.org/10.1002/jemt.23097
- Kim M, Kim J, Yoon M, Choi D-I, Lee K-M (2004) Origin of multicellular pollen and pollen embryos in cultured anthers of pepper (Capsicum annuum). Plant Cell, Tissue and Organ Culture 77: 63–72. https://doi.org/10.1023/B:TICU.0000016506.02796.6a
- Knapp S, Barboza GE, Romero MV, Vignoli-Silva M, Giacomin LL, Stehmann JR (2015) Identification and lectotypification of the Solanaceae from Vellozo’s Flora Fluminensis. Taxon 64(4): 822–836. https://doi.org/10.12705/644.14
- Knapp S, Barboza GE, Bohs L, Särkinen T (2019) A revision of the Morelloid Clade of Solanum L. (Solanaceae) in North and Central America and the Caribbean. PhytoKeys 123: 1–144. https://doi.org/10.3897/phytokeys.123.31738
- Knapp S, Chiarini F, Cantero JJ, Barboza GE (2020) The Morelloid clade of Solanum L. (Solanaceae) in Argentina: nomenclatural changes, three new species and an updated key to all taxa. PhytoKeys 164: 33–66. https://doi.org/10.3897/phytokeys.164.54504
- Kochieva EZ, Ryzhova NN, Van Dooijeweert W, Boukema IW, Arens P, Voorrips RE (2004) Assessment of genetic relationships in the genus Capsicum using different DNA marker systems. In: Voorrips RE (Ed.) Proceedings of the XIIth EUCARPIA Meeting on Genetics and Breeding of Capsicum and Eggplant. European Association for Research on Plant Breeding (EUCARPIA), Wageningen, 44–50.
- Kollmannsberger H, Rodríguez-Burruezo A, Nitza S, Nuez F (2011) Volatile and capsaicinoid composition of ají (Capsicum baccatum) and rocoto (Capsicum pubescens), two Andean species of chile peppers. Journal of the Science of Food and Agriculture 91: 1598–1611. https://doi.org/10.1002/jsfa.4354
- Konecsni I (1971) Contribution to the anatomy of Capsicum annuum L. III. Comparative study of the endocarp. Acta Agronomica Academiae Scientiarum Hungaricae 20(1–2): 3–15.
- Kong M-J, Lee J-S, Hong S-P (2011) Comparative seed morphology of Solanaceae in Korea. Korean Journal of Plant Taxonomy 41: 35–48. https://doi.org/10.11110/kjpt.2011.41.1.035
- Kraft KH, Brown CH, Nabhan GP, Luedeling E, Luna Ruiz J de J, d’Eeckenbrugge GC, Hijmans RJ, Gepts P (2014) Multiple lines of evidence for the origin of domesticated chili peppers, Capsicum annuum, in Mexico. Proceedings of the National Academy of Sciences 111: 6165–6570. https://doi.org/10.1073/pnas.1308933111
- Kraus J, De Sousa H, Rezende M, Castro N, Vecchi C, Luque R (1998) Astra Blue and Basic Fuchsin double staining of plant materials. Biotechnic and Histochemistry 73: 235–243. https://doi.org/10.3109/10520299809141117
- Kristjansson K, Rasmussen K (1991) Pollination of sweet pepper (Capsicum annuum L.) with the solitary bee Osmia cornifrons (Radoszkowski). Acta Horticulturae 288: 173–179. https://doi.org/10.17660/ActaHortic.1991.288.24
- Krstić B, Merkulov J, Gvozdenović D, Pajević S (2001) Anatomical and physiological characteristics of seed in pepper (Capscium annuum L.) varieties. Acta Agronomica Hungarica 49(3): 221–229. https://doi.org/10.1556/AAgr.49.2001.3.2
- Kuester B, Regel E, Rach L, Herder F [Eds] (1859) Annotationes Botanicae. Index Seminum [St. Petersburg (Petropolitanus)] 1858: 23–54.
- Kulkarni M, Borse T (2010) Induced polyploidy with gigas expression for root traits in Capsicum annuum (L.). Plant Breeding 129: 461–464. https://doi.org/10.1111/j.1439-0523.2009.01696.x
- Kunth KS (1818) Nova Genera et Species Plantarum (quarto ed.) [H.B.K.] Vol. 3. J. Cramer, Weinheim, 456 pp.
- Kuntze CEO (1891) Revisio generum plantarum. Vol. 2. Arthur Felix, Leipzig; Dulau & Co., London, 1011 pp.
- Kumar OA, Raja Rao KGR (2003) Cytomorphological studies in Gamma-ray induced autotriploids of Capsicum annuum L. Cytologia (Tokyo) 68: 45–50. https://doi.org/10.1508/cytologia.68.45
- Kumar VR, Kumar OA, Venkateswara Rao Y, Raja Rao KG (2004) Effect of selection for increased seed set and meiotic behaviour in colchicine induced autotetraploid chili pepper (Capsicum annuum L.). In: Voorrips RE (Ed.) Proceedings of the XIIth EUCARPIA Meeting on Genetics and Breeding of Capsicum and Eggplant. European Association for Research on Plant Breeding (EUCARPIA), Wageningen, 221.
- Kuriachan P (1981) A cytogenetic study of the wild and cultivated varieties of Capsicum baccatum L. Journal of the Indian Botanical Society. Madras 4(1): 27–32.
- Kurian AL, Starks AN (2002) HPLC analysis of capsaicinoids extracted from whole orange habanero chili peppers. Journal of Food Science 67(3): 956–962. https://doi.org/10.1111/j.1365-2621.2002.tb09435.x
- Laborde Cancino JA, Pozo Campodónico O (1982) Present and past of chile in Mexico. Ministry of Agriculture and Water Resources, National Agricultural Research Institute, DF, 80 pp.
- Lamarck J-B (1794) Tableau encyclopédique et méthodique des trois règnes de la nature. Botanique. Vol. 2. Panckoucke, Paris, 140 pp.
- Lengel PA (1960) Development of the pollen and the embryo sac in Capsicum frutescens L. var. Japanese variegated ornamental. The Ohio Journal of Science 60(1): 8–12.
- Lester RN, Durrands P (1984) Enzyme treatment as an aid in the study of seed surface structures of Solanum species. Annals of Botany 53(1): 129–132. https://doi.org/10.1093/oxfordjournals.aob.a086662
- Levan A, Fredga KL, Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52: 201–220. https://doi.org/10.1111/j.1601-5223.1964.tb01953.x
- Levey DJ, Tewksbury JJ, Cipollini ML, Carlo TA (2006) A field test of the directed-deterrence hypothesis in two species of wild chili. Oecologia 150: 61–68. https://doi.org/10.1007/s00442-006-0496-y
- Libreros D, van Zonneveld M, Petz M, Meckelmann SW, Ríos L, Peña K, Amaya K, Ramírez M (2013) Catálogo de ajíes (Capsicum spp.) peruanos promisorios conservados en el banco de semillas del INIA, Perú. Bioversity International, Cali, 49 pp.
- Libreros D, van Zonneveld M, Petz M, Meckelmann SW, Bejarano C, Avila T, Reyes X, Mayan C, Amaya K, Ramírez M (2014) Catálogo de ajíes nativos (Capsicum spp.) bolivianos promisorios. Bioversity International, Cali, 50 pp.
- Limaye VA, Patil VP (1989) Karyomorphological studies in the genus Capsicum Linn. Cytologia 54: 455–463. https://doi.org/10.1508/cytologia.54.455
- Linnaeus C (1753) Species Plantarum. Impensis Laurentii Salvii, Holmiae [Stockholm], 560 pp. https://doi.org/10.5962/bhl.title.669
- Linnaeus C (1767) Mantissa Plantarum. Holmiae, Stockholm, 142 pp.
- Link HF (1821) Enumeratio Plantarum Horti Regii Berolinensis Altera. G. Reimer, Berlin, 458 pp. https://doi.org/10.5962/bhl.title.66
- L’Obel M (1576) Plantarum seu stirpium historia. Ex officina Christophori Plantinii Architypographi Regii, Antuerpiae, 471 pp. https://doi.org/10.5962/bhl.title.7094
- Lohde G (1875) Ueber die Entwicklungsgeschichte und den Bau einiger Samenschalen. Mittheilungen aus dem Gesammtgebiete der Botanik 2(1): 43–80.
- López P, Gorzalczany S, Acevedo C, Alonso R, Ferraro G (2012) Chemical study and anti-inflammatory activity of Capsicum chacoense and C. baccatum. Brazilian Journal of Pharmacognosy 22(2): 455–458. https://doi.org/10.1590/S0102-695X2011005000187
- López Castilla L de C, Garruña Hernández R, Castillo Aguilar C de la C, Martínez-Hernández A, Ortiz-García MM, Andueza-Noh RH (2019) Structure and genetic diversity of nine important landraces of Capsicum species cultivated in the Yucatan Peninsula, Mexico. Agronomy 9: 1–11. https://doi.org/10.3390/agronomy9070376
- Luna-Ruiz J de J, Nabhan GP, Aguilar-Meléndez A (2018) Shifts in plant chemical defenses of chile pepper (Capsicum annuum L.) due to domestication in Mesoamerica. Frontiers in Ecology and Evolution 6: e48. https://doi.org/10.3389/fevo.2018.00048
- Manirakiza P, Covazi A, Schepens P (2003) Pungency principles in Capsicum-Analytical determinations and toxicology. In: De AK (Ed.) Capsicum, the genus Capsicum. Taylor & Francis, London and New York, 71–86.
- Martins KC, Souza S, Pereira TN, Rodrigues R, Pereira MG, da Cunha M (2013) Palynological characterization and genetic divergence between accessions of chilli and sweet peppers. Horticultura Brasileira 31: 568–573. https://doi.org/10.1590/S0102-05362013000400010
- Martins KC, Santana Pereira TN, Machado Souza SA, Rodrigues R, Teixeira do Amaral Jr A (2015) Crossability and evaluation of incompatibility barriers in crosses between Capsicum species. Crop Breeding and Applied Biotechnology 15: 139–145. https://doi.org/10.1590/1984-70332015v15n3a25
- Martínez Crovetto RN (1981) Plantas utilizadas en medicina en el noroeste de Corrientes. Miscelanea (Fundación Miguel Lillo) 69: 7–139.
- Martínez Crovetto RN (2014) Algunos datos sobre etnobotánica mocoví. Bonplandia 23(2): 119–131. https://doi.org/10.30972/bon.232259
- Martius CFP (1814) Plantarum Horti Academici Erlangensis Enumeratio. Typis Hilpertianis, Erlangen, 210 pp.
- Mason JR, Bean NJ, Shah PS, Clark L (1991) Taxon-specific differences in responsiveness to capsaicin and several analogues: Correlates between chemical structure and behavioral aversiveness. Journal of Chemical Ecology 17: 2539–2551. https://doi.org/10.1007/BF00994601
- Masud Parvez GM (2017) Current advances in pharmacological activity and toxic effects of various Capsicum species. International Journal of Pharmaceutical Sciences and Research 8(5): 1900–1912.
- Matsushima K, Saritnum O, Hamauzu Y, Adachi R, Harada K, Minami M, Nemoto K (2010) Evaluation of the functional properties of chili pepper varieties ‘rocoto’ (Capsicum pubescens Ruiz & Pav.) and ‘botankoshou’ (C. annuum L.), which are suitable for growing in cool areas. Horticultural Research (Japan) 9(2): 243–248. https://doi.org/10.2503/hrj.9.243
- Mazourek M, Pujar A, Borovsky Y, Paran I, Mueller L, Jahn MM (2009) A dynamic interface for capsaicinoid systems biology. Plant Physiology 150: 1806–1821. https://doi.org/10.1104/pp.109.136549
- McLeod MJ, Eshbaugh WH, Guttman SI (1979a) A preliminary biochemical systematic study of the genus Capsicum-Solanaceae. In: Hawkes JG, Lester RN, Skelding AD (Eds) The Biology and Taxonomy of the Solanaceae. Academic Press, New York, 701–713.
- McLeod MJ, Eshbaugh WH, Guttman SI (1979b) An electrophoretic study of Capsicum (Solanaceae): The purple flowered taxa. Bulletin of the Torrey Botanical Club 106(4): 326–333. https://doi.org/10.2307/2560359
- McLeod MJ, Guttman SI, Eshbaugh WH (1982) Early evolution of Chili Peppers (Capsicum). Economic Botany 36(4): 361–368. https://doi.org/10.1007/BF02862689
- McLeod MJ, Guttman SI, Eshbaugh WH (1983a) Peppers (Capsicum). In: Tanksley SD, Orton TJ (Eds) Isozymes in Plant Genetics and Breeding, part B. Elsevier Science Publishers B.V., Amsterdam, 189–201. https://doi.org/10.1016/B978-0-444-42227-9.50013-5
- McLeod MJ, Guttman SI, Eshbaugh WH, Rayle RE (1983b) An electrophoretic study of evolution in Capsicum (Solanaceae). Evolution 37(3): 562–574. https://doi.org/10.1111/j.1558-5646.1983.tb05573.x
- McMullen CK (1999) Flowering Plants of the Galápagos. Cornell University Press, Ithaca and London, 370 pp. https://doi.org/10.7591/9781501728761
- McNeill J (2014) Holotype specimens and type citations: general issues. Taxon 63(5): 1112–1113. https://doi.org/10.12705/635.7
- Meckelmann SW, Riegel DW, van Zonneveld MJ, Ríos L, Peña K, Ugas R, Quinonez L, Mueller-Seitz E, Petz M (2013) Compositional characterization of native Peruvian chili peppers (Capsicum spp.). Journal of Agricultural and Food Chemistry 61(10): 2530–2537. https://doi.org/10.1021/jf304986q
- Meckelmann SW, Jansen C, Riegel DW, van Zonneveld M, Rios L, Pena K, Mueller-Seitz E, Petz M (2015) Phytochemicals in native Peruvian Capsicum pubescens (Rocoto). European Food Research and Technology 241(6): 817–825. https://doi.org/10.1007/s00217-015-2506-y
- Meghvansi MK, Siddiqui S, Haneef Khana Md, Gupta VK, Vairale MG, Gogoi HK, Singh L (2010) Naga chilli: A potential source of capsaicinoids with broad-spectrum ethnopharmacological applications. Journal of Ethnopharmacology 132(1): 1–14. https://doi.org/10.1016/j.jep.2010.08.034
- Merrill ED (1918) Species Blancoanae, a critical revision of the Philippine species of plants described by Blanco and Llanos. Bureau of Printing, Manila, 423 pp. https://doi.org/10.5962/bhl.title.2116
- Metcalfe CR, Chalk L (1957) Anatomy of the Dicotyledons. Vol. 2. Clarendon Press, Oxford, 557 pp.
- Meyer GFW (1818) Primitiae florae essequeboensis. H. Dieterich, Göttingen, 318 pp.
- Miers J (1849) Contributions to the botany of South America. Annals and Magazine of Natural History, Ser. II, 3(16): 261–269. https://doi.org/10.1080/03745485909494760
- Miller M, Harrison J (1991) The Great Chile Book. Ten Speed Press, California, 156 pp.
- Miller P (1752) Das englische Gartenbuch. Vol 1. K.J.G. Lochners, Nürenberg, 548 pp.
- Miller P (1768) The Gardeners Dictionary, 8th edn. Printed for the author, London.
- Mitchell C, Eshbaugh WH, Wilson KG, Pittman BK (1989) Patterns of chloroplast DNA variations in Capsicum (Solanaceae): A preliminary study. International Organization of Plant Biosystematists Newsletter 12: 3–11.
- Moeller J (1886) Früchte und Samen. In: Moeller J (Ed.) Mikroskopie der Nahrungs- und Genussmittel aus dem Pflanzenreiche. Springer, Berlin, Heidelberg, 78–334. https://doi.org/10.1007/978-3-662-36441-3_4
- Moeller J (1905) Früchte und Samen. In: Moeller J (Ed.) Mikroskopie der Nahrungs- und Genussmittel aus dem Pflanzenreiche. Springer, Berlin, Heidelberg, 161–498. https://doi.org/10.1007/978-3-662-36442-0_6
- Molina RA (1975) Enumeración de las plantas de Honduras. Ceiba 19(1): 1–118.
- Montani MC, Scarpa GF (2016) Recursos vegetales y prácticas alimentarias entre indígenas tapiete del noreste de la provincia de Salta, Argentina. Darwiniana, nueva serie 4(1): 12–30. https://doi.org/10.14522/darwiniana.2016.41.684
- Montes Hernández S (2010) Recopilación y análisis de la información existente de las especies del género Capsicum que crecen y se cultivan en México. Informe final. Campo Experimental Bajío, INIFAP, 40 pp.
- Moreira AFP, Ruas PM, Ruas CF, Baba VY, Giordani W, Arruda IM, Rodrigues R, Gonçalves LSA (2018) Genetic diversity, population structure and genetic parameters of fruit traits in Capsicum chinense. Scientia Horticulturae 236: 1–9. https://doi.org/10.1016/j.scienta.2018.03.012
- Morison R (1669) Hortus regius blesensis. Jacobi Allestry, London, 499 pp.
- Moscone EA (1990) Chromosome studies on Capsicum (Solanaceae) I. Karyotype analysis in C. chacöense. Brittonia 42(2): 147–154. https://doi.org/10.2307/2807632
- Moscone EA (1992) Estudios de cromosomas meióticos en Solanaceae de Argentina. Darwiniana 31: 261–297.
- Moscone EA (1993) Estudios cromosómicos en Capsicum (Solanaceae) II. Análisis cariotípico en C. parvifolium y C. annuum var. annuum. Kurtziana 22: 9–18.
- Moscone EA (1999) Análisis cariotípico en Capsicum baccatum var. umbilicatum (Solanaceae) mediante bandeos AgNOR y de fluorescencia. Kurtziana 27: 225–232.
- Moscone EA, Lambrou M, Ehrendorfer F (1996a) Fluorescent chromosome banding in the cultivated species of Capsicum (Solanaceae). Plant Systematics and Evolution 202: 37–63. https://doi.org/10.1007/BF00985817
- Moscone EA, Matzke MA, Matzke AJM (1996b) The use of combined FISH/GISH in conjunction with DAPI counterstaining to identify chromosomes containing transgene inserts in amphidiploid tobacco. Chromosoma 105: 231–236. https://doi.org/10.1007/BF02528771
- Moscone EA, Lambrou M, Hunziker AT, Ehrendorfer F (1993) Giemsa C-banded karyotypes in Capsicum (Solanaceae). Plant Systematics and Evolution 186: 213–229. https://doi.org/10.1007/BF00940799
- Moscone EA, Loidl J, Ehrendorfer F, Hunziker AT (1995) Analysis of active nucleolus organizing regions in Capsicum (Solanaceae) by silver staining. American Journal of Botany 82: 276–287. https://doi.org/10.1002/j.1537-2197.1995.tb11495.x
- Moscone EA, Klein F, Lambrou M, Fuchs J, Schweizer D (1999) Quantitative karyotyping and dual-color FISH mapping of 5S and 18S-25S rDNA probes in the cultivated Phaseolus species (Leguminosae). Genome 42: 1224–1233. https://doi.org/10.1139/g99-070
- Moscone EA, Baranyi M, Ebert I, Greilhuber J, Ehrendorfer F, Hunziker AT (2003) Analysis of nuclear DNA content in Capsicum (Solanaceae) by flow cytometry and Feulgen densitometry. Annals of Botany 92: 21–29. https://doi.org/10.1093/aob/mcg105
- Moscone EA, Scaldaferro MA, Grabiele M, Cecchini NM, Sanchez García Y, Jarret R, Daviña JR, Ducasse DA, Barboza GE, Ehrendorfer F (2007) The evolution of chili peppers (Capsicum-Solanaceae): a cytogenetic perspective. Acta Horticulturae (ISHS) 745: 137–170. https://doi.org/10.17660/ActaHortic.2007.745.5
- Moses M, Umaharan P, Dayanandan S (2014) Microsatellite based analysis of the genetic structure and diversity of Capsicum chinense in the Neotropics. Genetic Resources and Crop Evolution 61: 741–755. https://doi.org/10.1007/s10722-013-0069-y
- Mueller R, Beck SG, Lara R (2002) Vegetación potencial de los bosques de Yungas en Bolivia, basado en datos climáticos. Ecología en Bolivia 37(2): 5–14.
- Munting AJ (1974) Development of flower and fruit of Capsicum annuum L. Acta Botanica Neerlandica 23(4): 415–432. https://doi.org/10.1111/j.1438-8677.1974.tb00959.x
- Murry LE, Eshbaugh WH (1971) A palynological study of the Solaninae (Solanaceae). Grana 11: 65–78. https://doi.org/10.1080/00173137109429859
- Nascimento-Filho HR, Barbosa RI, Luz FJF (2007) Pimentas do gênero Capsicum cultivadas em Roraima, Amazônia brasileira. II. Hábitos e formas de uso. Acta Amazonica 37: 561–568. https://doi.org/10.1590/S0044-59672007000400011
- Nascimento Sousa WR, Almeida Lopes AC, Carvalho R, Ferreira Gomes Rl, Peron AP (2015) Karyotypic characterization of Capsicum sp. accessions. Acta Scientiarum, Agronomy 37: 147–153. https://doi.org/10.4025/actasciagron.v37i2.19485
- National Research Council (1989) Lost Crops of the Incas: Little-known plants of the Andes with promise for worldwide cultivatation. National Academic Press, Washington, 409 pp.
- Nee M (1986) Solanaceae I. Fl. Veracruz 49. Instituto Nacional de Investigaciones sobre Recursos Bióticos, Xalapa, Veracruz, 191 pp.
- Nee M, Bohs L, Knapp S (2006) New species of Solanum and Capsicum (Solanaceae) from Bolivia, with clarification of nomenclature in some Bolivian Solanum. Brittonia 58(4): 322–356. https://www.jstor.org/stable/4497330
- Nees von Esenbeck CG (1834) Monographs of the East Indian Solaneae. Transactions of the Linnean Society of London 17(1): 37–82. https://doi.org/10.1111/j.1095-8339.1834.tb00017.x
- Nehren UM, Alfonso de Nehren SP, Heinrich JH (2009) Forest fragmentation in the Serra dos Órgãos: Historical and landscape ecological implications. In: Gaese H, Torrico JC, Wesenberg J, Schlüter S (Eds) Biodiversity and land use systems in the fragmented Mata Atlântica of Rio de Janeiro, Cuvellier Verlag, Göttingen, 39–65.
- Nelson CH (2001) [2002] Plantas descritas originalmente de Honduras y sus nomenclaturas equivalentes actuales. Ceiba 42(1): 1–71.
- Nicolaï M, Cantet M, Lefebvre V, Sage-Palloix AM, A Palloix (2013) Genotyping a large collection of pepper (Capsicum spp.) with SSR loci brings new evidence for the wild origin of cultivated C. annuum and the structuring of genetic diversity by human selection of cultivar types. Genetic Resources and Crop Evolution 60: 2375–2390. https://doi.org/10.1007/s10722-013-0006-0
- Nishiyama I (1939) Studies on artificial polyploid plants. l. Production of tetraploids by treatment with colchicine. Agriculture and Horticulture (Tokyo) 14: 1411–1422.
- Nishiyama I (1940) Studies on artificial polyploid plants. IV. Comparative studies on a polyploid series in red pepper. Botany and Zoology (Tokyo) 8: 905–913.
- Norman DM, Mason JR, Clark L (1992) Capsaicin effects on consumption of food by Cedar Wax wings and House Finches. Wilson Bulletin 104(3): 549–551. http://www.jstor.org/stable/4163197
- Noss CF, Levey DJ (2014) Does gut passage affect post-dispersal seed fate in a wild chili, Capsicum annuum? Southeastern Naturalist 13(3): 475–483. https://doi.org/10.1656/058.013.0308
- Nugroho LH (2016) Red Pepper (Capsicum spp.) fruit: A model for the study of secondary metabolite product distribution and its management. In: Setyobudi RH, Nuringtyas TR, Adinurani PG (Eds) Towards the sustainable use of biodiversity in a changing environment: From basic to applied research, Proceeding of the 4th International Conference on Biological Science 1744, AIP Publishing, Yogyakarta, 1744: 020034-1–020034-7. https://doi.org/10.1063/1.4953508
- OECD (2006) Consensus document on the biology of the Capsicum annuum complex (Chili peppers, hot peppers and sweet peppers). Series on Harmonisation of Regulatory Oversight in Biotechnology 36: 11–45. http://www.oecd.org/biotrack
- Olmstead R, Bohs L, Migid HA, Santiago-Valentín E, García VF, Collier SM (2008) A molecular phylogeny of the Solanaceae. Taxon 57: 1159–1181. https://doi.org/10.1002/tax.574010
- Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, D’amico JA, Itoua I, Strand HE, Morrison JC, Loucks CJ, Allnutt TF, Ricketts TH, Kura Y, Lamoreux JF, Wettengel WW, Hedao P, Kassem KR (2001) Terrestrial Ecoregions of the World: A New Map of Life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 51: 933–938. https://doi.org/10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
- Onus AN, Pickersgill B (2004) Unilateral incompatibility in Capsicum (Solanaceae): occurrence and taxonomic distribution. Annals of Botany 94: 289–295. https://doi.org/10.1093/aob/mch139
- Ohta Y (1962a) Genetical studies in the genus Capsicum. Kihara Institute for Biological Research 1962: 1–94.
- Ohta Y (1962b) Cytogenetical studies on polyploid red pepper. Seiken Ziho: Report of the Kihara Institute for Biological Research 13: 89–92.
- Ohta Y (1962c) Caryotypes of Capsicum. Seiken Ziho: Report of the Kihara Institute for Biological Research 13: 93–99.
- Ohta Y (1962d) Physiological and genetical studies on the pungency of Capsicum, IV. Secretory organs, receptacles and distribution of capsaicin in the Capsicum fruit. Japanese Journal Breeding 12(3): 179–183. https://doi.org/10.1270/jsbbs1951.12.179
- Pal BP, Ramanujam S (1939) Induction of polyploidy in chile (Capsicum annuum L.) by colchicine. Nature 143: 245–246. https://doi.org/10.1038/143245b0
- Pal BP, Ramanujam S, Joshi AB (1941) Colchicine-induced polyploidy in crop plants. II. Chilli (Capsicum annuum L.). Indian Journal of Genetics and Plant Breeding 1: 28–40.
- Palchetti MV, Barboza GE, Cosa MT (2014) Anatomía foliar en especies de Capsicum (Solanaceae) de diferentes ambientes biogeográficos sudamericanos. Boletín de la Sociedad Argentina de Botánica 49(3): 417–436. https://doi.org/10.31055/1851.2372.v49.n3.9473
- Palfi G, Visnyovszky Z, Tranger B (1961) The production of polyploid red peppers. Kisérletügyi Közlemények, Reports of the Hungarian Agricultural Experiment Stations 54/C: 3–13.
- Palma G, Quezada-Euan JJ, Melendez-Ramirez V, Irigoyen J, Valdovinos-Nuez GR, Rejn M (2008) Comparative efficiency on Nannotrigona perilampoides, Bombus impatiens (Hymenoptera: Apoidea), and mechanical vibration on fruit production of enclosed habanero pepper. Journal of Economic Entomology 101(1): 132–138. https://doi.org/10.1093/jee/101.1.132
- Panda RC, Kumar AO, Rao KGR (1984) Cytomorphology of induced octoploid chili pepper (Capsicum annuum L.). Theoretical and Applied Genetics 68: 567–570. https://doi.org/10.1007/BF00285015
- Pandhair V, Sharma S (2008) Accumulation of capsaicin in seed, pericarp and placenta of Capsicum annuum L. fruit. Journal of Plant Biochemistry & Biotechnology 17(1): 23–27. https://doi.org/10.1007/BF03263255
- Papoiu ADP, Yosipovitch G (2010) Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opinion on Pharmacotherapy 11(8): 1359–1371. https://doi.org/10.1517/14656566.2010.481670
- Park YK, Park KC, Park CH, Kim NS (2000) Chromosomal localization and sequence variation of 5S rRNA gene in five Capsicum species. Molecules and Cells 10: 18–24. https://doi.org/10.1007/s10059-000-0018-4
- Parkinson J (1640) Theatrum botanicum. Tho. Cotes. London, 1755 pp.
- Patel JD, Shah JJ (1977) Growth pattern in Solanum melongena L. and Capsicum annuum L. Flora 166(4): 327–332. https://doi.org/10.1016/S0367-2530(17)32151-5
- Patel K, Ruiz C, Calderon R, Marcelo M, Rojas R (2016) Characterisation of volatile profiles in 50 native Peruvian chili pepper using solid phase microextraction–gas chromatography mass spectrometry (SPME–GCMS). Food Research International 89(1): 471–475. https://doi.org/10.1016/j.foodres.2016.08.023
- Paxton J (1838) Paxton’s Magazine of Botany 5: 1–280.
- Peña-Yam LP, Muñoz-Ramírez LS, Avilés-Viñas SA, Canto-Flick A, Guzmán-Antonio A, Santana-Buzzy N (2019) Floral biology studies in Habanero pepper (Capsicum chinense Jacq.) to implement in a cross-breeding program. Agriculture 9(12): e249. https://doi.org/10.3390/agriculture9120249
- Peñuela M, Arias LL, Viáfara-Vega R, Rivera Franco N, Cárdenas H (2020) Morphological and molecular description of three commercial Capsicum varieties: a look at the correlation of traits and genetic distancing. Genetic Resources and Crop Evolution 68: 261–277. https://doi.org/10.1007/s10722-020-00983-8
- Peralta M, Liverotti O (2018) Pimientos picantes (Capsicum sp.) en el Mercado Central de Buenos Aires). Boletín de Frutas y Hortalizas/Convenio INTA-CMCBA 81: 1–12.
- Perera KDA, Poulos JM (1993) Studies on stigma position of seventeen pepper accessions. Capsicum and Eggplant Newsletter 12: 44–46. https://doi.org/10.1016/0278-2316(93)90033-N
- Pereira LA, Barboza GE, Bovini MG, Zélia de Almeida M, Guimarães EF (2011) Caracterización y uso de “pimientas” en una comunidad quilombola de la Amazonía Oriental (Brasil). Journal of the Botanical Research Institute of Texas 5(1): 255–272.
- Pérez-Pastrana J, Islas-Flores I, Bárány I, Álvarez-López D, Canto-Flick A, Canto-Canché B, Peña-Yam L, Muñoz-Ramírez L, Avilés-Viñas S, Testillano PS, Santana-Buzzy S (2018) Development of the ovule and seed of Habanero chili pepper (Capsicum chinense Jacq.): Anatomical characterization and immunocytochemical patterns of pectin methyl-esterification. Journal of Plant Physiology 230: 1–12. https://doi.org/10.1016/j.jplph.2018.08.005
- Perry L, Dickau R, Zarrillo S, Holst I, Pearsall DM, Piperno DR, Berman MJ, Cooke RG, Rademaker K, Ranere AJ, Raymond JS, Sandweiss DH, Scaramelli F, Tarble K, Zeidler JA (2007) Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp.) in the Americas. Science 315: 986–988. https://doi.org/10.1126/science.1136914
- Peter KV, McCollum GD (1984) Pollen-stigma compatibility in direct and reciprocal crosses of Capsicum species. Journal of Heredity 75(1): 70–70. https://doi.org/10.1093/oxfordjournals.jhered.a109869
- Pickersgill B (1966) The variability and relationships of Capsicum chinense Jacq. PhD Thesis, Indiana University, University Microfilms, Inc., Michigan.
- Pickersgill B (1969a) The domestication of chili peppers. In: Ucko PJ, Dimbleby GW (Eds) The domestication and explotation of plants and animals. Duckworth, London, 443–450. https://doi.org/10.4324/9781315131825-40
- Pickersgill B (1969b) The archaeological record of chili peppers (Capsicum spp.) and the sequence of plant domestication in Peru. American Antiquity 34: 54–61. https://doi.org/10.2307/278313
- Pickersgill B (1971) Relationships between weedy and cultivated forms of chili peppers (genus Capsicum). Evolution 25: 683–691. https://doi.org/10.1111/j.1558-5646.1971.tb01926.x
- Pickersgill B (1977) Chromosomes and evolution in Capsicum. In: Pochard E (Ed.) Capsicum 77. Comptes Rèndues 3ème Congrès Eucarpia Piment. INRA (Institut National de la Recherche Agronomique), Montfavet-Avignon, 27–37.
- Pickersgill B (1984) Migration of chili peppers, Capsicum spp. in the Americas. In: Stone D (Ed.) Pre-Columbian Plant Migration. Papers of the Peabody Museum of Archeology and Ethnology 76: 105–123.
- Pickersgill B (1988) The genus Capsicum: a multidisciplinary approach to the taxonomy of cultivated and wild plants. Biologisches Zentralblatt 107(4): 381–389.
- Pickersgill B (1989) Genetic resources of Capsicum for tropical regions. In: Green SK (Ed.) Tomato and pepper production in the tropics. Proceedings of the International Symposium on Integrated Management Practices. Asian Vegetable Research and Development Center, Shanhua, Tainan, 2–8.
- Pickersgill B (1991) Cytogenetics and evolution of Capsicum L. In: Tsuchiya T, Gupta PK (Eds) Chromosome engineering in plants: Genetics, breeding, evolution. Part B. Elsevier, Amsterdam, 139–160. https://doi.org/10.1016/B978-0-444-88260-8.50013-6
- Pickersgill B (2007) Domestication of plants in the Americas: insights from Mendelian and molecular genetics. Annals of Botany 100: 925–940. https://doi.org/10.1093/aob/mcm193
- Pickersgill B (2016) Chile Peppers (Capsicum spp.) In: Lira R, Casas A, Blancas J (Eds) Ethnobotany of Mexico, Ethnobiology, Springer Science+Business Media, New York, 417–437. https://doi.org/10.1007/978-1-4614-6669-7_17
- Pickersgill B, Heiser CB (1977) Origins and distribution of plants domesticated in the New World tropics. In: Reed CA (Ed.) Origins of Agriculture. Mouton, The Hague, 803–835. https://doi.org/10.1515/9783110813487.803
- Pickersgill B, Heiser Jr CB, Mc Neill J (1979) Numerical taxonomic studies on variation and domestication in some species of Capsicum. In: Hawkes JG, Lester RN, Skelding AD (Eds) The Biology and Taxonomy of the Solanaceae. Academic Press, New York, 679–700.
- Poeppig EF (1832) Doctor Pöppig’s naturhistorische Berichte aus Peru. Notizen aus dem Gebiete der Natur- und Heilkunde 32: 225–234.
- Poggio L (1996) Algunos aportes a la citogenética y especiación vegetal. Anales Academia Nacional de Ciencias Exactas, Físicas y Naturales 48: 79–92.
- Powis TG, Gallaga Murrieta E, Lesure R, Lopez Bravo R, Grivetti L, Kucera H, Gaikwad NW (2013) Prehispanic use of chili peppers in Chiapas, Mexico. PLoS ONE 8: e79013. https://doi.org/10.1371/journal.pone.0079013
- Pozzobon MT, Schifino-Wittmann MT (2006) A meiotic study of the wild and semi-domesticated Brazilian species of genus Capsicum L. (Solanaceae). Cytologia 71(3): 275–287. https://doi.org/10.1508/cytologia.71.275
- Pozzobon MT, Schifino-Wittmann MT, Bianchetti L (2006) Chromosome numbers in wild and semidomesticated Brazilian Capsicum L. (Solanaceae) species: do x = 12 and x = 13 represent two evolutionary lines? Botanical Journal of the Linnean Society 151: 259–269. https://doi.org/10.1111/j.1095-8339.2006.00503.x
- Prince JP, Lackney VK, Angeles C, Blauth JR, Kyle MM (1995) A survey of DNA polymorphism within the genus Capsicum and the fingerprinting of pepper cultivars. Genome 38(2): 224–231. https://doi.org/10.1139/g95-027
- Pruthi JS (2003) Chemistry and quality control of Capsicum and Capsicum products. In: De AK (Ed.) Capsicum. The genus Capsicum. Taylor & Francis, London and New York, 25–70.
- Purdie RW, Symon DE, Haegi L (1982) Solanaceae. Flora of Australia 29: 1–208.
- Purkayastha J, Alam SI, Gogoi HK, Singh L (2012) Capsicum assamicum sp. nov. (Solanaceae), from Assam, Northeastern India. Ozean Journal of Applied Sciences 5(1): 55–66.
- QGIS Development Team (2019) QGIS Version 3.8 Geographic Information System User Guide. Open Source Geospatial Foundation.
- Qin C, Yu C, Shen Y, Fang X, Chen L, Min J, Cheng J, Zhao S, Xu M, Luo Y, Yang Y, Wu Z, Mao L, Wu H, Ling-Hu C, Zhou H, Lin H, González-Morales S, Trejo-Saavedra DL, Tian H, Tang X, Zhao M, Huang Z, Zhou A, Yao X, Cui J, Li W, Chen Z, Feng Y, Niu Y, Bi S, Yang X, Li W, Cai H, Luo X, Montes-Hernández S, Leyva-González MA, Xiong Z, He X, Bai L, Tan S, Tang X, Liu D, Liu J, Zhang S, Chen M, Zhang L, Zhang L, Zhang Y, Liao W, Zhang Y, Wang M, Lv X, Wen B, Liu H, Luan H, Zhang Y, Yang S, Wang X, Xu J, Li X, Li S, Wang J, Palloix A, Bosland PW, Li Y, Krogh A, Rivera-Bustamante RF, Herrera-Estrella L, Yin Y, Yu J, Hu K, Zhang Z (2014) Whole genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proceedings of the National Academy of Sciences of the United States of America 111: 5135–5140. https://doi.org/10.1073/pnas.1400975111
- Quagliotti L (1979) Floral ecology of Capsicum and Solanum melongena. In: Hawkes JG, Lester RN, Skelding AD (Eds) The Biology and Taxonomy of Solanacae. Academic Press, London, 399–419. http://download.osgeo.org/qgis/doc/manual/<DOCUMENT [accessed 5.03.2021]
- Rabinowitch HD, Fahn A, Meir T, Lensky Y (1993) Flower and nectar attributes of pepper (Capsicum annuum L.) plants in relation to their attractiveness to honeybees (Apis mellifera L.). Annals of Applied Biology 123: 221–232. https://doi.org/10.1111/j.1744-7348.1993.tb04087.x
- Raghavan TS, Venkatasubban K (1940) Studies in the South Indian chillies. I. A description of the varieties, chromosome numbers and the cytology of some X-rayed derivatives in Capsicum annuum Linn. Proceedings of the Indian Academy of Sciences 12 (Sect. B): 29–46. https://doi.org/10.1007/BF03049084
- Raghuvanshi S, Joshi S (1964) Cytomorphological studies on the colchiploids of Capsicum frutescens L. Cytologia 29: 61–78. https://doi.org/10.1508/cytologia.29.61
- Rao KG (1987) Colchicine induced chromosome mosaicism in chili pepper (Capsicum annuum L.). Proceedings of the Indian Academy of Sciences, Plant-Sci 97(1): 55–61. https://doi.org/10.1007/BF03053340
- Raveendar S, Lee KJ, Shin M-J, Cho G-T, Lee J-R, Ma K-H, Lee G-A, Chung J-W (2017) Complete chloroplast genome sequencing and genetic relationship analysis of Capsicum chinense Jacq. Plant Breeding and Biotechnology 5(4): 261–268. https://doi.org/10.9787/PBB.2017.5.4.261
- Reddy KR, Kakani VG (2007) Screening Capsicum species of different origins for high temperature tolerance by in vitro pollen germination and pollen tube length. Scientia Horticulturae 112: 130–135. https://doi.org/10.1016/j.scienta.2006.12.014
- Richard A (1850) Tentamen Florae Abyssinicae. Vol. 2. A. Bertrand, Paris, 518 pp.
- Rick CM (1950) Capsicum pubescens, a little-known pungent pepper from Latin America. Missouri Botanical Garden, Bulletin 38: 36–42.
- Rodrigues IMC, Knapp S, Stehmann JR (2019) The nomenclatural re‐establishment of Athenaea Sendtn. (Solanaceae) with a nomenclatural synopsis of the genus. Taxon 68(4): 839–846. https://doi.org/10.1002/tax.12089
- Rodríguez-Burruezo A, Prohens J, Raigón MD, Nuez F (2009) Variation for bioactive compounds in ají (Capsicum baccatum L.) and rocoto (C. pubescens R. & P.) and implications for breeding. Euphytica 170: 169–181. https://doi.org/10.1007/s10681-009-9916-5
- Rodríguez-Burruezo A, Kollmannsberger H, Gonzalez-Mas MC, Nitz S, Nuez F (2010) HS-SPME comparative analysis of genotypic diversity in volatile fraction and aroma contributing compounds of Capsicum fruits from the annuum-chinense-frutescens complex. Journal of Agricultural and Food Chemistry 58: 4388–4400. https://doi.org/10.1021/jf903931t
- Roemer JJ, Schultes JA (1819) Systema Vegetabilium, 15th edn. [bis]. Vol. 4. J.G. Cotta, Stuttgart, 888 pp.
- Roman ALC, Ming LC, Pires Sablayrolles M das G (2020) Pimentas Capsicum L. no Brasil: notas sobre botânica, história, concepções indígenas e folklore. Sociedade Brasileira de Etnobiologia e Etnoecologia, Porto Alegre, 95 pp.
- Romero da Cruz MV, Forni-Martins ER (2015) IAPT chromosome data 20. Taxon 64(6): 1344–1350[E34–E36]. https://doi.org/10.12705/646.42
- Romero da Cruz MV, Urdampilleta JD, Forni Martins ER, Moscone EA (2016) Cytogenetic markers for the characterization of Capsicum annuum L. cultivars, Plant Biosystems 151(1): 84–91. https://doi.org/10.1080/11263504.2015.1103798
- Romero da Cruz MV, Forni-Martins ER, Urdampilleta JD (2017) IAPT chromosome data 24. Taxon 66(1): 275–277[E16–E17].
- Rosi A, Canavesi V, Segoni S, Dias Nery T, Catani F, Casagli N (2019) Landslides in the Mountain Region of Rio de Janeiro: A proposal for the semi-automated definition of multiple rainfall thresholds. Geosciences 9(5): e203. https://doi.org/10.3390/geosciences9050203
- Roth I (1977) Fruits of Angiosperms. In: Linsbauer K, Tischler G, Zimmermann W (Eds) Encyclopaedia of Plant Anatomy 10(1): 1–675.
- Roubik DW, Moreno PJE (1991) Pollen and spores of Barro Colorado Island. Monographs in Systematic Botany 36: 1–268.
- Roxburgh W (1814) Hortus Bengalensis. Mission Press, Serampore, 105 pp.
- Roxburgh W (1824) Flora indica, or descriptions of Indian plants. Vol. 2. Mission Press, Serampore, 588 pp.
- Rudrapal M, Sarwa KK (2020) Capsicum: Chemistry and medicinal properties of indigenous Indian varieties. In: Dekebo A (Ed.) Capsicum. Intechopen, 1–9. https://doi.org/10.5772/intechopen.92241
- Ruiz H (1940) Travels of Ruiz, Pavón, and Dombey in Peru and Chile (1777–1788), with an epilogue and official documents added by Agustín Jesús Barreiro. Botanical Series Field Museum of Natural History 21: 1–372[Publication 467]. [Translation by B.E. Dahlgren] https://doi.org/10.5962/bhl.title.2305
- Ruiz H, Pavón JJA (1799) Flora peruviana et chilensis. Vol. 2. Gabrielis de Sancha, Madrid, 76 pp.
- Ruiz-Lau N, Medina-Lara F, Martínez-Estévez M (2011) El chile habanero: su origen y usos. Ciencia 62(3): 70–78.
- Rumphius GE (1747) Herbarium amboinense. Vol. 5. F. Chaguion & H. Uytwerf, Amsterdam, 492 pp.
- Rusby HH (1912) New species from Bolivia, collected by R.S. Williams. Bulletin of the New York Botanical Garden 8(28): 89–135.
- Rusby HH (1927) Descriptions of new genera and species of plants collected on the Mulford Biological Exploration of the Amazon valley, 1921–1922. Memoirs of the New York Botanical Garden 7: 205–388.
- Ryding O (2002) Count F. C. Raben`s Brazilian herbaria. Taxon 51: 369–376. https://doi.org/10.2307/1554906
- Saleh BK, Omer A, Teweldemedhin B (2018) Medicinal uses and health benefits of chili pepper (Capsicum spp.): a review. MOJ Food Processing & Technology 6(4): 325–328. https://doi.org/10.15406/mojfpt.2018.06.00183
- Samuels J (2014) The rocoto pepper: a novel crop Capsicum. The Organic Grower 28: 10–12.
- Sanati S, Razavi BM, Hosseinzadeh H (2018) A review of the effects of Capsicum annuum L. and its constituent, capsaicin, in metabolic syndrome. Iranian Journal of Basic Medical Sciences 21: 439–448. https//doi.org/10.22038/IJBMS.2018.25200.6238
- Särkinen T, Bohs L, Olmstead RG, Knapp S (2013) A phylogenetic framework for evolutionary study of the nightshades (Solanaceae): a dated 1000-tip tree. BMC Evolutionary Biology 13: 214–229. https://doi.org/10.1186/1471-2148-13-214
- Särkinen T, Poczai P, Barboza GE, Weerden GM van der, Baden M, Knapp S (2018) A revision of the Old World Black Nightshades (Morelloid clade of Solanum L., Solanaceae). PhytoKeys 106(29): 1–223. https://doi.org/10.3897/phytokeys.106.21991
- Saur Palmieri V, López ML, Trillo C (2018) Aproximaciones etnobotánicas de las especies y prácticas de frutos nativos comestibles de la actualidad. Aportes para la interpretación del pasado prehispánico de Cerro Colorado (Córdoba, Argentina). Boletín de la Sociedad Argentina de Botánica 53(1): 115–133. https://doi.org/10.31055/1851.2372.v53.n1.19912
- Scaldaferro MA (2019) Molecular cytogenetic evidence of hybridization in the “purple corolla clade of the genus Capsicum (C. eximium × C. cardenasii). Plant Biosystems 154(5): 685–691. https://doi.org/10.1080/11263504.2019.1674403
- Scaldaferro MA, Moscone EA (2019) Cytology and DNA content variation of Capsicum genomes. In: Ramchiary N, Kole C (Eds) Compendium of Plant Genomes. The Capsicum Genome. Springer Nature Switzerland AG, Cham, 57–84. https://doi.org/10.1007/978-3-319-97217-6_4
- Scaldaferro MA, Seijo JG, Acosta MC, Barboza GE, Ducasse DA, Moscone EA (2006) Genomic characterization of the germplasm in peppers (Capsicum-Solanaceae) by fluorescent in situ hybridization. Plant Science 43: 291–297.
- Scaldaferro MA, Grabiele M, Moscone EA (2013) Heterochromatin type, amount and distribution in wild species of chili peppers (Capsicum-Solanaceae). Genetic Resources and Crop Evolution 60(2): 693–709. https://doi.org/10.1007/s10722-012-9867-x
- Scaldaferro MA, Romero da Cruz MV, Cecchini NM, Moscone EA (2016) FISH and AgNor-mapping of the 45S and 5S rRNA genes in wild and cultivated Capsicum species (Solanaceae). Genome 59: 95–113. https://doi.org/10.1139/gen-2015-0099
- Scaldaferro MA, Barboza GE, Acosta MC (2018) Evolutionary history of the chili pepper Capsicum baccatum L. (Solanaceae): domestication in South America and natural diversification in the Seasonally Dry Tropical Forests. Biological Journal of the Linnean Society 124(3): 466–478. https://doi.org/10.1093/biolinnean/bly062
- Scarpa GF (2002) Plantas empleadas contra trastornos digestivos en la medicina tradicional criolla del Chaco Noroccidental. Dominguezia 18(1): 36–50.
- Scarpa GF (2007) Plantas asociadas a la pesca y a sus recursos por los Indígenas Chorote del Chaco Semiarido (Argentina). Boletín de la Sociedad Argentina de Botánica 41(3–4): 333–345.
- Scarpa GF, Rosso CN (2014) La etnobotánica moqoit inédita de Raúl Martínez Crovetto I: Descripción, actualización y análisis de la nomenclatura indígena. Boletín de la Sociedad Argentina de Botánica 49(4): 623–647. https://doi.org/10.31055/1851.2372.v49.n4.9995
- Schnack B, Covas G (1947) Karyological studies in flowering plants. Haumania 1: 32–41.
- Sendtner O (1846) Solanaceae et Cestrineae. Flora Brasiliensis 10(1): 1–231.
- Shinners LH (1956) Technical names for the cultivated Capsicum peppers. Baileya 4(2): 81–83.
- Shopova M (1966) Studies in the genus Capsicum. I. Species differentiation. Chromosoma 19: 340–348. https://doi.org/10.1007/BF00326922
- Sinha NP (1950) The somatic chromosomes and meiosis in Capsicum. Indian Journal of Genetics and Plant Breeding 10: 36–42.
- Silva JLS, Cruz-Neto O, Peres CA, Tabarelli M, Lopes AV (2019) Climate change will reduce suitable Caatinga dry forest habitat for endemic plants with disproportionate impacts on specialized reproductive strategies. PLoS ONE 14(5): e0217028. https://doi.org/10.1371/journal.pone.0217028
- Siskovic M (1962) The production of autotetraploids of red pepper (Capsicum annuum). Savremena Poljoprivreda, Novi Sad 10: 227–239.
- Smith LB, Downs RJ (1966) Solanáceas. In: Reitz R (Ed.) Flora Ilustrada Catarinense. I Parte. Fasc. SOLA. Herbário “Barbosa Rodrigues”, Itajaí, 321 pp.
- Smith PG, Heiser Jr CB (1957) Breeding behavior of cultivated peppers. Proceedings of the American Society for Horticultural Science 70: 286–290.
- Song Y, Gu L, Liu J (2019) Pollen morphology of selected species from the family Solanaceae. Palynology 43(3): 355–372. https://doi.org/10.1080/01916122.2018.1458663
- Souèges R (1907) Développement et structure de tégument séminal chez les Solanacées. Annales des Sciences Naturelles, Botanique, sér. 9(6): 1–124.
- Sousa-Peña M (2001) Systematic and reproductive biology of the genus Witheringia L’Her. (Solanaceae). PhD. Thesis, The University of Connecticut, Connecticut.
- Spalink D, Stoffel K, Walden GK, Hulse-Kemp AM, Hill TA, Van Deynze A, Bohs L (2018) Comparative transcriptomics and genomic patterns of discordance in Capsiceae (Solanaceae). Molecular Phylogenetics and Evolution 126: 293–302. https://doi.org/10.1016/j.ympev.2018.04.030
- Sricharoen P, Lamaiphan N, Patthawaro P, Limchoowong N, Techawongstien S, Chanthai S (2017) Phytochemicals in Capsicum oleoresin from different varieties of hot chilli peppers with their antidiabetic and antioxidant activities due to some phenolic compounds. Ultrasonics Sonochemistry 38: 629–639. https://doi.org/10.1016/j.ultsonch.2016.08.018
- Srinivasan K (2016) Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: A review. Critical Reviews in Food Science and Nutrition 56(9): 1488–1500. https://doi.org/10.1080/10408398.2013.772090
- Stafleu FA, Cowan RS (1976) Taxonomic literature: a selective guide to botanical publications and collections with dates, commentaries and types. Vol. 1. Scheltema & Holkema, Utrecht-Bohn, 1136 pp. https://doi.org/10.5962/bhl.title.48631
- Stafleu FA, Cowan RS (1983) Taxonomic literature: a selective guide to botanical publications and collections with dates, commentaries and types. Vol. 4. Scheltema & Holkema, Utrecht-Bohn, 1214 pp.
- Stafleu FA, Mennega EA (1993) Taxonomic literature: a selective guide to botanical publications and collections with dates, commentaries and types, Supp. II: Be-Bo. Koeltz Scientific Books, Königstein, 464 pp.
- Stewart A (1911) A botanical survey of the Galapagos Islands. Proceedings of the California Academy of Sciences, Ser. 4, 1: 7–288. https://doi.org/10.5962/bhl.title.42392
- Stewart C, Mazourek M, Stellari GM, O’Connell M, Jahn M (2007) Genetic control of pungency in C. chinense via the Pun1 locus. Journal of Experimental Botany 58: 979–991. https://doi.org/10.1093/jxb/erl243
- Stommel JR, Griesbach RJ (2005) Capsicum annuum L. ‘Black Pearl’. HortScience 40(5): 1571–1573. https://doi.org/10.21273/HORTSCI.40.5.1571
- Sturtevant EL (1888a) Capsicum umbilicatum. Bulletin of the Torrey Botanical Club 15(5): 108–109. https://doi.org/10.2307/2476468
- Sturtevant EL (1888b) Capsicum fasciculatum. Bulletin of the Torrey Botanical Club 15(5): 133–134. https://doi.org/10.2307/2476521
- Suzuki T, Fujiwake H, Iwai K Plant (1980) Intracellular localization of capsaicin and its analogues, capsaicinoid, in Capsicum fruit I. Microscopic investigation of the structure of the placenta of Capsicum annuum var. annuum cv. Karayatsubusa. Plant and Cell Physiology 21(5): 839–853. https://doi.org/10.1093/oxfordjournals.pcp.a076058
- Svenson HK (1946) Vegetation of the coast of Ecuador and Peru and its relation to that of the Galapagos Islands. II. Catalogue of Plants. American Journal of Botany 33: 427–498. https://doi.org/10.2307/2437584
- Symon D (1981) The Solanaceous genera, Browallia, Capsicum, Cestrum, Cyphomandra, Hyoscyamus, Lycopersicon, Nierembergia, Physalis, Petunia, Salpichroa and Withania, naturalised in Australia. Journal of the Adelaide Botanic Gardens 3(2): 133–166.
- Tabernaemontanus JT (1588) Neu vollkommen Kräuter Buch. König-Werenfels, Basel.
- Takizawa K, Ishikawa K, Nunomura O, Ito T (2008) Ploidy level effect on physiology of pepper plant as affected by fruit loading. Acta Horticulturae 779: 689–697. https://doi.org/10.17660/ActaHortic.2008.779.89
- Tanaka Y, Ono M (1874) [1875] Shintei Somoku Dzusetsu, 2nd edn. Vol. 3. Mino, Ogaki, 143 pp.
- Tanaka Y, Hara M, Goto T, Yoshida Y, Yasuba K (2017) Variations in capsaicinoid contents in the chili pepper (Capsicum baccatum) and is non-pungent accessions. Scientific Reports of the Faculty of Agriculture Okayama University 106: 27–32.
- Tapia FF, Campos MA [Eds] (2016) Tumbo y Locoto en la región de Arica y Parinacota. Boletín del Instituto de Investigaciones Agropecuarias, Arica 329: 1–132.
- Terpó A (1966) Kritische Revision der wildwachsenden Arten und der kultivierten Sorten der Gattung Capsicum L. Feddes Repertorium 72(2–3): 155–191. https://doi.org/10.1002/fedr.19660720207
- Tewksbury JJ, Nabhan GP (2001) Directed deterrence by capsaicin in chillies. Nature 412(6845): 403–404. https://doi.org/10.1038/35086653
- Tewksbury JJ, Nabhan GP, Norman DM, Suzan H, Toxill J, Donovan J (1999) In situ conservation of wild chiles and their biotic associates. Conservation Biology 13: 98–107. https://doi.org/10.1046/j.1523-1739.1999.97399.x
- Tewksbury JJ, Manchego C, Haak DC, Levey DJ (2006) Where did the chili get its spice? Biogeography of capsaicinoid production in ancestral wild chili species. Journal of Chemical Ecology 32(3): 547–564. https://doi.org/10.1007/s10886-005-9017-4
- Tewksbury JJ, Levey DJ, Huizinga M, Haak DC (2008a) Costs and benefits of capsaicin-mediated control of gut retention in dispersers of wild chilies. Ecology 89: 107–117. https://doi.org/10.1890/07-0445.1
- Tewksbury JJ, Reagan KM, Machnicki NJ, Carlo TA, Haak DC, Calderón Peñaloza AL, Levey DJ (2008b) Evolutionary ecology of pungency in wild chilies. Proceedings of the National Academy of Sciences of the United States of America 105: 11808–11811. https://doi.org/10.1073/pnas.0802691105
- Thampi PSS (2003) A glimpse of the world trade in Capsicum. In: De AK (Ed.) Capsicum, The genus Capsicum. Taylor & Francis, London and New York, 16–24.
- Thiers B (2021) Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih/
- Tokunaga K (1934) Studies on the chromosome numbers of some species in Solanaceae. The Japanese Journal of Genetics 9: 231–238. https://doi.org/10.1266/jjg.9.231
- Tong N, Bosland PW (2003) Observations on interspecific compatibility and meiotic chromosome behavior of Capsicum buforum and C. lanceolatum. Genetic Resources and Crop Evolution 50: 193–199. https://doi.org/10.1023/A:1022986615694
- Tournefort JP (1719) Institutiones rei herbariae, 3rd edition, Vol 1. Typographia Regia, Paris, 695 pp. https://doi.org/10.5962/bhl.title.153849
- Tripodi P, Greco B (2018) Large scale phenotyping provides insight into the diversity of vegetative and reproductive organs in a wide collection of wild and domesticated peppers (Capsicum spp.). Plants 7(4): e103. https://doi.org/10.3390/plants7040103
- Tripodi P, Acquadro A, Lanteri S, D’Agostino N (2019) Genome sequencing of Capsicum species: Strategies, assembly, and annotation of genes. Biochemistry and Cell Biology of Ageing: Part I. Biomedical Science: 139–152. https://doi.org/10.1007/978-3-319-97217-6_8
- Tschirch A (1925) Fructus Capsici. Handbuch der Pharmakognosie: 867–878. Chr. Herm. Tauchnitz, Leipzig.
- Tschirch A, Oesterle O (1900) Fructus capsici annui. Anatomischer Atlas der Pharmakognosie und Nahrungsmittelkunde. Chr. Herm. Tauchnitz, Leipzig, 351 pp.
- Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF [Eds] (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code)-Regnum Vegetabile 159. Koeltz Botanical Books, Königstein. https://doi.org/10.12705/Code.2018
- Van Heurck HF, Müller Argoviensis J (1870) Solana nova. In: Van Heurck HF (Ed.) Observationes Botanicae et Descriptiones Plantarum Novarum Herbarii Van Heurckiani. Recueil d’Observationes Botaniques et de Descriptions de Plantes Nouvelles. Fasc. 1. F. Baggerman, Anvers [Antwerp], 38–95. https://doi.org/10.5962/bhl.title.127490
- van Zonneveld M, Ramirez M, Williams DE, Petz M, Meckelmann S, Avila T, Bejarano C, Ríos L, Peña K, Jäger M, Libreros D, Amaya K, Scheldeman X (2015) Screening genetic resources of Capsicum peppers in their primary center of diversity in Bolivia and Peru. PLoS ONE 10(9): e0134663. https://doi.org/10.1371/journal.pone.0134663
- Vazart J (1950) Nouvelles observadons sur les noyaux a calotte: étude caryologique comparée de Hordeum vulgare L., Capsicum annuum L., Pastinara urms Req. et Solanum ovigerum Dun. I. Noyau interphasique et mitosis. Revue Générale de Botanique 57: 517–553.
- Vazart J (1951) Nouvelles observations sur les noyaux a calotte: étude caryologique comparée de Hordeum vulgare L., Capsicum annuum L., Pastinaca urens Req. et Solanum ovigerum Dun. II. La méiose et la formation du pollen chez Capsicum annuum L. Revue Générale de Botanique 58: 42–61.
- Vellozo JM da C (1829) [“1825”] Florae Fluminensis. Typographia Nationali: Rio de Janeiro. https://doi.org/10.5962/bhl.title.745
- Vilagelim Costa L, Lopes R, Gomes Lopes MT, de Figueiredo AF, Silva Barros W, Marialva Alves SR (2009) Cross compatibility of domesticated hot pepper and cultivated sweet pepper. Crop Breeding and Applied Biotechnology 9: 37–44. https://doi.org/10.12702/1984-7033.v09n01a06
- Vogel S (1998) Remarkable nectaries: Structure, ecology, organophyletic perspectives. III. Nectar ducts. Flora 193: 113–131. https://doi.org/10.1016/S0367-2530(17)30827-7
- Voss A (1894) Vilmorin’s Blumengärtnerei Beschreibung, Kultur und Verwendung des Gesamten Pflanzenmaterials fur Deutsche Garten, 3rd edition. Vol. 1. Author’s edition, Berlin, 1264 pp.
- Votava EJ, Bosland PW (2004) ‘NuMex Suave Red’ and ‘NuMex Suave Orange’ Mild Capsicum chinense cultivars. HortScience 39(3): 627–628. https://doi.org/10.21273/HORTSCI.39.3.627
- Wahua C, Okoli BE, Edwin-Wosu NL (2014) Morphological, anatomical, cytological and phytochemical studies on Capsicum annuum Linn. (Solanaceae). European Journal of Experimental Biology 4(1): 464–471.
- Walpers GG (1844) Synopsis Solanacearum, Scrophularinarum, Orobanchearum et Labiatarum, Repertorium Botanices Systematicae Vol. 3. F. Hofmeister, Lipsiae, 1002 pp.
- Walsh BM, Hoot SB (2001) Phylogenetic relationships of Capsicum (Solanaceae) using DNA sequences from two noncoding regions: the chloroplast atpB-rbcL spacer region and nuclear waxy introns. International Journal of Plant Sciences 162: 1409–1418. https://doi.org/10.1086/323273
- Watson S (1890) Descriptions of new species of plants from northern Mexico, collected chiefly by Mr. C.G. Pringle, in 1888–1889. Proceedings of the American Academy of Arts and Sciences 25: 141–163.
- Weinemann JW (1745) Phytanthoza Iconographia Vol. 4. H. Lentzium, Regensburg.
- Weryszko-Chmielewska E, Michałojć Z (2011) Anatomical traits of sweet pepper (Capsicum annuum L.) fruit. Acta Agrobotanica 64(4): 181–188. https://doi.org/10.5586/aa.2011.059
- Wettstein R von (1891) Solanaceae. In: Engler HGA, Prantl KAE (Eds) Die natürlichen Pflanzenfamilien 4(3b): 4–38.
- Willdenow CL (1809) Enumeratio Plantarum Horti Regii Botanici Berolinensis. Taberna Libraria Scholae Realis, Berlin, 592 pp.
- Winton AL, Winton KB (1939) Fruits of the nightshade family (Solanaceae). In: The Structure and composition of foods 4: 443–461.
- Witasek J (1910) Solanaceae. Akademie der Wissenschaften in Wien Mathematisch-Naturwissenschaftliche Klasse 79(2): 313–375.
- Wojciechowska B (1972) Studia systematyczne nad nasionami rodziny Solanaceae Pers. (Systematic studies on the seeds of the family Solanaceae Pers.). Monographiae Botanicae 36: 117–197. https://doi.org/10.5586/mb.1972.004
- Yacovleff E, Herrera FL (1935) El mundo vegetal de los antiguos peruanos. Museo Nacional, Peru, 152 pp.
- Yamamoto K, Sakai K (1932) On the chromosome number in some Solanaceae. Japanese Journal of Genetics 8: 27–33. https://doi.org/10.1266/jjg.8.27
- Yamamoto S, Nawata E (2005) Capsicum frutescens L. in Southeast and East Asia, and its dispersal routes into Japan. Economic Botany 59: 18–28. https://doi.org/10.1663/0013-0001(2005)059[0018:CFLISA]2.0.CO;2
- Yamamoto S, Nawata E (2009) Use of Capsicum frutescens L. by the indigenous peoples of Taiwan and the Batanes Islands. Economic Botany 63(1): 43–59. https://doi.org/10.1007/s12231-008-9052-5
- Yamamoto S, Djarwaningsih T, Wiriadinata H (2013) Capsicum pubescens (Solanaceae) in Indonesia: its history, taxonomy, and distribution. Economic Botany 67(2): 161–170. https://doi.org/10.1007/s12231-013-9230-y
- Yang S, Zheng Z, Mao L, Li J, Chen B (2018) Pollen morphology of selected crop plants from southern China and testing pollen morphological data in an archaeobotanical study. Vegetation History and Archaeobotany 27: 781–799. https://doi.org/10.1007/s00334-018-0696-5
- Yaqub M, Smith PG (1971) Nature and inheritance of self-compatibility in Capsicum pubescens and C. cardenasii. Hilgardia 40: 459–470. https://doi.org/10.3733/hilg.v40n12p459
- Zamski E, Shoham O, Palevitch D, Levy A (1987) Ultrastructure of capsicinoid-secreting cells in pungent and non-pungent red pepper (Capsicum annuum L.) cultivars. Botanical Gazette 148(1): 1–7. https://doi.org/10.1086/337620
- Zhang Z-Y, Lu A-M (1999) A comparative study of Physalis, Capsicum and Tubocapsicum. In: Nee M, Symon DE, Lester RN, Jessop JP (Eds) Solanaceae IV: advances in biology and utilization. Royal Botanic Gardens, Kew, 81–96.
- Zhang Z-Y, Lu A-M, D’Arcy WG (1994) Solanaceae. In: Wu Z-Y, Raven PH (Eds) Flora of China 17: 300–332.
- Zhang Z-Y, Yang D-Z, Lu A-M, Knapp S (2005) Seed morphology of the Tribe Hyoscyameae (Solanaceae). Taxon 54(1): 71–83. https://doi.org/10.2307/25065303
- Zarlavsky G [Ed.] (2014) Histología Vegetal: técnicas simples y complejas. Sociedad Argentina de Botánica: Buenos Aires, 198 pp.
List of exsiccatae
Abbott, J.R. 16182, 16431 (baccatum var. baccatum)
Abruzzi, M.L. 576 (flexuosum)
Acevedo-Rodríguez, P. 2433, 11344 (annuum var. glabriusculum)
Acevedo-Rodríguez, P. & D. Chinea 2653 (annuum var. glabriusculum)
Acevedo-Rodríguez, P. & J. Knight 15650 (annuum var. glabriusculum)
Acevedo-Rodríguez, P. & A. Reilly 2122 (annuum var. glabriusculum)
Acevedo-Rodríguez, P. et al. 646, 5092, 5554, 5652, 7223, 14065 (annuum var. glabriusculum)
Acevedo, S. et al. 1331 (annuum var. glabriusculum)
Acosta B., L.E. 187 (annuum var. glabriusculum)
Acosta P., R. 1405 (lanceolatum)
Acosta P., R. & F. Vazquez B. 753 (rhomboideum)
Acosta P., R. et al. 39 (annuum var. glabriusculum)
Acosta Castellanos, S. 9417 (annuum var. annuum)
Acosta Solís, M. 6435, 6899, 6968 (lycianthoides); 8047 (pubescens); 8597 (rhomboideum); 8848 (annuum var. annuum); 8916, 11252, 11295 (rhomboideum); 11366 (annuum var. glabriusculum); 12925 (rhomboideum); 13577 (annuum var. annuum); 13927 (frutescens); 13989 (chinense); 14234, 14819, 14862 (rhomboideum); 16208 (baccatum var. pendulum); 16452 (rhomboideum)
Adams, C.D. 5961, 8049 (annuum var. glabriusculum)
Adrián, C.O. 84 (pubescens)
Adsersen, A. & H. Adsersen 1517, 2361 (frutescens)
Agra, M.F. 74, 625 (frutescens); 658 (parvifolium)
Agra, M.F. & G.E. Barboza 7075 (parvifolium); 7086 (longidentatum)
Agra, M.F. & L. Bohs 7257 (caatingae)
Agra, M.F. et al. 2569, 2639, 4776, 4903, 5171, 5249, 5297, 5479 (parvifolium); 7081 (caatingae); 7083 (longidentatum); 7085 (caatingae); 7244 (mirabile); 7268 (carassense); 7293, 7299 (friburgense); 7323, 7331 (mirabile); 7340 (carassense); 7360 (mirabile); 7400 (villosum)
Aguayo, A. 75 (flexuosum)
Aguayo C., J.A. & H.A. Gómez R. 191 (annuum var. glabriusculum)
Agudelo, C.A. et al. 899 (lycianthoides); 1596 (dimorphum)
Aguiar, L. & L. Martau 339 (baccatum var. baccatum)
Aguilar H., M. 64, 177 (annuum var. glabriusculum); 199 (frutescens)
Aguilar, I. 46 (rhomboideum)
Aguilar, M.H. 78 (annuum var. annuum)
Aguilar, R.M. 136, 568, 721 (chacoense)
Aguilar Zepeda, J.A. & S. Diez Martínez y Day 38, 363 (annuum var. glabriusculum)
Aguinda, R. et al. 1537 (regale)
Aguirre-Galviz, L.E. 1099 (frutescens)
Agúndez, J.M. 221, 633 (annuum var. glabriusculum)
Ahumada, O. 5013 (chacoense)
Ahumada, O. & A. Castellón 9065 (baccatum var. baccatum)
Alarcón, E. 102 (annuum var. glabriusculum)
Albert de Escobar, L.K. & A. Uribe 3645 (lycianthoides)
Alburquerque, L.B. et al. 49 (hunzikerianum)
Alcorn, J.B. 1406, 1903, 2369 (annuum var. glabriusculum)
Alegría O., P. 47 (frutescens)
Aliscioni, S.S. et al. 665 (chacoense)
Allard, H.A. 2265, 14542, 14697, 14787, 17784 (annuum var. glabriusculum); 21850 (pubescens)
Allemão, F. 1229 (parvifolium)
Allen, A. & Werneck 3505 (rabenii)
Allen, C. 484 (annuum var. glabriusculum)
Allen, P.H. 2006 (annuum var. glabriusculum)
Almeda, F. et al. 3119 (annuum var. glabriusculum)
Almeida, E.C. 48 (rabenii)
Almeida de Jesus, J. 1959, 2064 (campylopodium)
Almeida Scabbia, R.J. et al. 910 (mirabile); 5113 (cornutum)
Alonso Méndez, G. 7709 (rhomboideum)
Alston, A.H.G. 7688 (rhomboideum); 8157 (dimorphum)
Altamirano, S. et al. 3711 (coccineum)
Alvarado C., A. 198 (annuum var. annuum)
Alvarado Flores, F. 116 (annuum var. glabriusculum)
Álvarez, A. et al. 1584 (lycianthoides)
Álvarez, D. 89, 1491, 2075 (annuum var. glabriusculum)
Álvarez, D. et al. 11583 (annuum var. glabriusculum)
Álvarez, E. & O. Montañéz 1 (annuum var. annuum)
Alvaro M., P. 397 (annuum var. glabriusculum)
Alvaro M., P. & D. Álvarez 741 (annuum var. glabriusculum)
Alves de Paiva, G. 01 (baccatum var. pendulum)
Amancio, A.M.D. et al. 188 (flexuosum)
Amaral Santos, A. et al. 721 (frutescens)
Amaral, E.V.E. 38, 62 (rabenii); 63 (baccatum var. pendulum); 65 (baccatum var. umbilicatum); 139 (chinense)
Amarilla, L. et al. 31 (flexuosum)
Ambrosetti, J.A. 1898 (chacoense)
Amorim, A.M. et al. 4429, 5371 (mirabile); 6317 (caatingae); 7235 (mirabile); 8292 (longidentatum)
Ancuash Atsut, E. 296 (chinense); 297 (annuum var. annuum)
Anderson, D.L. 1447 (chacoense)
Anderson, L.O. et al. 99/36 (mirabile)
Anderson, W.R. 11718 (villosum); 12556 (annuum var. glabriusculum)
Anderson, W.R. et al. 35734 (frutescens)
Andino U., R. 69 (rhomboideum)
Andrade, P.M. & M.A. Lopes 109 (campylopodium)
Andrade, P.R. & R.M. Chagas 1188 (rabenii)
Andrade, R. 33001B (chinense)
André, A. 3317 (villosum)
André, E.F. 701, 1780, 2520 (rhomboideum); 3839, 4570 (geminifolium)
Andreata, R. et al. 522 (campylopodium)
Angel, S. et al. 465 (dimorphum)
Ângulo, F. 007 (frutescens)
Anonymous 67, 130 (annuum var. glabriusculum); 05/17 (rabenii); 1414/6 (G00131878) (annuum var. glabriusculum)
Antezana, C.721 (eximium)
Anzótegui-Benitez 109, 237 (annuum var. annuum)
Aona, L.Y.S. et al. 1220 A (baccatum var. baccatum); 2046B (pereirae)
Apolinar María (Nicholas Seiler), Bro. 114 (annuum var. glabriusculum)
Araki, D.F. et al. 105 (recurvatum); 116 (flexuosum)
Aranda et al., M. 94 (annuum var. glabriusculum)
Araque, J. 462 (lanceolatum)
Araque Molina, J. & F.A. Barkley 308 (rhomboideum); 18N.S.140 (annuum var. glabriusculum); 18M.050, 19 A053 (frutescens)
Araquistain, M. 5, 262, 278, 394 (annuum var. glabriusculum)
Araquistain, M. & P.P. Moreno 69, 505, 1276, 2856 (annuum var. glabriusculum)
Araujo, G.B. 642 (caatingae)
Araujo, G.B. et al. 205 (caatingae)
Araujo-M., A. et al. 1638 (frutescens)
Arbeláez S., G. et al. 1136, 2466 (lycianthoides)
Arbo, M.M. 341 (annuum var. annuum)
Arbo, M.M. et al. 2038 (flexuosum)
Archer, W.A. 64 (rhomboideum); 93 (annuum var. glabriusculum); 605, 761 (rhomboideum); 1170, 1273 (dimorphum); 1404 (chinense)
Arcos Vernet, R. 40 (annuum var. glabriusculum)
Areces-Mallea, A.E. 2233 (annuum var. glabriusculum)
Arenas, P. 327, 436, 555, 645, 1393, 1546, 1688, 1853, 1956, 2121, 2197, 2377, 3043, 3388, 3764 (chacoense)
Argañaraz, J.L. 210 (chacoense)
Argeñal, F. 130 (annuum var. glabriusculum)
Argüelles, E. 889 (rhomboideum)
Arias-G, J.C. & J. Garzón J. 667, 668 (frutescens)
Arias-G., J.C. et al. 682 (frutescens)
Aristaguieta 3372, 4442, 5415, 6139 (rhomboideum)
Ariza Espinar, L. 248, 1626 (chacoense); 3213 (annuum var. annuum)
Arroyo P., L. et al. 6053 (minutiflorum); 6108 (neei)
Arroyo, L. et al. 2880, 2930 (minutiflorum); 3313 (baccatum var. baccatum), 3317, 3333 (minutiflorum); 5252 (eximium)
Arsène, G. 10587 (chinense)
Arvigo, R. et al. 148 (frutescens)
Asplund, E. 6548, 6701, 6992, 7576, 8104 (rhomboideum); 9713 (geminifolium); 12491, 12940 (coccineum); 13115 (geminifolium); 15241, 15280, 15363 (hookerianum); 18050 (pubescens); 15822, 18272 (rhomboideum); 19430, 19746 (chinense); 19939 (rhomboideum); 20070 (Capsicum benoistii)
Atahuachi, M. et al. 773 (eximium); 1629 (caballeroi); 1632, 1634, 1635, 1636, 1666 (baccatum var. baccatum)
Atamp 7659 (muticum)
Atha, D.E. et al. 1022 (frutescens)
Attié, M.C.B. et al. 59 (recurvatum)
Atwood, J.T. 2488 (annuum var. glabriusculum)
August, L. 18 (chacoense)
Augusto, H.H. 4847 (annuum var. glabriusculum)
Avellaneda, M. 12 (annuum var. glabriusculum)
Avendaño R., S. & C. Durán E. 3143 (annuum var. glabriusculum)
Ávila et al., A. 783 (annuum var. glabriusculum); 817 (rhomboideum)
Ayala, F. 5786 (annuum var. glabriusculum)
Ayala, F. et al. 2792, 2793 (chinense)
Ayala, M.G. 232 (annuum var. glabriusculum)
Ayarde, H. & M. Sidán 299 (chacoense)
Aymard, G. 5108 (annuum var. annuum)
Aymard, G. et al. 473 (frutescens); 2721 (annuum var. glabriusculum)
Bacab W., G. 115 (annuum var. glabriusculum)
Bacigalupo, N.M. et al. 1136 (chacoense); 1163 (baccatum var. baccatum)
Báez, S. et al. 28 (chinense)
Badillo, V. 6379 (frutescens); 7159 (rhomboideum)
Bailetti, E. 207 (eximium)
Baitello, J.B. 547 (villosum)
Baizabal M., R.M. & M.G. Zola B. 11, 12, 13 (annuum var. annuum)
Baker, C.F. 148 (annuum var. glabriusculum)
Baksh, Y.S. 10 (annuum var. glabriusculum)
Balansa, B. 2079, 2079a (rabenii); 2107, 2110, 2120 (flexuosum); 3131 (baccatum var. baccatum); 4704 (flexuosum)
Balcazar, A. 74 (geminifolium)
Baldeón Malpartida, S.M. & J. Campos 5316 (longifolium)
Balderrama, J. 10 (annuum var. annuum)
Balegno, B. 1058, 1149 (chacoense)
Balick, M.J. 2211 (chinense)
Balick, M.J. et al. 1539 (annuum var. glabriusculum); 2272, 2391 (frutescens)
Balls, E. 7153 (rhomboideum)
Balslev, H. 4890, 4892, 4894, 4895, 4904 (chinense)
Balslev, H. & E. Asanza 4363 (annuum var. glabriusculum)
Balslev, H. & D. Irvine 4582 & 4598 (chinense)
Bamonte, A.G. 77, 79 (annuum var. annuum)
Bang, M. 200, 727 (pubescens); 1126 (eximium); 1185 (pubescens); 1474 (baccatum var. pendulum)
Banton, F.S. 6838 (frutescens)
Barbosa, C. 14060, 14924 (dimorphum)
Barbosa, M.R. et al. 2674, 2895, 3127 (parvifolium)
Barboza, G.E. 86 (chacoense); 164 (baccatum var. umbilicatum); 632, 638, 645 (chacoense); 795 (frutescens); 1123 (baccatum var. umbilicatum); 1793 (chacoense); 1805, 1807 (baccatum var. baccatum); 1901 (chinense); 1918 (baccatum var. umbilicatum); 1919 (eximium); 2064bis, 2271 (frutescens); 2431 bis (baccatum var. baccatum); 2504, 2505, 2506 (chinense); 3055 (baccatum var. baccatum); 4078, 4200, 4766, 4803, 4809 (chacoense); 4824 (baccatum var. pendulum); 4881, 4882, 4884 (cardenasii); 4885 (eximium); 4886 (baccatum var. pendulum); 4895, 4896, 4902, 4903 (eximium); 4907, 4908 (caballeroi); 4910 (chacoense); 4914 (eximium), 4927 (neei); 4975 (baccatum var. pendulum); 5038 (baccatum var. baccatum); 5041 (hunzikerianum); 5042, 5043 (schottianum); 5044 (tovarii); 5047 (dimorphum); 5163 (baccatum var. umbilicatum)
Barboza, G.E. & C. Carrizo García 3644 (minutiflorum); 3646 (baccatum var. baccatum); 3648 (baccatum var. umbilicatum); 3946 (muticum)
Barboza, G.E. & M.T. Cosa 2517 (cornutum); 2519 (schottianum); 2525 (cornutum); 2526 (schottianum); 2533 (mirabile)
Barboza, G.E. & R. Deanna 5000 (recurvatum); 5003 (rabenii); 5004 (recurvatum); 5006, 5007, 5009, 5014, 5015, 5017, 5019 (schottianum); 5020 (rabenii); 5021 (flexuosum); 5024 (mirabile); 5026 (recurvatum); 5026 bis (villosum); 5027 (rabenii); 5028 (mirum)
Barboza, G.E. & S. Leiva González 4819 (geminifolium); 4821 (longifolium); 4826 (hookerianum); 4829 (frutescens); 4831 (hookerianum); 4832 (annuum var. glabriusculum); 4841, 4842 (piuranum); 4845 (geminifolium); 4846, 4849, 4850, 4851 (longifolium); 4852 (geminifolium); 4854 (rhomboideum); 5053 (dimorphum);
Barboza, G.E. & X. Reyes 5040 (neei)
Barboza, G.E. et al. 415 (baccatum var. baccatum); 426 (flexuosum); 458 (baccatum var. baccatum); 905 (flexuosum); 915 (recurvatum); 927 (baccatum var. baccatum); 1027, 1034 (flexuosum); 1038 (baccatum var. pendulum); 1274 (baccatum var. baccatum); 1487 (flexuosum); 1622 (chinense); 1629, 1632 (recurvatum); 1638 (schottianum); 1640, 1646, 1646b, 1647 (rabenii); 1648, 1649 (mirum); 1650 (schottianum); 1651 (pereirae); 1653 (villosum); 1654 (rabenii); 1655 (villosum); 1656 (rabenii); 1657 (flexuosum); 1847 (pubescens);1849 (eximium × pubescens); 1860, 1905 (eximium); 1916 (coccineum); 1921 bis (baccatum var. baccatum); 2019 (frutescens); 2021 (recurvatum); 2022 (frutescens); 2023 (recurvatum); 2032, 2033, 2034, 2035, 2036, 2037 (schottianum); 2041 (frutescens); 2048 (friburgense); 2057 (campylopodium); 2058 (mirabile); 3419 (baccatum var. baccatum); 3543 (eximium); 3631 (flexuosum); 3632 (mirabile); 3633, 3634, 3635, 3636, 3637 (schottianum); 3655 (caballeroi); 3665 (frutescens); 4889, 4890 (pubescens); 4891 (baccatum var. pendulum); 4913 (baccatum var. baccatum); 4918 (minutiflorum); 4919 (baccatum var. baccatum); 4921 (coccineum); 4923 (pubescens); 4924, 4925, 4926 (eximium × pubescens); 5032 (muticum); 5049 (annuum var. glabriusculum); 5050 (rhomboideum)
Barkley, F.A. 6, 10, 262 (dimorphum); 13434 (annuum var. glabriusculum)
Barkley, F.A. & L.L. Arboleda R. 14 (rhomboideum)
Barkley, F.A. & C. Bostirronnois 39498 (annuum var. glabriusculum)
Barkley, F.A. & G. Gutiérrez V. 1765 (annuum var. annuum); 1776 (annuum var. glabriusculum)
Barkley, F.A. et al. 17C403 (rhomboideum); 17C405 (annuum var. glabriusculum)
Barlett, H.H. 10007 (annuum var. glabriusculum)
Barreto, K.D. et al. 980 (baccatum var. baccatum)
Barreto, V. et al. 218 (caatingae)
Barros, W.D. 728 (villosum)
Barth, O.M. I 214 (villosum)
Bartholomew, B. et al. 2719 (annuum var. glabriusculum)
Bartlett, H.H. 10806 (rhomboideum); 20417, 20435 (chacoense)
Bastión, E. 407 (chacoense); 681 (eximium); 779 (chacoense)
Bates, D. et al. 1481 (annuum var. glabriusculum)
Batista, E.R. et al. 129 (recurvatum)
Bautista, H.P. & G.C. Pinto 1023 (caatingae)
Beaman, J.H. 6014 (lanceolatum)
Becerra Gonzáles, E. & J. Perea 1152 (annuum var. glabriusculum)
Becerril Pérez, S. & N.L. Ortiz Cornejo 7 (frutescens)
Beck, H.T. et al. 87 (annuum var. glabriusculum); 90, 91 (chinense); 116 (frutescens)
Beck, S. 130 PG94 (cardenasii); 3051 (ceratocalyx); 4465 (eximium); 5362 (frutescens); 6255, 9789 (eximium); 13372 (annuum var. annuum); 17097 (frutescens); 17259 (eximium); 21356 (baccatum var. baccatum); 23451 (minutiflorum); 25261 (eximium); 28089 (ceratocalyx); 32066 (baccatum var. baccatum); 33135 (ceratocalyx); 33151 (pubescens)
Beck, S. & M. Liberman 16298 (baccatum var. baccatum)
Beck, S. et al. 19150 (annuum var. glabriusculum); 19317 (chinense); 22714 (baccatum var. baccatum)
Bedoya, M. 1, 2 (annuum var. glabriusculum); 5 (lycianthoides); 316 (rhomboideum)
Belinelo, R. 05/18 (recurvatum)
Belshaw, C.M. 3084 (coccineum); 3210 (baccatum var. pendulum)
Beltrán, G.D. et al. 24 (rhomboideum); 85, 87 (lycianthoides); 140 (dimorphum)
Benítez, B. & Anzotegui 76 (baccatum var. baccatum)
Benítez, S. 612, 688, 689, 724, 726, 732, 734, 735, 738, 744 (annuum var. annuum)
Benítez, S. et al. 386, 387 (annuum var. annuum)
Benítez B., B. 585 (flexuosum)
Benítez de Rojas, C. 13, 298 (frutescens); 330 (rhomboideum); 379 (frutescens); 970 (rhomboideum)
Benítez de Rojas, C. & M.J. Baldizán 4976, 4980 (rhomboideum)
Benítez de Rojas, C. & F. Rojas 4202 (parvifolium); 4786 (rhomboideum)
Benítez de Rojas, C. et al. 4821, 4898 (rhomboideum)
Bennett, B.C. & P. Gomez Andrade 3776 (frutescens); 3441 (chinense)
Bennet, B. et al. 4276 (chinense)
Benoist, M.R. 3478, 3580 (rhomboideum); 4204 (benoistii)
Bensman, R. 310 (chinense); 480 (lycianthoides)
Bentancur, J. 11063, 11315 (rhomboideum)
Bentley, P.S. 312, 312-a, 312-b, 456 (frutescens)
Berlandier, J.L. 97, 152, 1863, 1907, 3045 (annuum var. glabriusculum)
Berlin, B. 1572 (annuum var. annuum); 2016 (annuum var. glabriusculum)
Bermúdez, L.A. 34950 (rhomboideum)
Bermúdez, L.A. & F.A. Barkley 17C889 (annuum var. glabriusculum)
Bernacci, L.C. et al. 957 (recurvatum); 1449 (rabenii); 2816 (baccatum var. umbilicatum); 2820, 4467 (rabenii); 21468 (flexuosum)
Bernal, C. 642 (rhomboideum)
Bernal, C. & A. Pico 350 (rhomboideum)
Bernal, C. et al. 1098 (frutescens)
Bernal, R. 1828 (dimorphum)
Bernardello, G. 471 (chacoense); 932 (baccatum var. baccatum)
Bernardello, G. & L. Galetto 726 (chacoense)
Bernardello, G. et al. 498, 525 (chacoense)
Bernardi, L. 6105 (annuum var. glabriusculum)
Bernoulli, K.G. & O.R. Cario 2339 (lanceolatum); 2344, 2378, 2395 (annuum var. glabriusculum)
Berry, P.E. 1044, 1341 (rhomboideum)
Berti, H. & M. Escalante 431 (chacoense)
Bertoni, J.E. 400 (flexuosum)
Betancur, J. et al. 352 (annuum var. glabriusculum); 3417 (geminifolium); 6187 (dimorphum); 10158, 11258 (annuum var. glabriusculum); 11615 (frutescens)
Bianchetti, L. & P.G. Bustamante 1518 (mirabile)
Bianchetti, L. & G. Pereira Silva 978 (annuum var. glabriusculum)
Bianchetti, L. et al. 330, 331, 332, 335, 346, 347, 348, 349, 350, 351, 352, 354, 359, 363, 366, 379, 380, 381, 382, 383, 384 (mirabile); 391 (friburgense); 393 (friburgense); 394 (mirabile); 399, 400 (campylopodium); 406, 407, 408 (muticum); 409, 410, 411, 415, 416 (schottianum); 417, 418, 420, 422, 425, 429, 430, 431, 432, 433 (villosum); 435 (rabenii); 438, 439 (schottianum); 444 (villosum); 445, 446, 456, 457, 458, 459, 461, 462, 464, 473 (recurvatum); 476, 480, 481 (schottianum); 487 (villosum); 489 (schottianum); 490 (recurvatum); 491, 492, 493 (cornutum); 495, 496 (mirabile); 499, 500, 501 (schottianum); 504, 505 (rabenii); 510 (mirabile); 511 (campylopodium); 512 (carassense); 1270, 1273 (pereirae); 1299 (friburgense); 1333 (cornutum); 1346, 1347, 1348, 1349, 1350 (pereirae); 1363, 1364, 1367, 1368 (carassense); 1517, 1520, 1523 (recurvatum); 1524 (rabenii); 1526, 1527 (recurvatum); 1533, 1534 (flexuosum); 1535 (schottianum); 1537 (hunzikerianum); 1538 (mirabile); 1539 (villosum); 1540 (schottianum); 1542 (cornutum); 1543 (villosum); 1544, 1545 (schottianum); 1546 (recurvatum); 1549 (villosum); 1550, 1551 (mirabile); 1552 (flexuosum); 1553 (rabenii); 1554 (mirabile); 1555 (rabenii); 1556 (mirabile); 1557 (villosum);1558 (pereirae); 1559 (mirabile); 1560 (caatingae); 1561 (schottianum); 1562 (campylopodium); 1563 (schottianum); 1564 (mirabile); 1565 (friburgense); 1566 (campylopodium); 1567 (pereirae); 1568 (mirabile)
Bianchi, S. & C. Camardelli 951 (chacoense)
Bianco, C.A. 651, 1652, 3216, 3465 (chacoense)
Biganzoli, F. et al. 544 (flexuosum)
Biggs, N. et al. 38 (baccatum var. baccatum); 118 (eximium)
Billiet, F. 3183 (chacoense)
Bishop, A. & B. Holst CC0048, CC0053 (annuum var. glabriusculum)
Bittar, M. & J. Bessa 90 (villosum)
Biurrum, F. & E. Pagliari 3271 pp (chacoense)
Blanco-Macías, A. 1559 (annuum var. glabriusculum)
Blanchet, J.S. 2823 (parvifolium); 3098A (annuum var. glabriusculum)
Blanchet, M. 90 (chinense)
Bocayuva, M. & A. Calvente 91 (campylopodium)
Boeke, J.D. 2327 (lycianthoides)
Boeke, J.D. & S. Boeke 3027 (pubescens)
Bohs 2026, 2190, 3446 (rhomboideum)
Bohs, L. & M. Nee 2754 (coccineum)
Bohs, L. & C. Vogt 3167, 3171 (flexuosum)
Bohs, L. et al. et al. 2823 (minutiflorum); 3346, 3401, 3421 (geminifolium); 3929 (annuum var. glabriusculum)
Bolotin, D. 21 (chinense)
Bonifaz, C. & X. Cornejo 3126, 3249 (lycianthoides); 4158 (annuum var. annuum)
Bonilla Baes, R. & E. Monsalvo G. 346 (pubescens)
Bono, J. 5167, 6707 (rhomboideum); 6896 (frutescens)
Boom, B.M. & C. Brewer-Carias 10105 (annuum var. glabriusculum)
Boom, B.M. et al. 11158 (annuum var. glabriusculum)
Bordas, E. & G. Schmeda 4024 (baccatum var. baccatum)
Borges, R. 65 (frutescens); 66 (baccatum var. pendulum); 67 (chinense)
Borges, R.A.X. et al. 298 (mirabile); 517, 525 (caatingae); 707 (mirabile)
Bornmüller, A. 585 (flexuosum)
Bortolotto, I..M. & D.P. Rodrigues B-615 (frutescens)
Botta, S. & F. Ruiz de Angulo 115 (chacoense)
Botteri, M. 904 (frutescens); 1855 (annuum var. glabriusculum)
Bourdy, G. 2000 (chacoense)
Bovini, M.G. 1119 (recurvatum); 1142 (rabenii); 1211 (campylopodium);1234 (rabenii)
Bovini, M.G. & J.M. Braga 1566 (campylopodium)
Bovini, M.G. & L.C.S. Giordano 363 (cornutum)
Bovini, M.G. & C.M. Mynssen 2729 (campylopodium)
Bovini, M.G. et al. 695, 959 (schottianum); 1721 (villosum); 1993 (schottianum); 2100 (campylopodium); 2605 (mirabile); 4229 (friburgense)
Bowman, R.I. 63 (frutescens)
Box, H.E. 1058 (annuum var. glabriusculum)
Boyle, B. et al. 5020 (annuum var. glabriusculum)
Bradburn, A. & S. Darwin 1272 (annuum var. glabriusculum)
Brade, A.C. 12668, 15095 (cornutum); 17263, 17463 (villosum)
Brade, A.C. et al. 19148 (campylopodium)
Braem, S.W. NC0026 (annuum var. glabriusculum)
Braga, J.M.A. 3973 (recurvatum); 4361 (campylopodium)
Braga, J.M.A. et al. 867 (schottianum);1613, 2005, 2134, 3076, 3730, 3801, 3898 (villosum); 7273 (campylopodium)
Braga, P.I.S. 2441 (cornutum)
Braga, R. 1536 (baccatum var. baccatum)
Brand, J. & M. González 604 (annuum var. glabriusculum)
Brandegee, J. 2294 (rhomboideum)
Brandbyge, J. & E. Asanza 32813 (chinense)
Brandegee, T.S. 414 (annuum var. glabriusculum)
Bravo V., E. 615 (rhomboideum)
Breckon, G. 5574 (annuum var. glabriusculum)
Breedlove, D.E. 7155-A (annuum var. annuum); 10371 (annuum var. glabriusculum); 10591 (rhomboideum); 10689 (frutescens); 11248, 11249, 11716 (annuum var. annuum); 11805 (annuum var. glabriusculum); 11696 (pubescens); 16083 (annuum var. annuum); 19922 (frutescens); 20370 (annuum var. glabriusculum); 25238, 25667 (rhomboideum); 26097 (lanceolatum); 26362 (rhomboideum); 26482 (annuum var. glabriusculum); 26518 (annuum var. annuum); 26889, 28050, 33849 (annuum var. glabriusculum); 34836, 35237 (lanceolatum); 36628, 36682 (annuum var. glabriusculum); 36720 (rhomboideum); 37442 (annuum var. glabriusculum); 38789 (lanceolatum); 39818, 40592 (rhomboideum); 41082 (lanceolatum); . 44367 (rhomboideum); 48935 (annuum var. glabriusculum); 51520 (rhomboideum); 52096 (annuum var. glabriusculum); 53338, 56499, 68656 (lanceolatum); 69942, 70605, 70888 (rhomboideum)
Breedlove, D.E. & B. Anderson 62936 (annuum var. glabriusculum)
Breedlove, D.E. & P.H. Raven 13239 (annuum var. glabriusculum)
Breedlove, D.E. & A.R. Smith 22629, 31992 (lanceolatum)
Breedlove, D.E. & R.F. Thorne 31031(lanceolatum)
Brenckle, J.F. 47-418 (frutescens)
Breteler, F.J. 3207, 4059 (rhomboideum)
Briones V., O.L. 1866 (annuum var. glabriusculum)
Bristol, M.L. 702 (frutescens); 1115, 1335 (pubescens)
Britton, E.G. 2942 (annuum var. glabriusculum)
Britton, N.L. 350, 379, 1007, 6242 (annuum var. glabriusculum)
Britton, N.L. & L.J. Brace 660 (annuum var. glabriusculum)
Britton, N.L. & E.G. Britton 7344, 9848 (annuum var. glabriusculum)
Britton, N.L. & J.F. Cowell 9965 (annuum var. glabriusculum)
Britton, N.L. & A. Hollick 1829 (annuum var. glabriusculum)
Britton, N.L. & C.F. Millspaugh 3014, 5450, 6234 (annuum var. glabriusculum)
Britton, N.L. & W.M. Wheeler 231 (annuum var. glabriusculum)
Britton, N.L. & P. Wilson 256 (annuum var. glabriusculum)
Britton, N.L. et al. 112, 1785, 12444, 15299 (annuum var. glabriusculum)
Brizuela, A. 343, 627, 664, 821, 837, 936 (chacoense)
Brizuela, J. 70, 196, 229, 338, 410, 475, 875, 1009, 1066, 1127, 1174, 1217, 1225, 1284, 1312, 1609 (chacoense)
Broadway, W.E. 366, 4514-Ser. I, 4563 (annuum var. glabriusculum); 4498 (frutescens);
Brockmann, J. 103 (annuum var. glabriusculum)
Brooke, M.A. 5934 (eximium)
Brosehel, M. & J. Craig 406 (carassense)
Brown, S. 93 (annuum var. glabriusculum)
Brumbach, W.C. 7357, 7750, 7754, 8915, 8917 (annuum var. glabriusculum)
Brunner, D. 1569, 1692, 1741 (chacoense); 1804 (flexuosum)
Brunner, D. et al. 913 (flexuosum)
Bryant, P.T. & G. Bryant 94 (annuum var. glabriusculum)
Buchinger, M. & Rodríguez 3222 (flexuosum)
Buchtien, O. 337 (baccatum var. pendulum); 338 (pubescens); 2231 (minutiflorum); 3262 (eximium); 4033 (pubescens); 4698 (baccatum var. pendulum); 5542 (pubescens)
Buendia S., M.C. 2 (annuum var. glabriusculum)
Bueno, E.A. 47 (campylopodium)
Bueno, O. et al. 628 (flexuosum)
Búgola-Silva, F. 8041 (schottianum)
Bullock, S.H. 1231, 1937 (annuum var. glabriusculum)
Bunch et al., P. 685 (annuum var. glabriusculum)
Bünger, M.O. & M.C. Souza 635 (campylopodium)
Bunting, G.S. 8159 (annuum var. glabriusculum)
Burandt, C. et al. 27V (annuum var. glabriusculum); 193 (parvifolium)
Burandt Jr. V., C. 1003 (rhomboideum)
Buratovich, F. 117, 152, 303, 314, 396, 413, 477, 580, 606 (chacoense)
Burch, D. 2335 (annuum var. glabriusculum)
Burch, D. & M. Bialek 6839 (annuum var. annuum)
Burch, D. & N. Chevalier 3709, 3716 (annuum var. annuum)
Burch, D. & T. Doughty 6840 (annuum var. annuum)
Burch, D. & R.J. Machwart 3714 (annuum var. annuum)
Burch, D. & L. Schmoll 6838 (annuum var. annuum)
Burchell, W.J. 1319 (baccatum var. baccatum); 1913 (campylopodium); 2428 (schottianum); 2443 (recurvatum); 3460 (rabenii); 3472 (recurvatum); 3705 (cornutum)
Burkart, A. 7752, 12553, 13361, 13972, 20300, 21384, 22299, 23492, 25459, 26746 (chacoense)
Burkart, A. et al. 28424 (annuum var. annuum)
Búrquez, A. & E. Búrquez 548 (annuum var. glabriusculum)
Búrquez, A. et al. 638 (annuum var. glabriusculum)
Bush, B.F. 1205 (annuum var. glabriusculum)
Bye, R.A. et al. 13 (pubescens); 89 (chinense); QD 228, QD 246 (annuum var. annuum); 1875, 16386 (annuum var. glabriusculum)
Caballero Pardo, R. 40 (frutescens); 103 (eximium)
Caballero Serrano, V. 006 (chinense)
Cabrera 3365, 3366 (chinense)
Cabrera C., E.F. & H. de Cabrera 2939, 5819 (lanceolatum)
Cabrera, A.L. & A.A. Sáenz 29195 (flexuosum)
Cabrera, A.L. & J.M. Marchionni 12781 (chacoense)
Cabrera, A.L. et al. 21831, 23353 (chacoense); 23364, 31487 (baccatum var. baccatum); 31896 (chacoense)
Cabrera, E. & H. de Cabrera 3130, 6458, 7021, 7139, 8787, 8990, 9076, 9115, 9147, 9560, 9602, 9654, 10056, 10269, 11512, 11748, 13073, 13858, 14056, 14277, 14422 (annuum var. glabriusculum)
Cabrera, E. & O. Canul 5513 (annuum var. annuum)
Cabrera, E. & L. Cortéz 208, 427 (annuum var. glabriusculum)
Cabrera, E. & R. Torres 938 (annuum var. glabriusculum)
Cabrera, E. et al. 3001, 4578, 5643, 8523 (annuum var. glabriusculum)
Cabrera, I. 36 (annuum var. glabriusculum)
Cabrera, O. et al. 291 (dimorphum)
Cáceres, S. et al. 343 (baccatum var. baccatum)
Cadena-M., J.J. et al. 143 (dimorphum)
Caity, G. 190 (annuum var. annuum)
Calderón, S. 523 (annuum var. annuum); 1197 (annuum var. glabriusculum); 1198 (frutescens); 1658 (annuum var. glabriusculum)
Calderón, Y. 123 (annuum var. glabriusculum)
Calle A., J. et al. 70 (rhomboideum)
Callejas, R. 3151 (dimorphum)
Callejas, R. et al. 6373 (dimorphum)
Calónico S., J. 22503 (annuum var. glabriusculum)
Calónico S., J. et al. 5810, 21404 (annuum var. glabriusculum)
Calzada, J.I. 2367, 2368 (annuum var. annuum); 2369 (annuum var. glabriusculum); 04954 (annuum var. annuum); 5577, 10493 (annuum var. glabriusculum); 10856 (pubescens); 12646 (lanceolatum); 12662 (frutescens); 14823 (annuum var. annuum); 19320 (annuum var. glabriusculum)
Calzada, J.I. & H.V. Der Well 15483 (annuum var. annuum)
Calzada, J.I. et al. 3774 (annuum var. annuum); 3990, 9679 (annuum var. glabriusculum)
Calzada, J.J. 21036, 22605 (rhomboideum)
Calzadilla, E. et al. 111 (caballeroi)
Calzadilla, J.J. 2136, 2704 (rhomboideum)
Camargo, L.A. 7781 (dimorphum)
Cambar, I. 173 (rhomboideum)
Camp, W.H. E-1739 (pubescens); E-2373 (rhomboideum); E-3366, E-4312 (geminifolium); E-5031 (pubescens);
Campos de la Cruz, J. 4833 (annuum var. glabriusculum)
Campos de la Cruz, J. & L. Campos 5988 (rhomboideum)
Campos de la Cruz, J. & S. Corrales 3603 (geminifolium)
Campos de la Cruz J. & O. Díaz 2293 (annuum var. glabriusculum); 2333 (annuum var. annuum); 2339 (frutescens); 2486 (annuum var. glabriusculum)
Campos de la Cruz, J. & S. Nuñez 4266 (pubescens)
Campos de la Cruz, J. & W. Vargas 6945, 6947, 6981 (chinense)
Campos de la Cruz, J. et al. 3031 (geminifolium); 3732 (rhomboideum); 6607 (longifolium)
Campos V., A. 1176, 3203 (rhomboideum); 4107 (annuum var. glabriusculum)
Cândido, E.S. et al. 178, 290, 298 (villosum); 317, 320, 333 (mirabile); 404 (villosum); 750 (mirabile)
Cano, A. 374 (annuum var. annuum); 2278 (geminifolium)
Cantero, J.J. 6385, 6423, 6976, 7102, 7311 (chacoense)
Cantino, P. 698 (chacoense)
Capparelli, A. 158 (chacoense)
Carabia, J.P. 3633 (annuum var. glabriusculum)
Caranqui, J. et al. 517 (lycianthoides)
Carauta, P.P. & P. Maas 2730 (campylopodium)
Carbonó, E. 4128 (rhomboideum)
Cárdenas, D. 9325, 9328, 24632 (frutescens)
Cárdenas, D. et al. 9300 (frutescens); 9306 (annuum var. annuum); 9307 (frutescens); 9308 (chinense); 9310 (annuum var. annuum); 9311 (chinense); 9312 (annuum var. annuum); 9317 (chinense); 9319 (annuum var. annuum); 9331 (chinense); 9354 (frutescens); 9356 (chinense); 9360 (annuum var. annuum); 9361 (chinense); 9362 (frutescens); 9377 (chinense); 9380 (annuum var. annuum); 9384 (frutescens); 9385, 9393, (chinense); 9394 (frutescens); 9401, 9403 (baccatum var. baccatum); 9405 (frutescens); 9406 (annuum var. annuum); 9412, 9413 (frutescens); 9423 (baccatum var. baccatum); 9424 (annuum var. annuum); 9431 (chinense); 9434 (frutescens); 9438 (annuum var. annuum); 9439 (chinense); 9441 (frutescens)
Cárdenas, L. 3936 (rhomboideum)
Cárdenas, M. 2270, 4237 (eximium); 4701 (baccatum var. baccatum); 5114 (eximium); 5565 (baccatum var. pendulum)
Cárdenas R., R. PU. 0558 (annuum var. annuum)
Cardona, F.A. & H. David 1224 (dimorphum)
Cardoso, D. 625 (caatingae)
Cardoso, D. & R.M. Santos 1922 (parvifolium)
Cardoso, J. 150 (mirabile)
Cardoso, L.J.T. et al. 1130 (recurvatum)
Cardozo, A. et al. 231 (baccatum var. baccatum)
Carette, E. 28 (chacoense)
Carmo, F.F. & L.C. Ribeiro 3527 (carassense)
Carneiro, E.M. 371 (caatingae)
Carneiro-Torres, D.S. et al. 717 (caatingae)
Carnevali, G. et al. 531 (parvifolium)
Carnevali, R. 5290 (chacoense)
Caro, J.A. 1694 (chacoense)
Carranza González, E. 3266 (annuum var. glabriusculum)
Carranza, E. 736, 766, 772, 937, 2455, 3212 (rhomboideum)
Carranza, E. & S. Zamudio 5519 (rhomboideum)
Carrasco, J.A. et al. 147 (caballeroi)
Carrasquilla, L. et al. 281 (chinense)
Carrasquillo, J.A. 128 (annuum var. glabriusculum)
Carretero, A. et al. 824, 939, 998, 1067, 1085 (neei)
Carrillo, L. & D. Reyes 434, 591 (chinense)
Carrizo García, C. 61, 62 (minutiflorum); 63 (baccatum var. baccatum); 64 (minutiflorum); 67 (eshbaughii); 73 (lanceolatum); 84 (flexuosum); 101 (baccatum var. umbilicatum), 102 (annuum var. glabriusculum)
Carter, A. 4902, 4921 (annuum var. glabriusculum)
Carter, A. & R. Morán 5365 (annuum var. glabriusculum)
Carvajal, J. 1 (annuum var. glabriusculum)
Carvajalino & Díaz 11 (annuum var. glabriusculum)
Carvalho, B. et al. 24 (pereirae)
Carvalho, D.N. 283, 308, 515, 582 (caatingae)
Carvalho, D.N. & M.O. Matos 188 (caatingae)
Carvalho-Sobrinho, J.G. et al. 2518 (parvifolium)
Casadiego, J. 248 (rhomboideum)
Cascante, J. 6 (pubescens)
Castañeda, M.E. 79 (rhomboideum)
Castañeda Vega, R. 72 (chacoense)
Castellanos, A. 54, 212 (lycianthoides); 217 (chacoense); 239 (lycianthoides); 24386 (campylopodium)
Castiglioni, J.A. & A.E. Ragonese 7042, 7109, 8027 (chacoense)
Castiglioni et al. 7324 (chacoense)
Castillo, A. 1079 (frutescens)
Castillo C., G. 207 (annuum var. glabriusculum); 214 (pubescens); 4136, 9327 (annuum var. glabriusculum); 1757 (pubescens); 14287, 18139, 18164 (annuum var. glabriusculum)
Castillo, D. et al. 029 (annuum var. annuum)
Castillo, J. et al. 165 (rhomboideum)
Castillo Campos, G. et al. 19219 (rhomboideum)
Castillo Mont, J.J. 2235 (rhomboideum)
Castillón, L. 24, 3568, 14331 (chacoense)
Castro, M. & A. Magallanes 39 (parvifolium)
Castro, R.M. et al. 1229 (caatingae)
Castro, S. & N. Andoke 597, 606 (frutescens); 607 (annuum var. glabriusculum); 608 (frutescens)
Castro, S. & C. Bernal 32 (dimorphum)
Castro, S. & I. Matapí 561 (chinense); 562 (frutescens); 563, 564, 565 (chinense)
Castro, S. & R. Matapí 523 (annuum var. glabriusculum)
Castro, S. & A. Rodríguez 132, 307, 308, 309, 310, 311, 312, 313, 314 (chinense)
Castro, S. & Virgelina 305, 307 (annuum var. annuum)
Castro, S. et al. 238 (annuum var. annuum)
Catharino, E.L. 720 (rabenii)
Caxambu, M.G. & E.L. Siqueira 2890 (flexuosum)
Caxambu, M.G. et al. 5146, 5902 (flexuosum)
Cayola, L. et al. 42, 127 (baccatum var. baccatum)
Cedillo Trigos, R. & R. Torres C. 2309 (rhomboideum)
Cedillo Trigos, R. et al. 794 (rhomboideum)
Cerana, M.M. 823 (baccatum var. baccatum)
Cerón, C.E. 194 (frutescens); 11092 (rhomboideum); 14561 (frutescens); 15237, 15577 (pubescens); 15714 (rhomboideum); 18324 (lycianthoides); 18842 (annuum var. annuum); 18856 (rhomboideum); 20848 (chinense)
Cerón, C.E. & M. Cerón 4712 (chinense)
Cerón, C.E. & C. Reyes 62013 (chinense)
Cerón, C.E. & M. Montesdeoca 12519, 13138, 13171 (rhomboideum)
Cerón, C.E. & 3er Curso Biología U. Central 6953 (rhomboideum)
Cerón, C.E. et al. 1292, 1299, 5849 (chinense); 18258 (hookerianum)
Cerrate de Ferreyra, E. 7644, 7673 (pubescens)
Cervi, A.C. & F.C. Silva 2039 (recurvatum)
Chagas-Mota, E.C. & L.M. Leão 3144 (caatingae)
Chagas-Mota, E.C. 828, 4711, 4828, 6286, 7450, 7578, 8184, 9419 (caatingae)
Chagas-Mota, E.C. et al. 11047 (caatingae)
Chan, C. 4149 (frutescens); 7144 (annuum var. glabriusculum)
Chan, C. et al. 30, 253, 1406 (annuum var. glabriusculum)
Chandler, H. 7044 (annuum var. glabriusculum)
Charpin, A. & L. Ramella 21481 (baccatum var. baccatum)
Chase, V.H. 7075 (rhomboideum)
Chater, A.O. 41 (annuum var. glabriusculum)
Chautems, A. et al. 165 (parvifolium)
Chavarría, U. 16 (annuum var. glabriusculum)
Chaves, E.M.F. et al. 182 (parvifolium)
Cháves, J.L. 235 (annuum var. glabriusculum)
Chazaro B., M. 2558 (pubescens)
Chazaro B., M. et al. 5164 (annuum var. glabriusculum)
Chevez, S. 40 (annuum var. annuum)
Chiang, F. 155, 209 (annuum var. glabriusculum)
Chiarini, F. & G. Wahlert 875 (flexuosum)
Chiarini, F. 758 (chacoense); 545 (annuum var. annuum); 1223 (baccatum var. baccatum)
Chicharo, D. 278 (annuum var. glabriusculum)
Chiea, S.A.C. 223 (cornutum)
Chodat, R.H. 47 (flexuosum)
Christopher, D.K. et al. 64 (annuum var. glabriusculum)
Chuck, L. 137 (pubescens)
Clark, J.L. & C. Aulestia 12193 (geminifolium)
Clark, J.L. et al. 3831 (hookerianum); 5337 (regale); 7671 (lycianthoides); 9403 (regale); 9806 (geminifolium); 9965 (dimorphum)
Clark, O.M. 7135 (rhomboideum)
Clase, T. et al. 4656 (annuum var. glabriusculum)
Cleef, A.M. et al. 11091 (rhomboideum)
Clewell, A.F. & F.C. Craighead 543 (annuum var. glabriusculum)
Cocucci, A.A. 248, 477, 978, 4966 (chacoense)
Cocucci, A.A. & A. Sérsic 5469b, 5471 (baccatum var. baccatum); 5488, 5877, 5939 (chacoense)
Cocucci, A.A. et al. 973 (chacoense); 5258, 5262, 5263 (baccatum var. baccatum); 5969, 5977 (chacoense); 5992, 5996 (baccatum var. baccatum); 6000 (chacoense)
Cocucci, A.E. 77 (chacoense)
Colinvaux, P.A. 440 (frutescens)
Collares, J.E. 45 (schottianum)
Colonnello, G. 522 (annuum var. glabriusculum)
Combs, R. 149, 151 (annuum var. glabriusculum)
Commerson, P. 160 (rabenii)
Conceição, S.F. et al. 507 (longidentatum)
Conde, B.E. 186 (rabenii)
Conrad, J. & W. Dietrich 2041 (recurvatum)
Contreras, E. 48, 294, 7684 (annuum var. glabriusculum)
Conzatti, C. & V. González 1221 (rhomboideum)
Cordero-P., Z. 683 (annuum var. annuum)
Correa N., M. 006 (annuum var. annuum)
Cook, O.F. & G.B. Gilbert 1017 (pubescens); 1165 (geminifolium)
Coons, M.P. 77-162, 77-181, 77-182 (rabenii)
Cooper, G.P. 115, 177 (annuum var. glabriusculum)
Core, E.L. 1105 (dimorphum)
Cornejo S., X. 421 (lycianthoides); 1727 (hookerianum)
Cornejo S., X. & C. Bonifaz 1353, 1778 (hookerianum); 2584 (frutescens); 2958, 5009 (hookerianum)
Coro, M. 1016, 1126 (baccatum var. baccatum)
Coronado G., I. & I. Velázquez 24, 149 (rhomboideum)
Coronado G., I. et al. 5331 (rhomboideum)
Coro-Rojas 1534 (chacoense)
Correa A., M.D. 1124 (dimorphum)
Correa M. et al. 3982 (annuum var. glabriusculum)
Corrêa, A.M. et al. 93 (recurvatum)
Corrêa Gomes, J. & F. Mattos 1029 (flexuosum)
Correa Múnera, M.A. 858 (dimorphum)
Corredor, C. & E. Moncada 07 (annuum var. glabriusculum)
Correll, D.S. 5814, 14847, 41078, 43340, 43702, 45127, 45621, 48675, 49299 (annuum var. glabriusculum)
Correll, D.S. & V.L. Cory 14104 (annuum var. glabriusculum)
Correll, D.S. et al. 41913 (annuum var. glabriusculum)
Cortéz, A. 156 (chacoense)
Cortés, L. & R. Cortés C. 30 (lanceolatum)
Cortés, R. et al. 2432 (dimorphum)
Cortés-Vázquez, M.E. 26, 143, 552 (annuum var. glabriusculum)
Cory, V.L. 51407 (annuum var. glabriusculum)
Cosa, M.T. 50, 342, 399 (chacoense)
Cosentino, K. 98, 99 (frutescens)
Costa, A. 367, 405 (caatingae); 4882 (flexuosum)
Costa, A. & Viegas 4904 (schottianum)
Costa, C.B. et al. 494 (mirabile)
Costa, C.N.G. et al. 1650 (flexuosum)
Costa, G. & A.C.S. Moraes 1744 (longidentatum)
Costa, G. et al. 2617 (caatingae)
Costa, J.A. 61 (caatingae)
Costa, K.C. & M.J. Rodal 5 (caatingae)
Costa, K.C. et al. 91 (caatingae)
Costa, S.M. 606 (caatingae)
Coulleri, J.P. & S.M. Ferrucci 338 (chacoense)
Coulter, T. 1237 (rhomboideum)
Couto, A.P.L. et al. 89, 106 (caatingae)
Covas, G. 1184 (chacoense)
Cowan, C. 1722 (annuum var. glabriusculum); 1805 (chinense); 2352 (annuum var. glabriusculum)
Crepaldi, M.O. 59 (baccatum var. baccatum)
Croat, T.B. 21043 (coccineum); 23892, 39814 (annuum var. glabriusculum); 40738 (lanceolatum); 41934 (rhomboideum); 43653 (annuum var. glabriusculum); 49390, 50879(lycianthoides); 55349 (rhomboideum); 55459 (dimorphum); 55803 (geminifolium); 57028 (lycianthoides); 58309, 70550 (annuum var. glabriusculum); 70657 (lycianthoides); 70847 (annuum var. glabriusculum); 73052 (frutescens); 91102 (dimorphum); 96081, 101027, 104378 (lycianthoides)
Croat, T.B. & G. Ferry 93747 (geminifolium)
Croat, T.B. & J.F. Gaskin 80291 (lycianthoides)
Croat, T.B. & D.P. Hannon 62887 (annuum var. glabriusculum); 62946 (rhomboideum); 62956 (annuum var. glabriusculum); 62988 (rhomboideum); 62992, 63278 A, 64555 (annuum var. glabriusculum); 92746 (lycianthoides)
Croat, T.B. & J. Whitehill 82726 (lycianthoides)
Croat, T.B. et al. 93341, 93366, 95685, 96413, 99984 (lycianthoides)
Croft, M.B. 179 (annuum var. glabriusculum)
Crosby, M.R. & C.A. Crosby 4694 (frutescens)
Crosby, M.R. et al. 119 (annuum var. glabriusculum)
Crovetto, M. 5581, 5589 (baccatum var. baccatum)
Cruz, J. 27 (campylopodium)
Cruz D., R. & A. Reyes 5639, 5668 (annuum var. glabriusculum); 5674 (annuum var. annuum)
Cruz R., G. 92 (rhomboideum)
Cruz, S. 003, 004, 005, 007, 009, 011 (annuum var. annuum)
Cuadros V., H. & A.H. Gentry 3060 (annuum var. glabriusculum)
Cuadros V., H. 501 (annuum var. glabriusculum); 774 (rhomboideum); 4344, 4475 (annuum var. glabriusculum)
Cuatrecasas, J. 173 (pubescens); 3343, 3614 (annuum var. glabriusculum); 8254 (rhomboideum); 8456 (geminifolium); 8572, 8655 (dimorphum), 10519, 12926 (annuum var. glabriusculum); 14479 (chinense);14514 (annuum var. glabriusculum); 15354 (rhomboideum); 17773 (annuum var. glabriusculum); 17782 a, 19436 (rhomboideum); 19637 (annuum var. glabriusculum); 19689 (frutescens); 20456 (rhomboideum); 21664, 22216 (dimorphum); 22375, 22665 (lycianthoides); 22800 (annuum var. glabriusculum); 23690 (rhomboideum); 23996 (lycianthoides)
Cuatrecasas, J. & H. Cuadros 28926 (annuum var. glabriusculum)
Cuatrecasas, J. & H. García Barriga 3519 (baccatum var. pendulum); 3693 (annuum var. annuum)
Cuatrecasas, J. & R. Romero Castañeda 24894 (annuum var. glabriusculum)
Cuatrecasas, J. et al. 12062 (annuum var. glabriusculum); 12209 (rhomboideum); 12236 (annuum var. glabriusculum); 27335 (rhomboideum)
Cueva, M. 218 (dimorphum); 270 (geminifolium); 460, 462 (dimorphum); 480, 485, 569 (geminifolium); 628 (dimorphum); 2912 (piuranum)
Cueva, M. & R. Rivera 484 (pubescens)
Cueva, M. et al. 2655, 2656, 2657, 2658 (piuranum)
Cuevas, R. & D. Deniz 3081 (rhomboideum)
Cuezzo, A.R. 928, 976, 3379 (chacoense)
Curran, H.M. 318 (annuum var. glabriusculum)
Curran, H.M. & M. Haman 1181 (parvifolium)
Curtiss, A.H. 671, 2205, 5458 (annuum var. glabriusculum)
Cusato, L. 1667 (flexuosum)
Custódio Filho, A. 1327 (schottianum); 1849, 1860, 1914, 2056 (hunzikerianum); 2099, 2807 (mirabile)
Custódio Filho, A. et al. 678, 1004, 1144 (cornutum)
Custódio Filho, F. & A. Custódio Filho 448 (hunzikerianum)
Czerwenka, J. 453 (annuum var. glabriusculum)
D’Arcy, W. & B. Hammel 12252 (annuum var. glabriusculum)
D’Arcy, W. 4737, 5137, 11215, 11824, 12102 (annuum var. glabriusculum); 12130 (frutescens); 14822, 14823 (rhomboideum); 15777, 16423 (lycianthoides); 17716 (cardenasii)
D’Arcy, W. et al. 15644 (dimorphum)
d’Orbigny, A.D. 604 (baccatum var. baccatum)
da Costa Sacco, J. 1332 (baccatum var. baccatum)
da Cruz, N.D. et al. 6291 (carassense)
da Saldanha da Gama, J. 6075 (rabenii)
da Silva, M.F.B. et al. 18 (caatingae)
Dahlgren, B.E. & E. Sella 564 (frutescens)
Dalafene 2 (frutescens)
Dalla Tea, F. 37 (chacoense)
Daly, D.C. et al. 21 (caballeroi); 8711, 9979 (coccineum)
Damasceno, S.A. 56 (caatingae)
Daneu, L. et al. 632 (mirabile)
Daniel, Hno. 397, 1464, 3429 (dimorphum)
David, H. et al. 3119 (annuum var. glabriusculum)
Davidse, G. & W. D’Arcy 11227 (flexuosum)
Davidse, G. et al. 20126, 37476 (annuum var. glabriusculum)
Daviña, J. & A.I. Honfi 593 (baccatum var. baccatum); 597 (flexuosum)
Davis, E.W. 1066 (chinense)
Davis, E.W. & J. Yost 993 (chinense); 994 (annuum var. glabriusculum)
Dawe, J. 73-0627 (annuum var. glabriusculum)
Dawe, M.T. 774 (rhomboideum)
Dawson, G. 117 (chacoense)
Day, E.H. 329 (annuum var. glabriusculum)
de Ance, S.A. 81 (chacoense)
de Andrade-Lima, D. 79, 51-918, 62-4055, 71-6395 (caatingae); 72-7202 & 72-7203 (villosum); 75-8086 (caatingae)
de Barros, A.A.M. et al. 835, 2367 (campylopodium)
de Barros, F. 1230 (schottianum)
de Benavides, O. 2974, 3169, 3636 (rhomboideum); 4672 (chinense); 4673, 4674 (annuum var. annuum); 5088, 5308, 5406 (rhomboideum); 5698, 6387 (annuum var. annuum); 6463, 7157, 7332, 10603 (rhomboideum)
de Benavides, O. & C. Paredes 6238 (rhomboideum)
de Benavídez, O. 1819 (annuum var. glabriusculum); 1986, 2246 (pubescens); 4692 (frutescens); 5389 (annuum var. glabriusculum); 6190 (pubescens); 7875 (annuum var. glabriusculum); 8312, 10527 (frutescens)
de Campos Novaes, J. 170 (schottianum); 1941, 2179 (flexuosum)
de Camargo, C. 020 (chinense)
de Carvalho, A.M.V. et al. 6863 (frutescens)
De Egea, J. & M. Peña-Chocarro 769 (chacoense)
de Escobar, B. & G. Barrera 281 (annuum var. annuum)
de Escobar, L.A. 828 (frutescens)
de Garganta F., M. 818 (annuum var. glabriusculum); 857 (rhomboideum)
de Gasper, A.L. 1394 (flexuosum)
de Gasper, A.L. et al. 1018, 1363 (flexuosum); et al. 2610 (mirabile)
de Godoy, S.A.P. et al. 2047 (schottianum)
de Jeréz, Z.A. et al. 4919 C (eximium)
de Jong, W. 53 (annuum var. glabriusculum)
de la Sota, E. 104 (baccatum var. baccatum); 129, 747 (chacoense)
de Lima, H.C. et al. 2526 (friburgense)
de Lima, R.A.F. 85 (recurvatum)
de M.A. Carvalho, M.V.B. 175 (caatingae)
de Mello Filho, L.E. 502, 1187 (campylopodium)
de Mello-Silva, R. 1275 (baccatum var. baccatum)
de Menjivar, M.G. & E.A. Montalvo 3846 (rhomboideum)
de Michel, R. 159 (baccatum var. baccatum); 492 (baccatum var. pendulum); 517 (annuum var. annuum); 714 (baccatum var. baccatum); 2568 (chacoense)
de Moraes, P.L R. et al. 1164 (recurvatum); 23685 (flexuosum)
de Oliveira, A.A. 2881, 2911, 2916 (flexuosum)
de Oliveira, F. 4 (baccatum var. baccatum)
de Oliveira Filho, L.C. & J.C.A. Lima 131 (longidentatum)
de Paula, C.H.R. & R. Bacelar 203 (campylopodium)
de Queiroz, L.P. 12964 (parvifolium); 15495 (caatingae)
de Queiroz, L.P. et al. 1520 (caatingae); 5958 (baccatum var. baccatum); 10598 (caatingae)
de Romero, M. 72 (frutescens)
de Saint-Hilaire, A. Cat. A1-N° 651, A2-N° 58 (campylopodium); 584, 621 (villosum)
de Saldanha da Gama, J. 5163 (muticum); 5373 (campylopodium); 6075 (baccatum var. baccatum)
de Sampaio, A.J. 4314 (rabenii)
de Souza Silva, J. 608 (caatingae)
de T. Alvim, P. 310 (rabenii)
de Varela, F. & A. del Castillo 1197 (chacoense)
de Vásquez, M.E. 385 (rhomboideum)
Deam, C.C. 24 (frutescens); 60321 (annuum var. glabriusculum)
Deanna, R. 51 (baccatum var. pendulum); 53 (frutescens); 164, 168, 170 (lycianthoides)
Deanna, R. & S. Leiva González 3, 11, 77, 99 (geminifolium);
Deanna, R. et al. 133, 144 (lycianthoides)
Debouck, D.G. 3016 (chacoense)
Debouck, D.G. et al. 3016 (baccatum var. pendulum); 3019 (baccatum var. baccatum)
Degener, O. 18764 (annuum var. glabriusculum)
Deginani, N.B. 2093 (chacoense)
Deginani, N.B. et al. 918 (eximium); 1523, 1701 (flexuosum)
Del Aguila et al., M. 662 (frutescens)
del Castillo, A. & F. de Varela 847 (chacoense)
del Castillo, A. & R. Neumann 383 p.p. (chacoense), 383 p.p. (eximium)
Delgado, A. et al. 81 (annuum var. glabriusculum)
Delgado, L. 23, 251, 359, 505 (rhomboideum)
Delgado, T. & Grupo Post-Grado MO-QCNE 38 (geminifolium)
Delgado S., A. et al. 1542 (annuum var. glabriusculum)
Demuner, V. & E. Bausen 628 (pereirae)
Dent, H.C. 90 (rabenii)
Di Fulvio, E. 455 (chacoense)
Di Giácomo, A. 230, 607 (chacoense)
Dias, H.M. et al. 783 (mirabile)
Díaz, A.L. 246 (rhomboideum)
Díaz, C. et al. 10120 (geminifolium)
Díaz, J. et al. 106 (frutescens)
Díaz, S. et al. 659 (geminifolium)
Díaz P., S. et al. 717 (dimorphum); 3883, 3900 (lycianthoides)
Díaz Rico, A. ADR-48, ADR-221 (annuum var. annuum)
Díaz S., C. et al. 2548, 2767 (geminifolium); 3201, 4032, 6242, 7417, 7474, 7512 (hookerianum)
Diesel, S.1655 (flexuosum)
Dimitri, M.J. & B. Piccinini 56 (chacoense)
Diogo, J. 420 (muticum)
Dirección Gral. de Biodiversidad/SERNA 60 (annuum var. glabriusculum)
Dittrich, V.A.O. & C. Kozera 298 (flexuosum)
do Cavalo, P. et al. 162 (caatingae)
do Socorro, M. & G. Ferreira 150 (longidentatum)
Dodson, C.H. 9617, 12331 (hookerianum)
Dodson, C.H. & A.H. Gentry 6576 (frutescens); 12269 (lycianthoides)
Dodson, C.H. & K. Tan 5319 (annuum var. glabriusculum)
Dodson, C.H. & L.B. Thien 2101, 2131 (lycianthoides); 2144 (rhomboideum); 12778 (lycianthoides)
Dodson, C.H. et al. 9149 (lycianthoides); 9157 (geminifolium); 9161, 14163 (lycianthoides)
Domin, K. 8247 (frutescens)
Domínguez Cadena, R. 2606 (annuum var. glabriusculum)
Domínguez Mariani, A. 522, 601 (annuum var. glabriusculum)
Domínguez, J.A. 179 (chacoense)
Domínguez-Licona, E. et al. 1824 (annuum var. glabriusculum)
Donnell Smith, J. 1837 (lanceolatum); 7255 (frutescens)
Dorantes, J. et al. 1216 (rhomboideum)
Dorr, L.J. 6729 (pubescens)
Dorr, L.J. & L.C. Barnett 6801 (eximium); 7041 (caballeroi); 7678 (annuum var. glabriusculum)
Dorr, L.J. et al. 5275, 5390 (rhomboideum)
Dos Santos et al. 274, 275, 276, 277 (chinense)
dos Santos, M.B. et al. 6 (schottianum)
Dostert, N. 98/162 (pubescens)
Douglas, W.D. 6154, 6155 (annuum var. annuum)
Dressler, R.L. 1276, 2429 (annuum var. glabriusculum)
Dreveck, S. & F.E. Carneiro 1620 (flexuosum)
Dreveck, S. et al. 268, 403 (flexuosum); 1832 (recurvatum)
Drombrowski, L.T. & Y.S. Kuniyoshi 2817 (recurvatum)
Drummond, T. 243 (annuum var. glabriusculum)
Drushel, J.A. 2810, 4179 (annuum var. glabriusculum)
Dryander, E. 2178 (annuum var. glabriusculum)
Duarte, A.P. 5491 (schottianum); 7666 (campylopodium)
Dudey, D. 1101 (annuum var. glabriusculum)
Dugand, A. 478 (annuum var. glabriusculum)
Duke, J.A. 3815, 5080 (annuum var. glabriusculum); 14410 (annuum var. annuum)
Duke, J.A. & H.F. Winters 17330 (pubescens)
Dumont, K. et al. 7778 (rhomboideum)
Dumont, M. et al. 43 (annuum var. glabriusculum)
Dunlap, V.C. 178 (annuum var. glabriusculum)
Duque Jaramillo, J.M. 20 (rhomboideum); 2036 (annuum var. annuum); 3330 (dimorphum); 3549 (annuum var. glabriusculum); 3555 (annuum var. annuum); 4083-A (annuum var. glabriusculum)
Durán, R. et al. 2963, 3485 (annuum var. glabriusculum)
Durán, S. et al. 1771 (caballeroi)
Dusén, P. 3360 (recurvatum); 5072 (campylopodium); 7418 (recurvatum); 7613, 7825 (flexuosum); 10200 (rabenii); 14227 (cornutum); 14527, 16139 (recurvatum); 16337 (flexuosum)
Duss, A. 149, 349, 3574, 3575, 3681 (annuum var. glabriusculum)
Dwyer, J.D. 2559 (annuum var. glabriusculum)
Dziekanowski, C.T. et al. 1717 (annuum var. glabriusculum)
Eaton, A.A. 421 (annuum var. glabriusculum)
Echeverria, G. 915 (pubescens)
Edmundo, A 360 (campylopodium)
Edwards, M.T. 592 (annuum var. glabriusculum)
Eiten, G. & L.T. Eiten 10140 (frutescens)
Ekman, E. 806 (baccatum var. baccatum); 808 (flexuosum); H 2116, 11372, 16269 (annuum var. glabriusculum)
Elías, Bro. 570, 1461 (annuum var. glabriusculum)
Ellemann, L. 66689 (pubescens)
Ellenberg, H. 4501 (chacoense)
Elorsa C., M. 367, 3132, 6168, 6186 (annuum var. glabriusculum)
Emmons, L. 86 (coccineum)
Encarnación, F. 750 (coccineum)
Encina, J.A. et al. 2553 (annuum var. glabriusculum)
Enea A., G. 253 (annuum var. glabriusculum)
Eriksen, B. & B. Ståhl 19 (lycianthoides)
Ernst, W.R. 1264 (annuum var. glabriusculum)
Escalante, S. 627 (annuum var. glabriusculum)
Escobar, J.D. 19 (chacoense)
Escobar FM, F. 122 (lycianthoides)
Escolastico, R. 165 (annuum var. glabriusculum)
Eshbaugh, W.H. 575, 588 (eximium); 614 (cardenasii); 765 (pubescens); 1526, 1527 (cardenasii); 1943 a, 1943 C, 1943 d (eshbaughii); 2046 C, 2046 J (cardenasii); E924 (baccatum var. pendulum); E954 (baccatum var. baccatum); E1137 (MU, US) (tovarii)
Espejo, A. et al. 1260 (annuum var. glabriusculum)
Espina, J. 182 (chinense); 189 (annuum var. annuum); 276 (chinense); 571 (frutescens); 577 (annuum var. glabriusculum); 586 (frutescens); 609 (annuum var. glabriusculum)
Espina, J. et al. 191, 193 (annuum var. glabriusculum)
Espinal T., S. 521, 1059 (rhomboideum)
Espinal, M. 203 (annuum var. glabriusculum)
Espindola-Nascimento, L. & J.R.Pinheiro 13 (chinense)
Espindola-Nascimento, L. & L.S. Simoa 01, 02, 03 (annuum var. glabriusculum); 04, 05, 06 (chinense); 07, 08 (annuum var. glabriusculum)
Espinosa Jiménez, J.A. 143 (annuum var. glabriusculum)
Espinosa, R. 1080 (pubescens); 1120 (rhomboideum)
Estrada, E. 43 (annuum var. glabriusculum)
Estrada V., S. 11 (lycianthoides)
Estudiantes herbario MEDEL 158, 630 (dimorphum); 709 (pubescens); 976 (annuum var. glabriusculum)
Etter, A. & L.A. Villa 1016 (annuum var. glabriusculum)
Evans, D.K. 4384 (annuum var. annuum)
Evans, R.J. 1469 (lanceolatum)
Eyerdam, W.J. 4 (annuum var. glabriusculum); 24801 (pubescens); 25313 (baccatum var. pendulum)
Eyerdam, W.J. & A.A. Beetle 8661, 8720 (annuum var. glabriusculum)
Ezcurra, C. & M. Ponce 533 (chacoense)
Fabris, H.A. 3454 (baccatum var. baccatum)
Fabris, H.A. et al. 5229 (eximium); 5278 (chacoense)
Fagerlind, F. & G. Wibom 1016 (rhomboideum); 2632 (lycianthoides)
Falcao, B.F. et al. 10 (cornutum)
Falcão, J. 190 (flexuosum)
Falcone & Castellanos 262 & 5023 (chacoense)
Farney, C. et al. 364 (campylopodium)
Felger, R.S. 1502 (annuum var. glabriusculum)
Felger, R.S. et al. 226, 1403 (annuum var. glabriusculum)
Felitto, G. & V. Ariati 804 (flexuosum)
Félix, D.F. 71 (baccatum var. baccatum)
Félix, L.P. 4994 (parvifolium)
Fendler, A. 982 (rhomboideum); 1018 (annuum var. glabriusculum)
Fernández, E. 3998 (minutiflorum)
Fernández, S. 97 (annuum var. glabriusculum)
Fernández, A. & R. Gonto 33761 (annuum var. glabriusculum)
Fernández Alonso, J.L. & R. Jaramillo 5268, 5338 (annuum var. glabriusculum); 5401 (rhomboideum)
Fernández Alonso, J.L. & G. López 18335 (rhomboideum)
Fernández Alonso, J.L. & G. Morales 6777 (rhomboideum); 6793 (annuum var. glabriusculum)
Fernández Alonso, J.L. et al. 7542 (frutescens); 10725 (lycianthoides); 13640 (annuum var. glabriusculum); 16057 (frutescens); 20758 (chinense); 23798 (dimorphum)
Fernández Casas, F.J. & J. Molero 6063, 6131 A, 6248, 6274 (baccatum var. baccatum)
Fernández Casas, F.J. & R. Morales Valverde 10659 (annuum var. glabriusculum)
Fernández M., R. & J. Dorantes 1840 (annuum var. glabriusculum)
Fernández Nava, R. 2946, 3379, 3395 (rhomboideum)
Fernández Nava, R. & Z. Guadarrama 1395 (annuum var. glabriusculum)
Fernández Pérez, A. & L.E. Mora 1362 (rhomboideum)
Fernández T., E. et al. 1896, 2007 (caballeroi)
Ferrari, G. 185 (frutescens)
Ferraro, L. 554 (baccatum var. pendulum)
Ferraz, E. et al. 273 (caatingae)
Ferreira, C.D.M. & P. Feliz 233 (campylopodium); 241 (mirabile)
Ferreira, E.M. 31, 60, 64, 65 (frutescens)
Ferreira, E.V.R. 150 (caatingae)
Ferreyra, R. 922 (coccineum); 4495 (annuum var. glabriusculum); 10086 (frutescens); 13163 (coccineum); 16384 (chinense)
Ferreyra, R. & S. Sánchez 19637 (annuum var. glabriusculum)
Ferris, R.S. & C.D. Duncan 3188 (annuum var. glabriusculum)
Ferris, R.S. & Y. Mexia 5130 (annuum var. glabriusculum)
Fiebrig, K. 952 (rabenii); 1472 (chacoense); 2072 (eximium); 12649 (baccatum var. baccatum)
Figueiredo, C. et al. 892 (coccineum)
Filgueiras, T.S. 3614 (chinense); 3615 (baccatum var. baccatum)
Filskov, P. et al. 37318 (rhomboideum)
Fisher, G.L. 41153 (annuum var. glabriusculum)
Flaster, B.49, 1165, 1166 (mirabile)
Fleischmann, E. 143 (coccineum)
Fletes, E. & L. Angulo 239 (pubescens)
Fleury, M. 124 (chinense)
Florentín Peña, T. 29 (chacoense)
Flores, D. 89 (eshbaughii)
Flores, L. 23 (rhomboideum)
Flores, S. et al. 157 (annuum var. glabriusculum)
Flores M., A. & M.A. Arvizo Y. 4787, 4860 (annuum var. glabriusculum)
Flores M., A. & P. Ramos G., A. 2413 (rhomboideum)
Flügel, H. et al. 7054 (annuum var. glabriusculum)
Folli, D.A. 2995 (frutescens)
Fonnegra G., R. 2672 (dimorphum)
Fonnegra G., R. & F.J. Roldán 4952 (dimorphum)
Fonnegra G., R. et al. 4417, 4442 (lycianthoides); 5315 (dimorphum); 6243 (annuum var. glabriusculum)
Fonseca, W.O. & T.N. Alves 482 (caatingae)
Fonturbel, F. FR-17 (pubescens)
Forni Martins, E.R. et al. 05/01, 05/03, 05/10 (schottianum)
Forte, A. 6154 (recurvatum)
Fortunato, R. & R. Micheli 5090 (chacoense)
Fortunato, R. et al. 1445, 2399, 3030, 3080, 3215, 4687, 5959, 6520, 6667 (chacoense); 7013, 7127, 7128, 7129, 7130, 7162 (baccatum var. baccatum); 7184 (chacoense)
Forzza, R.C. et al. 3329, 3610, 4366 (pereirae)
Fosberg, F.R. 55377, 59385 (annuum var. glabriusculum)
Fosberg, F.R. & F. Prieto 22763 (geminifolium)
Foster, R.B. 6849, 8802 (coccineum)
Foster, R. & C. Augspurger 3164 (coccineum)
Foster, R.B. & B. d’Achille 11630, 12017 (coccineum)
Foster, R.B. & J. Terborgh 5043 (coccineum)
Foster, R.B. et al. 3484 (coccineum)
Fotius, G. & I.B. Sá 3769 (parvifolium)
Fournet, J.P. 4616, 4666 (annuum var. glabriusculum)
Fournet, J.P. et al. 4878 (annuum var. glabriusculum)
Fournier, L.A. 218 (frutescens)
Fraga, C.N. et al. 2877 (villosum)
Frame, D. et al. 217 (coccineum)
França, F. et al. 4955 & 4956 (caatingae); 5556 (longidentatum)
França, G.S. & R. Stehmann 289 (mirabile)
Francia, P. 42 (baccatum var. pendulum)
Franco, C. et al. 4390 (schottianum)
Franco Rosselli, M.P. et al. 1645, 1649 (dimorphum)
Fredholm, A. 5523 (annuum var. glabriusculum)
Freeborn, B. et al. 37 (annuum var. glabriusculum)
Freire, B. & D. Naranjo 612 (chinense)
Freire, B. & M. Ruales 2931 (annuum var. glabriusculum)
Freire, E. 7152 (rhomboideum)
Freitas, L. 268, 615 (villosum)
Freire Fierro, A. 1243 (geminifolium); 3181 (lycianthoides)
Freire Fierro, A. & M. Asanza 3087 (geminifolium)
Frías-Castro, A. et al. 673 (rhomboideum)
Fries, R. 1110 (eximium); 1224, 1547 (chacoense)
Froehner, C. 169 (coccineum)
Fryxell, P.A, 5085, 1119, 1213, 2953 (annuum var. glabriusculum)
Fuentes, A.F. 1385 A (baccatum var. baccatum)
Fuentes, Z. & E. Fuentes 480 (rhomboideum)
Funez, L.A. 1561, 3325 (recurvatum)
Funez, L.A. & A.E. Zermiani 1394 (flexuosum)
Furlan, A. et al. 1527 (schottianum)
Gadelha Neto, P. da C. 1748 (chinense); 1751 (frutescens)
Gadelha Neto, P. da C. & I.B. Lima 3993 (parvifolium)
Galán, S. & K. Cárdenas 12 (annuum var. glabriusculum)
Galander, C. 1877 (baccatum var. pendulum)
Galeano, G. et al. 2127 (dimorphum)
Galeotti, H.G. 1189 (rhomboideum)
Gallardo, C. et al. 115, 392, 608 (annuum var. glabriusculum)
Gallardo Hernández, C. & E. Pérez García 1869 (annuum var. glabriusculum)
Gallegos, S. 318 A (eximium)
Galué, N. 122 (annuum var. glabriusculum)
Galvão, R. 137 (campylopodium)
Gamarra, P. 439 (pubescens)
Ganchozo, W. 007 (annuum var. annuum)
García Barriga, H. 3119 (rhomboideum); 4344 (annuum var. glabriusculum); 4708 (rhomboideum); 5056, 6426 (annuum var. glabriusculum); 6460-6462 (rhomboideum); 7114 (annuum var. glabriusculum); 11750, 12164 (rhomboideum); 13265 (annuum var. glabriusculum); 15258 (dimorphum); 20144 (rhomboideum); 20315A (frutescens); 20563 (annuum var. glabriusculum); 21269 (dimorphum); 26957 (annuum var. glabriusculum)
García Cañizares, F. 127 (annuum var. glabriusculum)
García, A. 2203 (rhomboideum)
García, A.A. 3190 (annuum var. annuum)
García, L.C. 133 (flexuosum)
García, P. 950 (chacoense)
García, R. 6600 (annuum var. glabriusculum)
García, I. & H. Cortéz 7235 (rhomboideum)
García, M. & F. Ramírez 242 (annuum var. glabriusculum)
García, R. et al. 4464 (annuum var. glabriusculum)
García, R.J.F. et al. 726 (cornutum)
García-Bielma, M.A. & P. Domínguez H. 626 (annuum var. glabriusculum)
García P., J. & A. Delgado S. 827 (annuum var. glabriusculum)
García R., J. & I. Montaño M. 348 (pubescens)
García Mendoza, A. et al. 4023 (annuum var. glabriusculum)
Garnier, A. 665 (pubescens)
Garvizu, M. et al. 1156 (eximium)
Garzón, G. et al. 3214 (frutescens)
Gatti, F.E. 24 (flexuosum)
Gaudichaud, C. 513 (campylopodium)
Gaumer, G.F. 864, 1546, 1546 bis (annuum var. glabriusculum); 1019 (frutescens); 2006, 24042 (annuum var. glabriusculum)
Gensini, J.G. 103 (dimorphum); 136, 180, 199 (lycianthoides)
Gentry, A.H. 3761 (annuum var. glabriusculum); 48174 (dimorphum); 12638 (rhomboideum)
Gentry, A.H. & P.E. Berry 14774 (rhomboideum); 15038 (annuum var. glabriusculum)
Gentry, A.H. & C. Bonifaz 28785 (rhomboideum)
Gentry, A.H. & C. Díaz S. 58262 (hookerianum)
Gentry, A.H. & C. Josse 72356 (hookerianum)
Gentry, A.H. & H. León 20187 (rhomboideum)
Gentry, A.H. & A.L. Peixoto 913 (campylopodium)
Gentry, A.H. et al. 8976 (annuum var. glabriusculum); 8998 (rhomboideum); 12095 (lycianthoides); 34774 (annuum var. glabriusculum); 37623 (coccineum); 44206, 53586 (annuum var. glabriusculum); 63614 (lycianthoides); 70189 (rhomboideum)
Gentry, H.S. 373, 949, 1541, 2359, 14323 (annuum var. glabriusculum)
Germán R., M.T. et al. 736 (pubescens); 1005 (annuum var. glabriusculum)
Gerold, R. 175 (eximium); 284 (chacoense)
Giacomelli, A. 130, 4806 (chacoense)
Giacomin, L.L. & T.E. Almeida 1688, 1691 (recurvatum)
Giacomin, L. et al. 200 (frutescens); 373 (villosum); 899 (mirabile); 1107 (schottianum); 1248, 1481 (mirabile); 1709 (recurvatum); 1946, 1959 (pereirae); 2014 (schottianum); 2018 (cornutum)
Gil, A. et al. 310 (dimorphum)
Gil, M. & R.D. López 21 (rhomboideum)
Gillies, J. 25 (chacoense)
Gilman, M.F. 142 (annuum var. glabriusculum)
Gilmartin, A. 554 (hookerianum)
Ginés, Bro. (P. Mandazen Soto) 2716 (annuum var. glabriusculum); 3901 (rhomboideum)
Giraldo, L.F. et al. 667, 900, 1631 (dimorphum)
Giraldo-Cañas, D. et al. 3485 (frutescens)
Glaziou, A. 1002 (rabenii); 3074 pp (recurvatum); 3074 pp (schottianum); 4168 (campylopodium); 8841 (rabenii); 8872, 11385, 12108 (campylopodium)
Glenboski, L.L. C-51 (annuum var. glabriusculum), C-87 (baccatum var. umbilicatum)
Godoy, S.A.P. et al. 352 (cornutum)
Goll, G.P. 271, 273 (annuum var. annuum); 378 (annuum var. glabriusculum)
Gomes Guarino, E. de S. et al. 978 (flexuosum)
Gomes, P. et al. 826 (caatingae)
Gómez, A. 511 (annuum var. glabriusculum)
Gómez, P. 3 (pubescens); 006 (frutescens)
Gómez, S. 190 (rhomboideum)
Gómez-Lorence, F. 877 (annuum var. glabriusculum)
Gonçalves, F.B. 267 (caatingae)
Gonçalves, J.M. et al. 175 (longidentatum)
Gonçalves, S.L. & M. Koketsu 1, 2 (chinense); 3 (frutescens)
Gontijo, F.D. et al. 589 (carassense)
González 89 (chinense)
González, A.C. 993 (annuum var. glabriusculum)
González, E. 874 (rhomboideum)
González, M. 2398 (rhomboideum)
Gonzáles, P. 341 (geminifolium); 344 (pubescens)
González, S. & R. Fernández 2186 (annuum var. glabriusculum)
González, V. 975 (annuum var. annuum)
González G., F. et al. 1553 (dimorphum)
González M., R. et al. 13 (dimorphum)
González Medrano, F. 4181, 9454 (rhomboideum); 9692 (annuum var. glabriusculum); 10492, 12578, 12872 (rhomboideum)
González Medrano, F. & P. Hiriart 12945 (annuum var. glabriusculum)
González Medrano, F. et al. 1823 (annuum var. glabriusculum); 4148 (rhomboideum)
González Olivares, S. 494 (annuum var. glabriusculum)
Gonzalez Ortega, J. 697 (annuum var. glabriusculum)
González Parini, F. 170, 1176, 1533 (flexuosum)
González Parini, F. et al. 324, 1706 (flexuosum)
Gonzatti, F. & E. Valduga 1779 (baccatum var. baccatum)
Goodding, L.N. 405-45 (annuum var. glabriusculum)
Gopar Vasquez, F. 122 (annuum var. glabriusculum)
Gordillo, E. & L. Forero 41 (rhomboideum)
Goudot, J. 4 (rhomboideum); 55 (lycianthoides)
Gould, F.W. et al. 2687 (annuum var. glabriusculum)
Gouvêa, Y.F. 213 (villosum); 215 (cornutum); 227 (villosum)
Gragson, T.L. 124 (baccatum var. baccatum)
Graham, J. & J. Schunke V. 271, 460 (annuum var. glabriusculum); 4633 (coccineum)
Granados-Tochoy, J.C. & J. Garzón 675 (annuum var. glabriusculum); 691 (dimorphum)
Granados-Tochoy, J.C. et al. 411 (dimorphum)
Grandi, T.S.M. 261 (frutescens)
Grases, C. 10 (rhomboideum)
Grassi, D. 2063 (chacoense)
Grayum, M. 4207, 12111 (annuum var. glabriusculum)
Grayum, M. & R. Warner 8366 (annuum var. glabriusculum)
Greenman, J.M. & M.T. Greenman 5629 (annuum var. glabriusculum)
Gregory et al. 10508 (frutescens)
Grijalva, A. 706, 759 (annuum var. glabriusculum)
Grijalva, A. et al. 2712, 2921 (rhomboideum)
Grijalva, R. 259 (lycianthoides)
Groth, B.H.A. 40 (annuum var. glabriusculum)
Gruhn, J. et al. 221 (annuum var. glabriusculum)
Grupo P. do Cavalo 162, 331, 438, 453, 694, 771 (caatingae)
Guadarrama, M.A. et al. 865, 6857 (annuum var. glabriusculum)
Guaglianone, E.R. 2255 (chacoense)
Guaglianone, E.R. et al. 309 (chacoense)
Guareco, I. 457 (coccineum); 627 (chinense)
Guareco, I. & J. Balderrama 464 (pubescens)
Guedes, F. 98 (longidentatum)
Guedes, M.L. et al. 10847 (caatingae); 12268 (parvifolium); 13245, 13297 (caatingae); 16183, 16187 (longidentatum); 30361 (caatingae)
Guerrero, O.D. 408 (rhomboideum)
Guerrero-Nuño, J.J. 586 (annuum var. glabriusculum)
Guilcapi, D. 19 (frutescens)
Guillemin, M. 939 (schottianum)
Guio 80 (baccatum var. baccatum)
Guízar Nolazco, E. & L. Pimentel B. 2884, 2954 (annuum var. glabriusculum)
Gustafsson, C. & C. Bonifaz 412 & 477 (lycianthoides)
Gutiérrez, E. 24 (chinense); 25 (annuum var. glabriusculum); 26, 109 (annuum var. annuum); 388 (chinense)
Gutiérrez, J. 75 (chacoense)
Gutiérrez, J.P. 85-23, 85-26, 85-27, 85-41, 85-71(annuum var. annuum)
Gutiérrez, M. & T. Acero 76 (annuum var. glabriusculum)
Gutiérrez B., C. 2227, 4475 (annuum var. glabriusculum)
Gutiérrez R., J. 1004, 1072 (neei)
Gutierrez V., G. 414 (annuum var. glabriusculum); 487 (rhomboideum)
Gutierrez V., G. & F.A. Barkley 17C080 (rhomboideum)
Guzmán, L. & F.J. Santana 303 (rhomboideum)
Guzmán, M. et al. 396, 667 (annuum var. glabriusculum); 1011 (annuum var. annuum); 1307 (annuum var. glabriusculum)
Haber, W.A. & E. Bello C. 2260 (rhomboideum)
Haber, W.A. & W. Zuchowski 9930 (annuum var. glabriusculum); 10105 (rhomboideum)
Hage, J.L. & E. B. dos Santos 356 (chinense)
Hahn, W.J. 802 (baccatum var. baccatum); 1405 (chacoense); 4830 (frutescens)
Hahn, W.J. & J.C. Sandino 420 (annuum var. glabriusculum)
Hahn, W.J. et al. 937 (flexuosum); 1024 (baccatum var. baccatum); 1213, 2506 (chacoense)
Hall, E. 495 (annuum var. glabriusculum)
Hall, J.S. & S.M. Bockus 7802 (annuum var. glabriusculum)
Hamann, M. & O. Hamann 645, 1418 (frutescens)
Hamann, O. & S. Serberg 1700 (frutescens)
Hamblett, R.B. 1538 (annuum var. glabriusculum)
Hanan Alipi, A.M. et al. 1386 (annuum var. glabriusculum)
Handro, O. 552 (cornutum); 860 (rabenii); 1106 (mirabile); 2184 (recurvatum)
Hansen, B.F. & M. Nee 7430 (annuum var. glabriusculum)
Hansen, B.F. & R.P. Wunderlin 12049 (annuum var. glabriusculum)
Hansen, B.F. et al. 1497 (annuum var. glabriusculum)
Hanson, H.C. 468 (annuum var. glabriusculum)
Harker, M. et al. 2432 (rhomboideum)
Harley, R.M. 16184 (longidentatum); 16297 (caatingae)
Harley, R.M. & A.M. Giulietti 54171 (parvifolium)
Harley, R.M. et al. 3445 (caatingae); 10986 (rabenii); 22736 (baccatum var. baccatum)
Harling, G. 316 (frutescens); 519, 1680 (lycianthoides); 4272 (rhomboideum); 5867 (longifolium)
Harling, G. & L. Andersson 12250 (lycianthoides); 13320, 13334 (rhomboideum); 13680 (geminifolium); 14414, 15209 (rhomboideum); 16487 (lycianthoides); 18283, 18316 (hookerianum); 18756 (lycianthoides); 21464 (longifolium); 22180, 22344 (geminifolium); 21478, 21665 (rhomboideum); 23121, 23265 (lycianthoides)
Harling, G. & B. Ståhl 26441 (geminifolium); 26470 (rhomboideum)
Harling, G. et al. 9226 (geminifolium); 15092, 15351, 20329, 20630, 20725 (rhomboideum)
Harmon, W.E. & J.D. Dwyer 3543 (annuum var. glabriusculum)
Harris, W. 5110, 9327, 10051 (annuum var. glabriusculum)
Hart, J. 594 (annuum var. glabriusculum); 621, 953 (pubescens)
Hartley, W. 5H 132 (chacoense)
Hartweg, K.T. 1296 (rhomboideum)
Hassler, É. 215 (baccatum var. baccatum), 1607 (flexuosum); 1926 (baccatum var. baccatum), 2891 (rabenii); 5134 (flexuosum); 5703 (baccatum var. baccatum); 5742, 5893 (flexuosum); 6070 (baccatum var. baccatum), 6498 (rabenii); 8633 (flexuosum); 12385 (baccatum var. baccatum)
Hasting, J.R. & R.M. Turner 65-84, 64-229 (annuum var. glabriusculum)
Hatschbach, G. 52 (recurvatum); 3952, 7352 (flexuosum); 10180 (recurvatum); 12593 (flexuosum); 17491 (recurvatum); 18030, 18412 (flexuosum); 20262, 25714, 26000, 30892 (recurvatum); 34886 (chinense); 38096 (recurvatum); 58780 (baccatum var. baccatum); 61141 (pereirae)
Hatschbach, G. & L.Z. Ahumada 31246 (baccatum var. baccatum)
Hatschbach, G. & A.R. Campos 58235 (recurvatum)
Hatschbach, G. & D.D. Guimarães 54794, 54797 (recurvatum)
Hatschbach, G. & O. Guimarães 25528 (rabenii)
Hatschbach, G. & C. Koczicki 20077, 20877 (recurvatum)
Hatschbach, G. & R. Kummrow 38441 (annuum var. glabriusculum)
Hatschbach, G. & L. Landrum 40412 (recurvatum)
Hatschbach, G. & O. Ribas 61697 (flexuosum)
Hatschbach, G. & J.M. Silva 61582 (villosum)
Hatschbach, G. et al. 58241 (rabenii); 58827 (baccatum var. baccatum); 71589 (flexuosum); 77668 (baccatum var. baccatum)
Hattori, E.K.O. et al. 917 (villosum)
Haught, O. 2413 (rhomboideum); 3041, 3514 (hookerianum); 5148 (rhomboideum); 6134 (geminifolium); 6307 (rhomboideum); 6559 (annuum var. glabriusculum)
Haught, O. & H.K. Svenson 11621 (rhomboideum)
Haumán, M. et al. 0317, 320 (chinense)
Hawkes, J.G. et al. 2003 (annuum var. glabriusculum); 3308 (chacoense); 6558 (eximium)
Hawkins, T. & D. Mejía 702 (lanceolatum)
Haxaire, G. 5143 (chinense); 5144, 5147 (annuum var. glabriusculum)
Heilborn, O. 426 (rhomboideum)
Heilbron, T.J. & D. Sanredjo 6 (annuum var. glabriusculum)
Heinonen, S. 59 (flexuosum)
Heinonen, S. et al. 143, 152 (chacoense)
Heinrich, M. 7800 (annuum var. annuum)
Heinrichs, E. 182 & 184 (rhomboideum)
Heiser, C.B. BGH 952 (baccatum var. pendulum); C19 (annuum var. glabriusculum); C 29 (baccatum var. pendulum); C36, C229, C230, C256, C259, C260 (frutescens); C271 (cardenasii); C272, C273 (pubescens); C276a, C279a, C280a (baccatum var. baccatum); C281a, C282a, C290 (baccatum var. pendulum); C 301 (eshbaughii); E 1130 (annuum var. glabriusculum); 4196 (cardenasii); 4197 (eximium); 4675e (annuum var. glabriusculum); 4804, 4806 (baccatum var. pendulum); 4813, 4828 (rhomboideum); 5041, 6097, 6100, 6247, (frutescens); 7801 (flexuosum); 7802 (coccineum)
Heiser Jr., C.B. C1 (pubescens); C6 (frutescens); C10 (chacoense); 4196 (cardenasii)
Hekker, F. & W.H. Hekking 10014, 10023 (rhomboideum)
Helme, N, 80 (chinense)
Heller, A.A. 98, 227, 4526 (annuum var. glabriusculum)
Henao, A. 4373 (annuum var. glabriusculum)
Henao, C.I. 167 (frutescens); 170 (annuum var. glabriusculum)
Henao, C.I. & L. Buraiño 26 (chinense); 169 (annuum var. glabriusculum)
Henao, C.I. & R. Kuiru 172 (annuum var. annuum)
Henao, C.I. & D. Padd 168 (annuum var. glabriusculum)
Henao, C.I. & K. Rubiela 316 (annuum var. annuum)
Henao, C.I. & Zɨueche 247 (annuum var. glabriusculum)
Henkel, T.W. & R. James 3520 (annuum var. glabriusculum)
Henz, E. 35360 (flexuosum)
Herb. A. van Royen n. 908.244-150 (frutescens)
Herb. Biltmore 10175 (annuum var. glabriusculum)
Herb. Linn. N° 249.5 (annuum var. annuum)
Herb. Sag. 465 (chinense)
Heredia, M.D. et al. 563 (lycianthoides)
Heriberto, Bro. 15, 231 (frutescens)
Heringer, E.P. 6472 (rabenii)
Hernández, A. & F. Crespo 82, 83 (annuum var. annuum)
Hernández, L. 1206 (rhomboideum); 1944 (annuum var. annuum); 2016 (annuum var. glabriusculum); 3099 (rhomboideum)
Hernández, L. & P. Ceballos 675 (annuum var. glabriusculum)
Hernández A., F. & J.A. Gutiérrez G. 65 (annuum var. glabriusculum)
Hernández García, L. & G.J. Martín 166 (pubescens)
Hernández M., H.M. & R. Torres C. 413 (rhomboideum)
Hernández Magaña, R. 5302 (rhomboideum); 12093, 12130 (annuum var. glabriusculum)
Hernández Magaña, R. et al. 6045 (rhomboideum)
Hernández Najarro, F. & C. Méndez Morales 1310 (annuum var. glabriusculum)
Hernandez Ortega, R. 481, 482, 484 (annuum var. annuum)
Hernani A., L. & A. Peña C. 379 (geminifolium)
Herrera 543 (chacoense)
Herrera, D.A. 16 (chinense)
Herrera, G. & J. Bittner 9479 (dimorphum)
Herrera Ch., G. & Grupo Estudiantes de Biodiversidad 2924 (annuum var. glabriusculum)
Herzog, T. 2289 (ceratocalyx)
Heyde, E. & E. Lux 3436 (rhomboideum); 3439 (annuum var. glabriusculum); 4545 (rhomboideum)
Hieronymus, G. 356, 427 (chacoense)
Hilgert, N. 1363, 1373, 1374, 2028 (baccatum var. pendulum); 2060 (chinense); 2061 (eximium)
Hill, S.R. 18388 (annuum var. glabriusculum)
Hill, S.R. et al. 12996, 27189 (frutescens)
Hinojosa, I. & A. Wásra 1133 (baccatum var. pendulum)
Hinton, G.B. 4336 (chinense); 4339, 9311, 11977 (rhomboideum); 23896 (annuum var. glabriusculum)
Hinton, G.B. et al. 24206 (annuum var. glabriusculum)
Hinton, G.S. 21500 (rhomboideum)
Hinton, J.C. 17588, 17943 (rhomboideum)
Hiriart, P. 404, 417 (rhomboideum)
Hiriart V., P. & G. Ortiz C. 22 (rhomboideum)
Hitchcock, A.S. 242 (annuum var. glabriusculum); 20841 (rhomboideum)
Hodges, V. & J. Gorham 96 (chinense)
Hodge, W.H. 6882 (annuum var. glabriusculum)
Hoehne, W. 3539 (frutescens); 30971 (annuum var. annuum)
Holm, R.W. & H. Iltis 75, 297 (annuum var. glabriusculum)
Holm-Nielsen, L. 4905, 16643, 16676 (rhomboideum)
Holm-Nielsen, L. & J.L. Jaramillo 28836, 28876 (rhomboideum)
Holm-Nielsen, L. & S. Jeppesen 1240 (geminifolium)
Holm Nielsen, L. et al. 21109 (chinense)
Holston, I.F. 569 (rhomboideum)
Holt, E.G. & W. Gehriger 109 (frutescens)
Holton, I. 13 (annuum var. glabriusculum)
Homeier, J. 2555 (geminifolium)
Homeier, J. & N. Cumbicus 4060 (dimorphum)
Honfi, A. et al. 1284 (baccatum var. pendulum); 1286 (annuum var. annuum); 1287, 1288 (baccatum var. pendulum)
Hoogte, L.v.d. & C. Roersch 724 (baccatum var. baccatum)
Hoover, W.S. 1896, 2093, 2227 (lycianthoides)
Hormia, K. 2194 (chinense); 2195 (annuum var. glabriusculum); 2228 (annuum var. annuum)
Horta, M.B. et al. 126 (parvifolium)
Horton, O.B. & J.L. Morrison 8858 (annuum var. glabriusculum)
Hosseus, C.C. 103, 340, 424 (chacoense)
House, P.R. 866 (lanceolatum)
Howard, R.A. 4867 (annuum var. glabriusculum); 6278 (chinense); 6558, 11136, 14049 (annuum var. glabriusculum)
Howard, R.A. & E.S. Howard 8761, 10055 (annuum var. glabriusculum)
Howard, R.A. & G.R. Proctor 13367 (annuum var. glabriusculum)
Howell, J.T. 9009, 9175A (frutescens); 10289 (annuum var. glabriusculum)
Hoyos, D. et al. 118, 127, 146 (regale)
Hoyos, S.E. et al. 348 (chinense)
Hoyos-Gómez, S.E. et al. 355 (annuum var. annuum)
Huamantupa, I. 15251 (regale)
Huaylla, H. et al. 2178 (neei)
Huber, O. 252 (annuum var. glabriusculum)
Huft, M.J. & E. Cabrera 2436 (annuum var. glabriusculum)
Humbert, H. et al. 26957 (annuum var. glabriusculum)
Humbles, J.E. 6146 (rhomboideum)
Hunnewell, F.W. 18784 (campylopodium)
Hunn, E. OAX-1341, OAX-1342, OAX-1343, OAX-1344 & OAX-1345 (annuum var. annuum)
Hunziker, A.T. 1578 (chacoense);1579 (baccatum var. baccatum); 1907 (eximium); 1985, 1987, 2010, 2024 (baccatum var. baccatum); 2052 (baccatum var. pendulum); 2104, 2452 (chacoense); 2776, 2786 (baccatum var. baccatum); 4795, 4797, 4807, 4812, 4820, 5011, 5071, 5088, 5299, 5941, 6016, 6734, 7315 (chacoense); 7336, 7337, 7338 (baccatum var. baccatum); 7339 (baccatum var. pendulum); 7340, 7341(chacoense); 7342 (baccatum var. pendulum); 7343 (baccatum var. baccatum); 7344 (chacoense); 7345, 7346 (eximium); 7347, 7348, 7349, 7350, 7351, 7352, 7353, 7354, 7355 (baccatum var. baccatum); 7356, 7357, 7358, 7359, 7360 (chacoense); 7605 (pubescens); 7624, 8530, 8764, 8842, 8915, 9111, 9153, 9170, 9222, 9519, 9805 (chacoense); 10320 (flexuosum); 10376, 10381, 10642, 10700, 10816 (chacoense); 11491 (baccatum var. pendulum); 11492, 11493 (annuum var. annuum); 11695, 13271, 13300, 13520, 13912, 14160, 14842, 15834, 16516, 17972, 18343, 18572 (chacoense); 19546 (recurvatum); 19557 (cornutum); 19558 (schottianum); 19562 (mirabile); 19563, 19564 (rabenii); 19566, 19567 (villosum); 19572 (campylopodium); 19577 (schottianum); 25202, 20229 (rabenii); 20230 (recurvatum); 20232 (flexuosum); 20762 (baccatum var. pendulum); 20775 (recurvatum); 21016 (chacoense); 23481 (baccatum var. baccatum); 24992, 24994, 24995 (flexuosum); 24997, 24998, 24999, 25001, 25002, 25003 (recurvatum); 25013 (flexuosum); 25116, 25129, 25130, 25134, 25135 (campylopodium); 25137 (pereirae); 25140, 25142, 25143, 25155, 25156, 25157 (mirabile), 25160 (campylopodium); 25165 (schottianum); 25166 (campylopodium); 25167, 25168 (schottianum); 25169, 25173, 25174, 25175, 25176 (villosum); 25179, 25180 (schottianum); 25183, 25184, 25186, 25187 (recurvatum); 25190, 25192, 25193 (schottianum); 25196 (recurvatum); 25197, 25198, 25199 (cornutum); 25200, 25201 (mirabile); 25219 (chacoense); 25233 (caatingae); 25234 (chinense); 25235, 25236, 25237, 25238, 25239 (mirabile); 25240, 25241, 25242 (schottianum); 25243 (mirabile); 25244, 25245 (campylopodium); 25246 (muticum); 25247, 25248, 25249 (pereirae); 25250, 25251, 25252, 25253, 25254, 25255, 25260, 25264, 25267 (mirabile); 25380, 25381 (baccatum var. baccatum); 25383 (baccatum var. umbilicatum); 25382, 25383 (baccatum var. pendulum); 25481 (annuum var. glabriusculum); 25484 (pubescens); 25485, 25486, 25487 (baccatum var. umbilicatum); 25488 (baccatum var. pendulum); 25489 (frutescens); 25490 (chinense); 25491 (baccatum var. baccatum); 25492 (annuum var. annuum); 25496 (baccatum var. pendulum); 25498 (annuum var. annuum); 25505 (frutescens); 25550, 25501 (annuum var. annuum); 25502 (chinense); 25552, 25559 (baccatum var. pendulum); 25653, 25654, 25655 (tovarii); 29428 (annuum var. annuum)
Hunziker, A.T. & J.A. Caro 13495, 13610 (chacoense)
Hunziker, A.T. & A.E. Cocucci 14666, 14698, 14913, 15050 (chacoense)
Hunziker, A.T. & E. Di Fulvio 16995, 17129 (chacoense)
Hunziker, A.T. & P. Maldonado 16263 (chacoense)
Hunziker, A.T. & R. Subils 24963, 24964 (chacoense)
Hunziker, A.T. et al. 23390, 24346 (chacoense); 24993 (flexuosum); 25161 (muticum); 25181 (villosum); 25206 (carassense); 25239 (mirabile); 25256 (carassense); 25334, 25388 (chacoense);
Hunziker, J.H. 2450 (annuum var. annuum); 2532, 9031 (frutescens); 9032, 9034 (annuum var. glabriusculum)
Hunziker, J.H. et al. 12745, 13036 (baccatum var. baccatum)
Hürlimann, H. 6032 (mirabile)
Hurtado, F. 715 (chinense)
Hurtado, R. 698 (pubescens)
Hurtado, R. & R. Zenteno 1636 (baccatum var. baccatum)
Hutchinson, P.C. & K. von Bismarck 6396 (geminifolium)
Hutchinson, P.C. & J.K. Wright 3129, 3541 (annuum var. glabriusculum); 3549 (rhomboideum); 4892 (pubescens); 6905 (rhomboideum)
Hutchinson, P.C. et al. 3078 (rhomboideum)
Ibarra Manríquez, G. 3616 (annuum var. glabriusculum)
Ibarrola, T.S. 2988 (chacoense); 3911, 4051, 4281 (baccatum var. baccatum)
Idobro, J.M. 10379 (dimorphum)
Iltis, H.H. 257 (baccatum var. pendulum); 1067 (pubescens)
Iltis, H.H. & M.G. Iltis 29 (annuum var. glabriusculum)
Imaguive, N. 5276 (flexuosum)
Insfrán, P. 765 (chacoense)
Insua, R. 513 (chacoense)
Irvine, D. 773 (annuum var. glabriusculum)
Irwin, H.S. et al. 19117, 19158, 19189, 19438, 25203 (rabenii)
Isern, J. 8094, 8240 (chacoense)
Itow, S. 136 (frutescens)
Ivanauskas, N.M. et al. 4532 (schottianum); 4631 (hunzikerianum)
Jack, J.G. 4376, 5223 (annuum var. glabriusculum)
Jahn, A. 667 (rhomboideum)
Jaime, N.A. 23 (chacoense)
Jameson, W. 328 (rhomboideum)
Jansen-Jacobs, M.J. et al. 119 (annuum var. glabriusculum); 1809 (frutescens); 1810, 1811, 3465 (annuum var. glabriusculum)
Jaramillo, J. 6425 (6423?), 6426, 6774, 6965-B, 8209 (lycianthoides); 8926 (pubescens); 10265 (geminifolium); 26136 (rhomboideum); 26264 (annuum var. annuum)
Jaramillo, J. & F. Cohello 29118 (rhomboideum)
Jaramillo, J. & I. Tapia 18452 (lycianthoides); 18525 (dimorphum)
Jaramillo, J. & V. Zak 7856 (lycianthoides)
Jaramillo, P. et al. 1775 (frutescens)
Jaramillo, R. et al. 351 (frutescens); 2698 (dimorphum)
Jardim, A. et al. 1995 (caballeroi)
Jardim, J.G. 4435 (parvifolium)
Jardim, J.G. et al. 4345, 4860 (pereirae)
Jarenkow, J.A. 2158 (flexuosum)
Játiva, C. & C.C. Epling 552 (rhomboideum)
Jensen, E. MK0040 (annuum var. glabriusculum)
Jermy G. 189 (annuum var. glabriusculum)
Jiménez, E. et al. 1126 (lycianthoides)
Jiménez, I. et al. 7517 (eximium)
Jiménez, J. 02 (annuum var. annuum)
Jiménez, R. 1 (annuum var. glabriusculum)
Jiménez, B. & G. Marín 1651 & 5893 (flexuosum)
Jiménez, F. et al. 722 (annuum var. glabriusculum)
Jiménez L., A. 50 (baccatum var. baccatum)
Jiménez-Osornio, J.J. 106 (chinense)
Jimeno Sevilla, D. et al. 967 (pubescens)
Job, M.M. 874, 1210 (chacoense)
Jörgensen, P. 4370 (flexuosum); 7679 (rabenii)
Johns et al. 142 (coccineum); 201, 343 (baccatum var. pendulum)
Johnson, A. 70 (annuum var. annuum)
Johnston, I.M. & C.H. Mueller 949 (annuum var. glabriusculum)
Johnston, J.R. 75 (parvifolium)
Jönsson, G. 330 a (flexuosum)
Jordán, C.G. et al. 517 (caballeroi)
Jørgensen, P.M. 56290 (rhomboideum)
Jørgensen, P.M. et al. 1401, 1476, 1501 (geminifolium); 61307 (rhomboideum)
Joseph, A. 28 (annuum var. glabriusculum)
Josse, C. 480, 487, 539 (rhomboideum)
Joyal, E. 1815, 1926, 1931 (annuum var. glabriusculum)
Joyal, E. et al. 1761 (annuum var. glabriusculum)
Juárez de Varela, F. 2076 (baccatum var. baccatum)
Juárez García, G. 1630 (annuum var. glabriusculum)
Juárez García, G. et al. 2678 (annuum var. glabriusculum)
Judd, W.S. et al. 8388 (annuum var. glabriusculum)
Juncosa, A. 2018, 2228, 2236 (rhomboideum)
Juncosa, A. & G. Misas 1071 (dimorphum)
Jung, S.L. & A. Barros 374 (recurvatum)
Junge, C. 5755 (annuum var. annuum)
Jung-Mendacolli, S.L. et al. 1421 (flexuosum)
Jurgensen, C. 861 (annuum var. glabriusculum)
Kalliola, R. et al. P3-051 (coccineum)
Katán, T. 349 (geminifolium)
Kayap, R. 652 (chinense)
Kegler, A. 1256, 1354, 1426, 1565 (flexuosum)
Keller, H.A. 288, 1144, 1349, 2957, 5570 (flexuosum); 8340 (baccatum var. baccatum)
Keller, H.A. & A. Ferreira 2135 (flexuosum)
Keller, H.A. & M. Franco 4940 (baccatum var. baccatum)
Keller, H.A. & N.G. Paredes 7798 (flexuosum)
Keller, H.A. et al. 1945 (flexuosum); 5232 (baccatum var. baccatum); 6943 (pubescens); 10099 (flexuosum)
Kellerman, W.A. 4854, 7674 (annuum var. glabriusculum)
Kelly, I. 858 (annuum var. glabriusculum)
Kermes, E. 500 (baccatum var. baccatum)
Kessler, M. 2496 (rhomboideum); 2712 (hookerianum)
Kiesling, R. 8964 (chacoense)
Killeen, T. et al. 3415 (coccineum)
Killip, E.P. 93 (annuum var. glabriusculum); 605 (rhomboideum); 5395 (annuum var. glabriusculum); 5911, 34823 (lycianthoides)
Killip, E.P. & H. García 33781 (lycianthoides)
Killip, E.P. & T.E. Hazen 11019 (annuum var. glabriusculum)
Killip, E.P. & A.C. Smith 14251, 14252, 14534 (annuum var. glabriusculum); 20853 (rhomboideum); 22637 (baccatum var. pendulum); 23023, 26351, 26386 (coccineum); 27820 (pubescens); 28864 (baccatum var. pendulum); 42473 (annuum var. glabriusculum)
Killip, E.P. et al. 11069, 38142, 38146 (rhomboideum); 39889 (dimorphum)
King, D.O. 284 (chacoense)
King, R.M. 1525, 1613 (rhomboideum)
Kinupp, V.F. et al. 3169, 3229 (baccatum var. baccatum)
Kirizawa, M. 2087 (cornutum)
Kirizawa, M. & J.A. Correa 2164 (recurvatum)
Kirizawa, M. et al. 771, 2551 (cornutum); 3288 (villosum); 4321 (cornutum)
Klein, R.M. 2063 (rabenii); 2232 (recurvatum); 2774 (rabenii); 3190, 5628, 8050 (flexuosum)
Klein, R.M. & A. Bresolin 6447 (frutescens); 8346, 9216 (baccatum var. baccatum)
Klitgaard, B.B. & G.P. Lewis 386 (rhomboideum)
Klitgaard, B.B. et al. 411 (lycianthoides)
Klug, G. 4248 (coccineum)
Knab-Vispo, C. et al. 1288 (annuum var. glabriusculum)
Knapp, S. 5946 (annuum var. glabriusculum); 6164 (rhomboideum)
Knapp, S. & J. Mallet 3899, 6254 (annuum var. glabriusculum)
Knapp, S. et al. 3342 (annuum var. glabriusculum); 9103 (pubescens)
Knight, D.H. 446, 447 (rhomboideum); 641 (lycianthoides); 764 (rhomboideum)
Knuden, J.T. & B.Ståhl 381, 439 (lycianthoides)
Koch, S.D. & P.A. Fryxell 83123 (annuum var. glabriusculum)
Koch, S.D. et al. 79532 (annuum var. glabriusculum)
Køie, M.E. 5149 (rhomboideum)
Kohn, E. 1225 (annuum var. annuum); 1611, 1612, 1613, 1896, 1910 (chinense)
Kollmann, L. et al. 3497 (baccatum var. baccatum)
Korte, A. & A. Kniess 2051 (flexuosum)
Kozera, C. et al. 128 (flexuosum)
Kral, R. 1884, 61242 (annuum var. glabriusculum)
Krapovickas, A. 762, 1215 (chacoense); 1575 (baccatum var. baccatum); 1709, 1905, 6021, 6093, 6671 (chacoense); 8435 (frutescens); 12446 (baccatum var. baccatum); 15554 (annuum var. glabriusculum); 22180, 23686 (annuum var. annuum); 47426, 47449, 47466 (eximium)
Krapovickas, A. & C. Cristóbal 11848 (baccatum var. baccatum); 34271 (baccatum var. pendulum); 35425 (schottianum); 40806 (annuum var. annuum); 44232 (chacoense)
Krapovickas & A. Schinini 28514 (baccatum var. baccatum); 30421, 30457, 30494, 30655 (chacoense); 30952 (baccatum var. baccatum); 31054, 31056 (baccatum var. pendulum); 31057 (annuum var. annuum); 31154 (chacoense); 31459 (baccatum var. baccatum); 31659 (baccatum var. pendulum); 31689 (frutescens); 31721 (baccatum var. pendulum); 32132 (frutescens); 32133, 32141 (baccatum var. pendulum); 32144, 32244, 32360 (baccatum var. baccatum); 32488 (chinense); 32764 (annuum var. annuum); 34819, 36348 (frutescens); 36971 (flexuosum); 39010, 39012, 39018 (baccatum var. baccatum); 39321 (baccatum var. pendulum); 39244 (baccatum var. umbilicatum)
Krapovickas, A. et al. 13996, 14230 (baccatum var. baccatum); 14900 (chacoense); 18340, 21558 (flexuosum); 23427 (frutescens); 25312 (flexuosum); 25551 (baccatum var. baccatum); 27442, 27769, 27879, 27991, 28010, 28162, 28164 (chacoense); 41070 (baccatum var. baccatum); 45375 (chacoense); 47263 (baccatum var. baccatum); 47484 (eximium)
Krieger, L. 34 (frutescens); 7470 (rabenii); 13410 (campylopodium); 21364 (mirabile); 22976 (frutescens); 22981 pp. (frutescens); 22981 pp. (chinense)
Krukoff, B.A. 10584 (ceratocalyx)
Kruse, H. 1906 (annuum var. glabriusculum)
Kufer, J. 99 (annuum var. glabriusculum); 100 (frutescens)
Kuhlmann, J.G. 722 (annuum var. glabriusculum); 6288 (campylopodium)
Kuhlmann, M. 144 (flexuosum); 272 (recurvatum); 665 (flexuosum); 1710 (schottianum); 2643 (recurvatum); 2741 (villosum); 2785 (hunzikerianum); 2809 (recurvatum); 3026 (baccatum var. baccatum); 3556 (recurvatum); 4311 (hunzikerianum)
Kuhlmann, M. & S. Jimbo 80 (annuum var. glabriusculum)
Kummritz, S. & D. Ohashi 99 (flexuosum)
Kummrow, R.1307, 2449 (flexuosum)
Kuntz et al. 78 (chacoense)
Kuntze, O. 487 (annuum var. annuum); 1235 (rhomboideum); 1811 (parvifolium)
Kurtz, F. 192, 958, 4221, 4615, 6503, 8510, 16056 (chacoense)
Kvist, L.P. & E. Asanza 40201, 40356, 40456, 40565 (frutescens); 40566, 40586 (chinense)
La Rotta, C. 147, 273 (frutescens)
La Rotta, C. & H. Martínez 737 (annuum var. annuum)
La Rotta, C. et al. 435 (chinense)
Labiak, P.& S.R. Ziller 403 (flexuosum)
Lacerda, A.V. & F.M. Barbosa 415, 471, 486 (parvifolium)
Lakela, O.K. 25539, 27780a (annuum var. glabriusculum)
Lakela, O.K. & F. Almeda 30154 (annuum var. glabriusculum)
Lakela, O.K. & D. Laker 29097 (annuum var. glabriusculum)
Lakela, O.K. et al. 25447 (annuum var. glabriusculum)
Lamboray, O. 22 (annuum var. annuum)
Landrum, L.R. 2881 (recurvatum)
Landrum, L.R. et al. 10868 (rhomboideum)
Lanfranchi, A.E. 1125, 1144 (chacoense)
Langlassé, F. 16 (rhomboideum)
Lanjouw, J. 1040 (frutescens)
Lanjouw, L. & J.C. Lindeman 1462 (annuum var. glabriusculum)
Lanna, J. & A. Castellanos 26542 (rabenii)
Lanstyak, L. 49 (villosum)
Lara, M.E. 191 (rhomboideum)
Lasseigne, A.A. 4450 (rhomboideum)
Laughlin, R.M. 1116 (annuum var. annuum); 1296 (annuum var. glabriusculum); 1324 (pubescens); 1325, 1667, 2012 (annuum var. glabriusculum); 2013 (annuum var. annuum); 2018 (frutescens); 2117, 2118, 2119, 2652, 2653 (annuum var. annuum); 2654 (annuum var. glabriusculum); 2657 (annuum var. annuum); 2937 (pubescens); 3017, 3018 (annuum var. annuum)
Lawesson, J.E. et al. 39639, 39640, 39646 (chinense)
Lazor, R.L. et al. 2525 (annuum var. glabriusculum)
Leavenworth, W.M. & H. Hoogstrael 1339 (annuum var. glabriusculum)
Legname, P.R. 49, 117, 165, 204, 5786, 7218, 7316, 8186 (chacoense)
Legname, P.R. & A.R. Cuezzo 7204 C (eximium)
Legname, P.R. et al. 5322 (chacoense); 5901 (baccatum var. baccatum); 7356 (flexuosum)
Lehmann, F.C. 233, 234, 438 (rhomboideum); 2603 (dimorphum); 3577 (annuum var. annuum); 4730 (annuum var. glabriusculum); 4732, 4745 (rhomboideum); 4949 (pubescens)
Leisner, R.L. & V. Medina 13444 (rhomboideum)
Leitão, H.F. et al. 1997 (flexuosum)
Leitão Filho, H.F. et al. 9546, 9725 (carassense); 33146 (recurvatum)
Leite, J.E. 745 (flexuosum); 3792 (mirabile)
Leiva González, S. 6531, 6580, 6581 (longifolium)
Leiva González, S. & G.E. Barboza 5647 (geminifolium); 6555 (annuum var. glabriusculum); 6561, 6562 (piuranum); 6576 (geminifolium); 6585 (rhomboideum)
Leiva González, S. et al. 720 (frutescens); 834 (annuum var. glabriusculum); 1214, 1216 (baccatum var. pendulum); 1320 (chinense); 1427, 1429 (pubescens); 1740, 2018 (geminifolium); 2105 (annuum var. glabriusculum); 2788 (geminifolium)
Lélis, N.S. et al. 1, 2, 12 (chinense)
Lemes, F.O.A. & G.P. Freitas 602 (villosum)
León, Bro. 626, 7855 (annuum var. glabriusculum)
León, H.A. 20 (chinense); 178, 278, 591 (annuum var. glabriusculum)
León de Luz, J.L. 2019 (annuum var. glabriusculum)
León S., S. 036 (annuum var. annuum)
Leonard, E.C. 3318, 3461, 8495 (annuum var. glabriusculum); 9700 (frutescens); 11113 (annuum var. glabriusculum)
Leonard, E.C. & G.M. Leonard 12089, 14735 (annuum var. glabriusculum)
Leoni, L.S. 3625 (mirabile)
Letterman, G.W. 96 (annuum var. glabriusculum)
Lévy, P. 31, 1087 (annuum var. glabriusculum)
Lewis, G.P. & B.B. Klitgaard 3730 (rhomboideum)
Lewis, M. 88629 (pubescens)
Lewis, W.H. 2925, 2980 (chinense); 7187, 7407 (annuum var. glabriusculum); 14011 (frutescens)
Lewis, W.H. et al. 839, 2939 (annuum var. glabriusculum); 10922 (annuum var. annuum); 11196 (annuum var. glabriusculum); 11207 (chinense); 12552, 12906 (annuum var. glabriusculum); 13607, 13960, 14010 (chinense)
Liberman, M. et al. 1833 (eximium); 1883 (chacoense); 2051 (baccatum var. baccatum); 2052 (eximium)
Liesner, R.L. 2239, 4432 (annuum var. glabriusculum); 20013 (frutescens)
Liesner, R.L. & A. C. González 5735 (annuum var. glabriusculum); 10728 (rhomboideum); 12024 (annuum var. glabriusculum)
Liesner, R.L. & G. Vega 2885 (annuum var. glabriusculum)
Liesner, R.L. et al. 5417, 7613 (annuum var. glabriusculum)
Lighthipe, L.H. 738 (annuum var. glabriusculum)
Lillo, M. 325, 327, 610, 1949, 2533 (chacoense); 3850 (baccatum var. baccatum); 6206 (chacoense)
Lima, A.S. & L. da Silva 5894 (schottianum)
Lima, C.T. et al. 38 (caatingae)
Lima, I.B. & J.R. Lima 526 (parvifolium)
Lima, J.M. 332 (baccatum var. pendulum)
Lima, L.R. et al. 389 (schottianum)
Lima, R.A.F. 420 (recurvatum)
Lima, V.C. et al. 75 (parvifolium)
Linares, E. 846 (annuum var. annuum)
Linares, J.L. 556 (frutescens); 1840, 3856, 6186 (rhomboideum)
Linares, J.L. & C.A. Martínez 859 (annuum var. glabriusculum)
Lindberg, G.A. 176d, 177 (rabenii)
Lindeman, J.C. & J.H. de Haas 578, 3581, 5029 (flexuosum)
Lindheimer, F. 482, 1036 (annuum var. glabriusculum)
Linneo F., I. 476 (baccatum var. pendulum)
Linneo F., I. et al. 1558 (minutiflorum)
Lins, E. 3 (caatingae)
Liogier, A.H. & P. Liogier 18713, 19207, 23115, 23679, 24230 (annuum var. glabriusculum)
Liogier, A.H. et al. 28206, 31084, 32113, 32122, 34006, 34488 (annuum var. glabriusculum)
Lira Neto, J.A. et al. 444 (campylopodium)
Llanos, F. 421 (rhomboideum)
Llanos H., F. & J. Camacho 1827 (annuum var. glabriusculum)
Llatas Quiroz, S. 1745 (geminifolium); 3452 (frutescens); 3454 (chinense); 4147 (frutescens); 4300, 4650 (annuum var. annuum)
Lleras Perez, E. 2135 (rabenii)
Lleras Pérez, E. et al. 1942, 1944 (rabenii); 1947, 1956, 1957, 1961, 1964, 1970, 1973 a, 1974, 1975, 1976, 1981, 1989 (flexuosum); 1992, 1993, 1994, 1995 (baccatum var. pendulum); 2009, 2010, 2015, 2020, 2023, 2029 (recurvatum); 2036, 2037 (flexuosum); 2047 (rabenii); 2050, 2140 (mirabile); 2142 (rabenii); 2152, 2161, 2172 (campylopodium); 2179, 2181 (pereirae)
Lloyd, C.G. 435 (annuum var. glabriusculum)
Loefgren, A. 438 (rabenii); 4321 (flexuosum); 5878 (villosum); 15427, 15430 (flexuosum)
Løjtnant, B. & U. Molau 14076 (rhomboideum)
Lombardi, J.A. 1570 (baccatum var. baccatum)
Lombardi, J.A. et al. 6217 (hunzikerianum); 8320 (recurvatum); 8333 (mirabile)
Lomelí Sención, J.A. 2908 (annuum var. glabriusculum)
Long, R.W. & R.P. Wunderlin 4079 (annuum var. glabriusculum)
Long, R.W. et al. 2231, 2271 (annuum var. glabriusculum)
Lopes et al. 1422 (caatingae)
Lópes, M. et al. 416 (mirabile)
López, A. c14 (rhomboideum)
López, A. & E. Saravia 203 (eximium)
López, A. et al. 4252 (annuum var. glabriusculum)
López, C.A. 83 (annuum var. glabriusculum); 99 (dimorphum)
López, J.C. et al. 78 (pereirae)
López, R. et al. 1756, 5943, 5997 (frutescens)
López Figueiras, T.M. 45, 223 (annuum var. glabriusculum)
López Jurado, G. & J.S. Riascos 511 (rhomboideum); 613 (pubescens)
López L., L. & G.J. Martín 135 (pubescens)
Lopez Luna, R. 0579 (frutescens)
López M., A. 7873 (annuum var. annuum)
López Moreno, J. & E. Cedillo Portugal 29 (annuum var. annuum)
López-Palacios, S. 1302, 2137 (rhomboideum); 2143 (annuum var. glabriusculum)
López-Palacios, S. & J.A. Bautista B. 3473 (pubescens); 3557 (rhomboideum)
López-Palacios, S. & J.M. Idrobo 3828 (annuum var. glabriusculum)
Lorence, D.H. 5560 (annuum var. glabriusculum)
Lorence, D.H. & R. Cedillo 3078 (annuum var. glabriusculum)
Lorentz, P.G. 105, 1318 (chacoense)
Lorentz, P.G. & G. Hieronymus 240 (baccatum var. baccatum)
Lorenzi, H. et al. 5656 (flexuosum)
Lossen, W. 323 (chacoense)
Losser, G. & L. Aristeguieta 3438 (rhomboideum)
Lot, A. et al. 2075 (annuum var. glabriusculum)
Lott, E.J. 711 (annuum var. glabriusculum)
Lott, E.J. & J.A. Magallanes 659 (annuum var. glabriusculum)
Lozano et al. 577A (chinense)
Lozano, G. et al. 642 (chinense); 4820 (rhomboideum)
Lozano, P. et al. 942 (rhomboideum)
Lozano, R. 1207 (neei)
Lozano C., G. 3270, 4036 (dimorphum)
Lozano C., G. & R. Schnetter 2925 (annuum var. glabriusculum)
Lozano C., G. et al. 575, 5167 (frutescens)
Lucas, E.J. et al. 667 (mirabile)
Lucas, J. 135 (lanceolatum)
Lucca, C.F. et al. 18 (caatingae)
Lucena, R.F. & U.P. Alburquerque 91, 92 (parvifolium)
Lucena, R.F. & A.C. Silva 227, 228 (parvifolium)
Lugo S., H. 1249, 2444 (rhomboideum)
Luján, M.C. 327 (chacoense)
Luna, A. 356 (annuum var. glabriusculum)
Luna, F.E. 957 (chacoense)
Lundell, C.L. 87 (frutescens); 3754 (annuum var. annuum); 15376 (annuum var. glabriusculum); 16480 (frutescens)
Lundell, C.L. & E. Contreras 19773, 20417, 20971 (lanceolatum)
Lundell, C.L. & A.A. Lundell 7998 (annuum var. glabriusculum)
Lundell, P.H. 4766 (frutescens)
Luschnath de la Tour, J.B.L.T. 3098 (annuum var. glabriusculum)
Luteyn, J.L. 12554 (dimorphum)
Luteyn, J.L. & R. Callejas 11796 (dimorphum)
Luteyn, J.L. & O. Rangel 13146 (dimorphum)
Luteyn, J.L. et al. 6595 (geminifolium); 12547 (lycianthoides)
Luti, R. & E. Bücher 4004 (chacoense)
Lutz, B. 1007 (mirabile)
Lyra-Lemos, R.P. 11567 (caatingae)
Lyra-Lemos, R.P. & E.M. Duarte 5771 (caatingae)
Lyra-Lemos et al., R.P. 6827, 9402, 13147, 13177, 14027 (caatingae)
Maas, P.J. & P. Carauta 3275 (campylopodium)
Macbride, J.F. 4831 (geminifolium)
MacDougal, J.M. & F.J. Roldán 3547 (lycianthoides); 3557 (dimorphum)
Macedo, A. 3079 (schottianum)
Macedo, I.C.C. 5 (flexuosum)
Machado, R.F. et al. 271 (baccatum var. baccatum); 368 (longidentatum)
Machado, T.M. et al. 297 (mirabile)
Machado, W.J. & J.B. Jesus 738, 787, 800 (caatingae)
Machuca, J.A. N. 6889 (rhomboideum)
Madison, M.T. et al. 4675 (annuum var. annuum); 4967 (annuum var. glabriusculum)
Madrigal Sánchez, X. 4875 (annuum var. glabriusculum)
Madsen, E.B. 84312 (rhomboideum)
Madsen, J.E. 50214 (rhomboideum); 63565 (frutescens); 85610, 87010 (rhomboideum)
Madsen, J.E. & L. Ellemann 75101 (dimorphum)
Madsen, J.E. et al. 7040 (frutescens); 7601 (geminifolium)
Magallanes, J.A. 2691 (annuum var. glabriusculum)
Magaña, M.A. 1579 (annuum var. glabriusculum); 1699 (annuum var. annuum); 1957 (frutescens); 2006 (lanceolatum); 2326 (annuum var. glabriusculum)
Magaña Rueda, P. et al. 340 (annuum var. glabriusculum)
Magnoni 10 (chacoense)
Malda B., G. 111 (annuum var. glabriusculum)
Maldonado B., R. 504, 1250 (chacoense)
Malme, R. 156 (chacoense)
Málvarez, M.R. 583 (chacoense)
Mamaní M., F. 583 (chacoense)
Mamaní M., F. et al. 1435 (baccatum var. baccatum)
Manchego, C. CBP 01, 03, 04, 05, 11, 12, 13, 14, 15, 16, 17, 18 (baccatum var. baccatum); CBNP 01, 02, 03, 04, 05, 06 (baccatum var. baccatum); CBP A1 (baccatum var. baccatum); CBP T1, T2, T4, T7, T8, T9, T10, T16, T17, T18, T19, T20, T29 (baccatum var. baccatum); CBP NT2, NT3, NT5 (baccatum var. baccatum); CCP 01, CCP 03, CCP A1, CCP T5, CCP T6, CCP T11, CCP T13, CCP T14, CCP T25, CCP T26, CCP T27, CCP T28, CCP NT 4, CCNP A1, CCNP NT s.n., CCNP T3, CCNP T12, CCNP T15, CCNP T23, CCNP T24 (chacoense); CEP T21, CENP T22 (eximium)
Manchego, C. & M. Simon 60062B, 60065B, 60068B, 60076B, 60087B (chacoense)
Manchego, C. et al. 60194B, 60198B (chacoense)
Mandon, G. 427 (eximium); 428 (pubescens)
Maranta, A. 185 (chacoense)
Marchessi, E. et al. 1205 (chacoense)
Marín, C. et al. 2482 (frutescens)
Marín, C. & F. Rodríguez 504, 509 (frutescens); 525 (annuum var. annuum)
Marín, G. et al. 776 (flexuosum)
Markgraf, F. & A.C. Brade 3753 (villosum)
Marques de Lima, J. 273 (rabenii)
Marquete, R. et al. 260 (campylopodium)
Marquez R., W. 472 (pubescens)
Márquez, S. & B. Vecino 119 (annuum var. annuum)
Marrugo G., J.C. 296 (annuum var. glabriusculum); 1021 (geminifolium)
Marrugo G., J.C. & J.M. Vélez P. 55, 265 (dimorphum)
Marrugo G., J.C. et al. 709 (rhomboideum)
Marsh, E. 256 (annuum var. glabriusculum)
Marshall, S.A. & D.A. Neill 6624 (annuum var. glabriusculum)
Martin, R.T. 75, 168 (chinense)
Martin, R.T. & C.A. Lau-Cam 1242 (frutescens)
Martinelli, G. 1107 (schottianum)
Martinelli, G. et al. 2461, 2472, 2492 (mirabile); 10405 (campylopodium)
Martínez, G.J. 136, 190, 1185 (chacoense)
Martínez, M. 1145 (annuum var. glabriusculum); 1329, 2183, 3431 (rhomboideum); 5105 (annuum var. glabriusculum); 5332 (annuum var. glabriusculum); 5243 (rhomboideum)
Martínez, O. 85 (rhomboideum)
Martínez A., M.A. 346 (frutescens)
Martínez C., G. 1287 (annuum var. glabriusculum); 1643 (annuum var. annuum);1897, 2011, 3087, 3094 (annuum var. glabriusculum)
Martínez Crovetto, R. 31 (chacoense); 100 (baccatum var. baccatum); 135 (flexuosum); 11125 (baccatum var. baccatum)
Martínez Crovetto, R. & A. Schinini 10857 (baccatum var. baccatum)
Martínez Gallegos, J.F. 79 (rhomboideum)
Martínez Ojeda, E. 263 (annuum var. glabriusculum)
Martínez R., C. 44 (rhomboideum); 76 (annuum var. glabriusculum)
Martínez S., E. 2262 (annuum var. glabriusculum); 9066 (lanceolatum); 11240, 16073, 29417 (frutescens)
Martínez S., E. & A. García 22066 (annuum var. glabriusculum)
Martínez S., E. & A. Ibarra 40296 (annuum var. glabriusculum)
Martínez S., E. & F. Martínez 6316 (frutescens)
Martínez S., E. & L. Rico 6062 (annuum var. glabriusculum)
Martínez S., E. & J.C. Soto 3640 (annuum var. annuum)
Martínez S., E. et al. 246 (chinense); 4476 (annuum var. glabriusculum); 23039 (lanceolatum); 29428 (annuum var. annuum); 29562, 36224 (annuum var. glabriusculum)
Martínez Torres, H.L. 57, 82, 98 (annuum var. glabriusculum)
Martins, C. 242 (caatingae)
Marulanda, O. 395 (dimorphum)
Marulanda, O. & S. Churchill 490 (chinense)
Maruñak, V. et al. 564, 626 (chacoense)
Mateus Gutierrez, C. 28 (annuum var. glabriusculum)
Mathews 854 (pubescens)
Matos, F. 1076 (rhomboideum)
Matos, G.M.A. et al. 308 (caatingae)
Matos, J.R. 32 (frutescens)
Matthes, B. 139 (annuum var. glabriusculum)
Mattos, J. 11459 (schottianum); 12156 (rabenii); 12165 (mirabile); 12778 (cornutum)
Mattos, J. & M. Mattos 14254 (hunzikerianum); 14776 (villosum)
Matuda E. 247 (annuum var. glabriusculum); 2406 (pubescens); 4656, 4868 (rhomboideum); 5177 (lanceolatum); 6976 (rhomboideum); 17590 (annuum var. glabriusculum); 17642 (frutescens); 18120 (annuum var. glabriusculum)
Maturo, H. 245 (chacoense)
Maxon, W.R. 2160 (frutescens)
May, O. 39 (annuum var. annuum)
Mayer, M. 24 (parvifolium)
Mayworm, M.A. et al. 135 (cornutum)
McCart, W. L. 11158 (annuum var. glabriusculum)
McCarten, N.F. & R.L. Bittman 2585 (rhomboideum)
McDaniel, S. 14075 (coccineum); 27181 (annuum var. glabriusculum)
McDaniel, F. & L. Santiago 2556 (coccineum)
McDaniels, L.H. 698 (pubescens)
McDowell, T. et al. 796 (annuum var. annuum); 1940, 2174 (annuum var. glabriusculum)
McVaugh, R. 23267 (rhomboideum)
Meave del Castillo, J. & E. Pérez-García 1837, 2350 (annuum var. glabriusculum)
Medina A., M.E. & F. Vazquez B. 542 (rhomboideum)
Medina E. 939 (annuum var. glabriusculum)
Medrano, F.G. et al. 1823 (annuum var. glabriusculum)
Meier, W. et al. 6621 (annuum var. glabriusculum)
Meireles, L.D. et al. 1047 (recurvatum)
Mejía, F. 001 (annuum var. annuum); 002 (pubescens)
Mejía, M.M. 183 (annuum var. glabriusculum)
Mejía, M.M. & C. Ramírez 11039, 11137 (annuum var. glabriusculum)
Mejía, M.M. & T. Zanoni 6351, 6619, 7524, 8100 (annuum var. glabriusculum)
Mejía, M.M. et al. 8925, 10386, 10396, 10490 (annuum var. glabriusculum)
Mejía Pimentel, M. & T. Zanoni 7738 (annuum var. annuum)
Melo, E. 9364 (longidentatum); 11840, 11878, 13140 (caatingae)
Melo, E. & B. Marques da Silva 6081 (longidentatum)
Melo, E. et al. 1859, 1920 (longidentatum); 1969 (caatingae); 3646 (parvifolium); 3862, 4336 (caatingae); 4344 (longidentatum); 4693 (longidentatum); 5440 (caatingae); 5571 (baccatum var. baccatum); 5658 (parvifolium); 5664 (baccatum var. baccatum); 5926, 6230, 6295 (caatingae); 6545 (rabenii); 6909 (longidentatum); 7695 (parvifolium); 7813 (flexuosum)
Melo, G.F.A. et al. 221 (parvifolium)
Melo, P.H.A. 5030 (baccatum var. baccatum)
Melo, P.H.A. & J.A. Lombardi 362 (baccatum var. baccatum); 381 (rabenii)
Melo, P.H.A. & T.R. Peixoto 6080 (longidentatum)
Mencho, M. & E. Gutiérrez 586 (baccatum var. baccatum)
Mendes, M.R.A. & R.S. Albino 509 (parvifolium)
Mendes, M.R.A. et al. 292, 539 (parvifolium)
Méndez, M. et al. 476 (annuum var. glabriusculum)
Méndez Girón, A. 7745 (lanceolatum)
Mendonça et al. 5971 (baccatum var. baccatum)
Mendoza, H. 661, 814 (lycianthoides); 1800 (annuum var. glabriusculum); 17394 (dimorphum)
Mendoza, H. et al. 14946, 15269 (dimorphum)
Mendoza, J.E. & L. Quevedo 407, 525 (dimorphum)
Mendoza, J.E. et al. 4202 (lycianthoides)
Mendoza, M. 788 (chacoense)
Mendoza, M. & M. Balcazar 2021 (eximium)
Mendoza, M. & E. Calzadilla 362, 363, 801 (eximium)
Mendoza, M. & R. Lozano 827, 828 (eximium)
Mendoza, M. & D. Vidal 1265 (eshbaughii)
Mendoza, M. et al. 2805 (chacoense)
Mendoza, W. et al. 6570 (annuum var. glabriusculum)
Meneces, E. & W. Terceros 1004 (eximium)
Mentz, L.A. et al. 263 (baccatum var. baccatum); 278 (flexuosum); 289 (recurvatum); 290 (flexuosum); 309, 400, 401 (baccatum var. baccatum)
Mercado, M. & R. Navia 1811 (eximium)
Mereles, F. 2920, 5536, 8651 (chacoense)
Mereles, F. & R. Degen 5136 (chacoense)
Mereles, F. & M. Soloaga 7568 (baccatum var. pendulum)
Mereles, F. et al. 7498 (flexuosum); 7499 (baccatum var. pendulum); 7502 (baccatum var. pendulum); 7503 (flexuosum)
Merino, B. & T. Delgado E-1340, E- 1569 (geminifolium)
Messias, M.C.T. & R. M. Carrillo 2443 (baccatum var. baccatum)
Metz, M.C. 111 (annuum var. glabriusculum)
Mexia, Y. 4148, 4205 (rabenii); 4328 (villosum); 4493 (rabenii); 7195 (pubescens); 8246 (geminifolium)
Meyer, F.G. & P.M. Mazzeo 11988 (frutescens)
Meyer, R. 13848 (chacoense)
Meyer, T. 92, 858, 2457, 2458 (chacoense); 5477 (baccatum var. baccatum); 5506 (flexuosum); 5976 (baccatum var. baccatum); 6694 (flexuosum); 6790 (baccatum var. baccatum); 8561, 9795 (chacoense); 11550 (flexuosum); 11918 (baccatum var. baccatum); 13128, 13325 (chacoense); 15922 (baccatum var. baccatum); 17527 (eximium); 18144 (baccatum var. baccatum); 19927 (coccineum); 20304 (chacoense)
Meyer, T. & A.R. Cuezzo 22440 (baccatum var. baccatum)
Meyer, T. et al. 9170C (chacoense)
Meza E., P. 2 (annuum var. glabriusculum)
Miers, J. 4541 pp (campylopodium); 4541 pp (mirabile)
Miguel, A. 2006 (lanceolatum)
Mill, H. 614 (lanceolatum)
Miller, G.S. 149 (frutescens); 198 (annuum var. glabriusculum)
Miller, G.S. Jr. 199 (frutescens)
Miller, J.S. et al. 2404 (chinense); 5792 (annuum var. glabriusculum)
Miller, J. & P. Yépez 651, 652, 667 (chinense)
Miller, O.O. & J.R. Johnston 35 (rhomboideum); 81 (frutescens); 82 (annuum var. glabriusculum); 255 (parvifolium)
Mione, T. 466 (geminifolium); 812 (piuranum)
Miranda, A. 8 (frutescens)
Miranda, A. et al. 1519, 1519 A, 1519 B (annuum var. annuum); 1644 (annuum var. glabriusculum)
Miranda, A.M. 6695 (caatingae)
Miranda, A.M. & Félix 1642 (caatingae)
Miranda, A.M. et al. 6148 (parvifolium)
Miranda, F. 4021 (rhomboideum); 5482 (annuum var. glabriusculum); 6374 (rhomboideum); 8097, 8224 (annuum var. glabriusculum)
Miranda-Moyano, N. 225 (frutescens)
Miranda-Moyano, N. & G. Moya 334 (annuum var. annuum); 436, 437 (chinense)
Moina, S. Z 23 (pubescens)
Molas, L. 792 (flexuosum)
Molas, L. & V. Vera 1067 (chacoense)
Moldenke, H.N. 727 (annuum var. glabriusculum)
Molina, A.M. & J. Hilfer 2788, 2993, 3046 (chacoense)
Molina, A.M. et al. 1328 (chacoense)
Molina, D. 34, 110 (annuum var. glabriusculum)
Molina R., A. 262 (annuum var. glabriusculum); 561 (rhomboideum); 594 (annuum var. glabriusculum); 1054, 1177 (frutescens); 1457, 1820, 2784, 3200, 3212, 3248, 3875 (rhomboideum); 22452, 23062 (annuum var. glabriusculum); 24525 (chinense); 34008 (annuum var. annuum)
Molina R., A. & A.R. Molina 14053 (lanceolatum); 34535 (annuum var. annuum)
Molina R., A. et al. 33572 (annuum var. annuum)
Monetti, L. 1975 (chacoense); 2257 (eximium)
Monro, A.K. et al. 2164 (annuum var. glabriusculum)
Monsalvo J., C.B. 12 (pubescens)
Monteagudo, A. et al. 3475 (geminifolium); 9371(dimorphum); 9470 (geminifolium); 13706 (dimorphum); 15087 (geminifolium)
Monteiro, H. 1918, 2276 (baccatum var. baccatum)
Montenegro, O. 137 (frutescens)
Montenegro, V. 1576 (rhomboideum)
Montenegro Valls, J.F. et al. 8782 (chinense)
Montes, J.E. 398, 1635, 1689, 2139, 2140, 2155, 2213, 2305 b, 2353, 2375, 2411, 2411b, 2476, 3280, 4026, 4277, 4289, 12314, 14727 (flexuosum); 15164, 15188, 15202 (baccatum var. baccatum); 15418 (chacoense); 15612, 15633, 15716, 15900 (baccatum var. baccatum); 16310, 16536, 27474, 27476 (flexuosum)
Moonlight, P.W. & T. Särkinen 709 (parvifolium)
Moore, A.D. 1546 (annuum var. glabriusculum)
Moore Jr., H.E. 1820, 3831 (rhomboideum)
Mora, L.E. 332 (rhomboideum)
Moraes, A.O. et al. 159, 160 (caatingae); 161 (longidentatum)
Moraes, M.S. 18 (campylopodium)
Moraes, M.V. 173 (baccatum var. baccatum); 790 (caatingae)
Morales, G. 2914 (geminifolium)
Morales, M. et al. 015 (annuum var. glabriusculum)
Mora-López, J.L. 134 (annuum var. glabriusculum)
Moreira, H.P. et al. 84 (campylopodium)
Morel, I. 452, 722, 776, 910, 1746, 1845, 2867, 3375, 4171, 4417, 6746, 7127, 7691, 8909 (chacoense)
Morello, J. & A.E. Cuezzo 157, 174 (baccatum var. baccatum); 284, 402, 1004 (chacoense)
Moreno, P.P. 733, 763, 788, 1180, 1240, 1271, 1408, 1480, 1544, 1605, 1725, 1791, 1891, 1926, 1948, 1949, 2037, 2089, 2123, 2170, 2243, 2313, 2348, 2481, 2544, 2636, 2685, 2738, 3044, 3292, 3590, 3727, 3919, 4220, 4328 (annuum var. glabriusculum); 10196, 21241, 21761, 21824, 21875 (rhomboideum); 22007 (annuum var. glabriusculum)
Moreno, P.P. & J. Henrich 8445, 8793 (annuum var. glabriusculum); 8954 (annuum var. annuum)
Moreno, P.P. & J.C. Sandino 12134 (frutescens); 12153 (annuum var. annuum)
Moreno V., D.I. 34 (rhomboideum)
Mori, S.A. et al. 11603 (baccatum var. baccatum)
Morillo, G. & R. Smith 6537 (rhomboideum)
Morillo, G. et al. 4297, 4299, 8448 (rhomboideum)
Morong, T. 388 (baccatum var. baccatum); 961 (chacoense)
Morrill, S. & J. Harvey 154 (annuum var. glabriusculum)
Morrone, O. 780 (flexuosum)
Morrone, O. et al. 932, 1444 (flexuosum); 2884 (baccatum var. baccatum)
Morrow, C.F. 10, 15 (annuum var. glabriusculum)
Morton, C.V. 4713, 10259 (annuum var. glabriusculum); 10907 (eximium)
Moscone, E.A. 98, 104 (chacoense); 148 (flexuosum); 194, 195 (chacoense); 197 (baccatum var. umbilicatum); 198 (pubescens); 200 (frutescens); 202 (pubescens); 204 (annuum var. annuum); 207 (chacoense); 229 (flexuosum); 237 (frutescens)
Moscone, E.A. & R. Neumann 192, 205, 206, 209 (baccatum var. pendulum); 210 (baccatum var. baccatum); 211 (baccatum var. pendulum); 212, 213 (chacoense); 214, 215 (baccatum var. baccatum)
Mosén, H. 984 (baccatum var. baccatum); 1563 (schottianum); 3443 (recurvatum)
Mostacedo, B. 495 (minutiflorum)
Mostacedo C., B. 2699 (baccatum var. baccatum); 2975 (chacoense)
Mostacero, J. et al. 1768 (rhomboideum)
Mota, R.C. 106, 2260, 2653 (carassense)
Moura, S. 1016 (caatingae)
Moya, J.G. 84 (rhomboideum)
Moya, G. & D. Reyes 206, 207 (chinense)
Múlgura, M. et al. 1779, 2112, 3322, 3391 (flexuosum)
Müller, C.H. 2674 (rhomboideum)
Müller, C.H. & M.T. Müller 131, 647 (annuum var. glabriusculum)
Müller, G. & P. Gutte 8422 (frutescens)
Müller, J.K. 174 (annuum var. annuum)
Muñoz, L. 257 (annuum var. annuum)
Muñoz C., E. 300 (annuum var. glabriusculum)
Múrcia, C. 129, 191 (dimorphum); 371 (lycianthoides); 470 (dimorphum)
Múrcia, G. 77, 77 B (dimorphum)
Mutis, J.C. 2010 (dimorphum); 3590 (rhomboideum)
Mynssen, C.M. et al. 1404 (mirabile)
Naboulet 140 (chacoense)
Nadal, F.H. et al. 28 (friburgense); 43 (recurvatum)
Nadruz, W.M. et al. 2251 (campylopodium)
Naranjo, D. & B. Freire 329 (chinense)
Narváez, C. 018 (annuum var. annuum)
Narvaez, E. 17 (annuum var. glabriusculum)
Narváez Montes, M. 861 (annuum var. glabriusculum)
Nash, G.V. 985, 1287 (annuum var. glabriusculum)
Nash, G.V. & N. Taylor 1456 (annuum var. glabriusculum)
Nates Parra, G. 214 (annuum var. glabriusculum); 225 (rhomboideum)
Nava Zafra, A. et al. 1812 (annuum var. glabriusculum)
Navarro, G. & I.G. Vargas C. 261 (chacoense)
Navarro, J.A. et al. 288 (dimorphum)
Navarro Sánchez, G. 1618 (baccatum var. baccatum)
Nee, M. 3424, 10103 (baccatum var. baccatum); 6789, 6989, 6990, 23723, 26700, 27745, 28210, 28288, 32699 (annuum var. glabriusculum); 33493, 33715 (minutiflorum); 34184 (baccatum var. baccatum); 34412 (annuum var. glabriusculum); 35427 (minutiflorum); 36049 (coccineum); 36164 (eshbaughii); 36304, 36514 (baccatum var. baccatum); 37233, 37305 (coccineum); 37575, 37585 (eximium); 37759 (baccatum var. baccatum); 38180 (coccineum); 38361 (eximium); 38899, 39360 (coccineum); 39545, 40110, 40103 (minutiflorum); 40886 (coccineum); 41131 (frutescens); 42027 (minutiflorum); 42191 (baccatum var. baccatum); 42218 (minutiflorum); 43027 (frutescens); 43167 (baccatum var. baccatum); 44503 (minutiflorum); 44837, 45842, 47937, 48439, 48489, 48553 (baccatum var. baccatum); 49201 (minutiflorum); 51786 (pubescens); 52197 (eshbaughii); 52917 (minutiflorum); 55645 (frutescens)
Nee, M. & L. Bohs 50775 (baccatum var. baccatum); 50807 (eximium)
Nee, M. & J.I. Calzada 33201 (annuum var. glabriusculum)
Nee, M. & G. Coimbra S. 33969 (baccatum var. baccatum)
Nee, M. & G. Diggs 24413 (annuum var. glabriusculum)
Nee, M. & R. Flores S. 54782 (baccatum var. baccatum); 54790, 54863, 54882 (chacoense)
Nee, M. & A. Molina O. 56085 (chacoense)
Nee, M. & S. Mori 4270, 4271 (rhomboideum)
Nee, M. & M. Saldías P. 35956, 36874 (coccineum)
Nee, M. & Solomon 30265 (annuum var. glabriusculum); 30265 (frutescens); 32018 (pubescens); 36558, 36578 (eximium)
Nee, M. & M. Sundue 51994 (minutiflorum)
Nee, M. & K. Taylor 25938 (pubescens); 28811 (rhomboideum)
Nee, M. & W.R. Téllez 28018 (annuum var. glabriusculum)
Nee, M. & I. Vargas C. 37448, 38323 (eximium); 43483 (eshbaughii); 44747 (eximium)
Nee, M. & S. Vega 27926 (annuum var. glabriusculum)
Nee, M. et al. 19489, 27750, 28175 (annuum var. glabriusculum); 47215, 47257 (rhomboideum); 51778 (ceratocalyx); 52407 (caballeroi); 55030 (baccatum var. baccatum); 55070 (eximium); 55227 (frutescens); 55779 (chacoense)
Neill, D. & T. Núñez 10491 (hookerianum)
Nelson, C. 3908 (rhomboideum)
Nelson, C. et al. 7102 (annuum var. glabriusculum)
Nelson, E.B. 4343, 5379, 7809 (annuum var. glabriusculum)
Nesom, G. et al. 6009 (annuum var. glabriusculum)
Neubert, H. 51, 216 (flexuosum)
Nevling, L. & A. Gómez Pompa 196 (annuum var. glabriusculum); 645 (annuum var. annuum); 2317 (rhomboideum)
Nichols, C.E. 1139 (annuum var. glabriusculum)
Nicolau, S.A. 1050 (recurvatum)
Nicolau, S.A. et al. 1237 (villosum)
Noblick, L.R. 3207 (caatingae)
Noblick, L.R. & Lemos 4227 (caatingae)
Norris, D.H. 17477 (annuum var. glabriusculum)
Northrop, J.I. & A.R. Northrop 256 (annuum var. glabriusculum)
Novara, L. & S. Bruno 9295 (baccatum var. baccatum); 9898 (chacoense)
Novara, L. 1936, 3069, 3353 (chacoense); 5102, 8346 (eximium); 8423 (chacoense)
Novara, L. & R. Neumann 3121 (eximium)
Novara, L. et al. 12205 (baccatum var. baccatum); 12206 (eximium); 12504 (chacoense)
Núñez V., P. 6203 (coccineum); 7537 (pubescens); 8600 (baccatum var. pendulum); 8720 (pubescens); 14114 (coccineum)
Núñez V., P. et al. 6780 (annuum var. glabriusculum); 6799 (baccatum var. pendulum); 10024 (coccineum); 16906 (frutescens)
Núñez, T. et al. 39 (rhomboideum); 336 (annuum var. glabriusculum)
O’Donell, C.A. 351, 901, 4328, 4410, 4436 (chacoense)
O’Donell, C.A. & J.M. Rodríguez V. 920, 978 (chacoense)
Obando, S. et al. 46 (dimorphum)
Ochoa, C. 602 (pubescens); 11995 (baccatum var. baccatum)
Odonne, G. 25 (annuum var. glabriusculum); 0522 (frutescens); 561, 563, 626, 627 (annuum var. glabriusculum); 0641 (frutescens); 664 (annuum var. glabriusculum)
Olivares Hernández, D. 1 (annuum var. glabriusculum)
Oliveira, A.R.S. et al. 675 (coccineum)
Oliveira, D.G. et al. 158 (caatingae)
Oliveira, G.B. 360, 371, 387, 395 (pereirae)
Oliveira, M. 7 (caatingae); 5795 (parvifolium)
Oliveira, M. & J.R. Silva 5862 (parvifolium)
Oliveira, M. et al. 5408 (caatingae)
Oliver, R.L. et al. 1106 (annuum var. glabriusculum)
Øllgaard, B. & H. Balslev 9348 (geminifolium)
Øllgaard, B. et al. 90357 (dimorphum)
Omawale & R. Persaud 130 (annuum var. annuum)
Orcutt, C.R. 626, 1467 (annuum var. glabriusculum)
Ordones, J. et al. 2229 (carassense)
Ordonez, M.J. 53, 239 (annuum var. glabriusculum)
Ordóñez, M.T. & H. Valencia 10 (annuum var. glabriusculum)
Ordóñez, P. 016 (frutescens)
Orejuela R., A. & E. Calderón Sáenz 166 (lycianthoides)
Orejuela R., A. 109, 174 (lycianthoides)
Orejuela R., A. et al. 2640 (regale); 2685 (dimorphum); 2688 (geminifolium); 3034, 3035 (regale)
Orlandi, R. 251 (caatingae)
Orozco, C.I. & R. Kuiru 173 (annuum var. glabriusculum)
Orozco, C.I. et al. 3371, 3675, 3717, 3838, 3863, 3865 (dimorphum); 3922, 3931, 3944 (geminifolium); 3960, 3972, 3989 (lycianthoides)
Orozco, O.L. 122 (pubescens)
Orozco-Segovia, A.D. 368 (annuum var. glabriusculum)
Ortega, A. et al. 51 (frutescens)
Ortega, H.J. & L.R. Ortega 865 (annuum var. glabriusculum)
Ortega, R. et al. 876 (frutescens)
Ortega, R.V. 494 (rhomboideum)
Ortega O., R. et al. 870 (annuum var. glabriusculum)
Ortega-Torres, L.M. 87 (annuum var. annuum); 88 (annuum var. glabriusculum)
Ortega-Torres, L.M. & J. Tzuc 272 (annuum var. glabriusculum)
Ortiz, C. et al. 3 (caballeroi)
Ortiz, E. & R. Francis J. 736 (geminifolium)
Ortiz, E.V. et al. 752 (dimorphum)
Ortiz, F.J. 11 (lycianthoides)
Ortíz, G. 01, 07, 20 (annuum var. annuum)
Ortiz, J.J. & S. Avendaño 1055 (annuum var. glabriusculum)
Ortiz, M. 1283 (flexuosum)
Ortiz, R. et al. 591 (rhomboideum)
Ortíz, R.T. 341 (annuum var. glabriusculum)
Ortuño, T. 271 (cardenasii); 769 (chacoense)
Otero J., J.I. 284 (annuum var. glabriusculum)
Ovando, A. 18 (baccatum var. baccatum)
Pabón, M.A. E. 257, 258, 259, 277 (chinense)
Pabst, G. & E. Pereira 7225 (campylopodium)
Pachano, A. 78 (rhomboideum)
Padilla, I. 219 (annuum var. glabriusculum)
Padilla, I. et al. 3088 (frutescens)
Paixão, J.L. 951 (mirabile)
Paixão, J.L. et al. 863 (pereirae)
Palacios, R.A. 733 (chacoense)
Palacios, W. 6318 b (geminifolium); 6837 (rhomboideum)
Palacios, W.A. & D. Rubio 9956 (hookerianum)
Palacios, W. & M. Tirado 13256 (dimorphum)
Palacios, W. & H. van der Werff 3591 (lycianthoides)
Palacios, W. et al. 9746 (lycianthoides)
Palacios E., E. 636 (annuum var. glabriusculum)
Palma Gutiérrez, J. 85-24, 85-25, 85-69 (chinense)
Palmer, E. 135 (annuum var. annuum), 135, 136 (chinense); 137, 138, 139 (annuum var. annuum); 144 (annuum var. glabriusculum); 173 (rhomboideum); 215, 230 (annuum var. glabriusculum); 272 (rhomboideum); 394 (annuum var. glabriusculum); 456 (rhomboideum); 638 (annuum var. glabriusculum); 639, 640, 642 (annuum var. annuum); 772, 931, 1135, 10789 (annuum var. glabriusculum)
Palombo, N. 3 (baccatum var. pendulum); 19 (eshbaughii); 21, 22, 23 (pubescens)
Panero, J.L. & I. Calzada 4029 (rhomboideum)
Parada, G.A. et al. 2760 (eximium); 3523 (neei); 4355 (baccatum var. baccatum); 4456 (minutiflorum); 5445 (chacoense)
Paredes, D. 845 (annuum var. annuum)
Parodi, L.R. 3102, 14133 (chacoense)
Parra V., G. 26, 089 (rhomboideum)
Pascali, A.H.G. 8 (annuum var. annuum)
Pascual, J. 1535 (rhomboideum)
Pastore, J.F. & R.M. Harley 2603 (longidentatum)
Patzlaff, R. 15 (frutescens)
Paula, M.F.R. et al. 18 (recurvatum)
Paula-Souza, J. 7254 (baccatum var. baccatum); 7981, 8175 (chacoense)
Paula-Souza, J. et al. 7336 (flexuosum)
Pavajeau, L. 60, 174 (dimorphum)
Pavetti, C. & T. Rojas 10716 (flexuosum); 10821 (chacoense)
Pedersen, T.M. 4670 (chacoense); 5315 (baccatum var. baccatum); 5517 (chacoense); 7712 (baccatum var. baccatum); 7787 (schottianum); 10239 (chacoense); 10996 (flexuosum); 15395 (chacoense); 15791 (flexuosum)
Pedralli, G. 87 (flexuosum)
Pedralli, G. et al. 3045 (flexuosum)
Pedraza-Peñalosa, P. et al. 59, 2201 (dimorphum)
Penereiro 11062 (chinense)
Penland, C.W. & R.H. Summers 44, 841 (rhomboideum)
Pennell, F.W. 2715 (rhomboideum); 9318, 10316 (dimorphum); 10989 (annuum var. glabriusculum); 17941 (rhomboideum); 19497 (annuum var. glabriusculum)
Pennell, F.W. & E.P. Killip 5761 (dimorphum)
Pennell, F.W. & H.H. Rusby 187 (rhomboideum)
Penneys, D. 502 (pubescens)
Pennington, T.D. 60SD (rhomboideum)
Pensiero, J. 5245 (chacoense)
Pensiero, J. & J. Tivano 3261 (chacoense)
Pensiero, J. et al. 7434, 7511 (chacoense)
Peña-Chocarro, M. 2536 (chacoense)
Peñaloza, H. 4856 (frutescens)
Peñaranda, J.A. 458 (eximium)
Peñaranda, J.A. et al. 817 (eximium)
Peredo, I. 348 (baccatum var. baccatum)
Pereira Duarte, A. 979 (schottianum)
Pereira, E. 2245 (pereirae); 5305 (flexuosum)
Pereira, E. et al. 4444 (campylopodium)
Pereira, F.G. et al. 406 (recurvatum)
Pereira, L.A. et al. 1672 (frutescens); 1699 (chinense); 1701, 1747, 1780, 1807 (annuum var. glabriusculum); 1809 (frutescens); 1816, 1817 (chinense); 1819 (baccatum var. pendulum); 1820 (frutescens); 1821 (chinense);1822 (annuum var. glabriusculum); 1824 (baccatum var. umbilicatum); 1827, 1828 (chinense); 1829 (frutescens); 1830 (baccatum var. umbilicatum); 1831, 1833 (chinense); 1834 (baccatum var. umbilicatum); 1835 (chinense); 1836 (frutescens); 1837, 1839 (chinense); 1840, 1841, 1842 (annuum var. glabriusculum); 1846, 1851, 1852 (chinense);1863 (baccatum var. pendulum); 1864 (chinense); 1865, 1866 (frutescens); 1867 (annuum var. glabriusculum); 1868, 1870, 1873, 1896 (chinense);1899 (baccatum var. pendulum); 1900 (annuum var. glabriusculum); 1901, 1902 (chinense); 1903 (annuum var. glabriusculum); 1904, 1905, 1906, 1908, 1918, 1919, 1920 (chinense)
Pereira, L.A. & W.M.S. Severino 1851 (baccatum var. pendulum); 1853 (annuum var. glabriusculum); 1854 (chinense); 1855 (frutescens)
Pereira, R. et al. 115, 2607 (caatingae)
Pereira, S.M. 3219 (frutescens)
Pereira-Silva, G. 9995 (flexuosum); 13172 (frutescens)
Pereira-Silva, G. & G.A. Moreira 12751 (annuum var. glabriusculum)
Pereira-Silva, G. et al. 2100, 4678 (rabenii); 5761 (frutescens); 7216 (rabenii); 8481 (caatingae); 10880, 11175, 11207 (flexuosum); 13175 (rabenii)
Pérez, A.J. et al. 3833 (chinense); 5386 (lycianthoides)
Pérez, K. & D. Tea 18 (chacoense)
Perez Arbeláez, E. & J. Cuatrecasas 6511 (annuum var. glabriusculum); 8297 (rhomboideum); 8305 (annuum var. glabriusculum)
Pérez Arbeláez, E. 443 (annuum var. glabriusculum); 2085 (rhomboideum); 2491 (annuum var. glabriusculum); 3119 (rhomboideum)
Pérez C., E. & E. Carranza 2888 (rhomboideum)
Pérez Cruz, G. 245, 250 (rhomboideum)
Pérez-García, E. & B. Reyes Días 920 (annuum var. glabriusculum)
Pérez J., A. 521, 766 (annuum var. glabriusculum)
Pérez Z., J.A. et al. 1661 (rhomboideum)
Perrottet, S. 218 (frutescens)
Pesha Baca, V. 95 (chinense)
Pessoa, M.C. & J.R. Lima 323 (parvifolium)
Pessôa, S. de V.A. et al. 127 (schottianum)
Petersen, E. & J.P. Hjerting 947 (chacoense)
Petersen, P.M. 6454 (annuum var. glabriusculum)
Peterson, P.M. & C.R. Annable 7043 (annuum var. glabriusculum)
Pfeifer, H.W. 1283 (annuum var. glabriusculum)
Philipson, W.R. et al. 1456 (annuum var. glabriusculum)
Phillips, O. & P. Núñez 163 (coccineum)
Piccinini, B.G. & J. Hilfer 4123, 4293 (chacoense)
Piccinini, B.G. & C.A. Petetin 2961, 2980 (chacoense)
Pickel, B.J. 2492, 3892 (parvifolium); 3964 (chinense)
Pickersgill, B. Cb 4 (annuum var. glabriusculum); 401 (tovarii); 462 (recurvatum); 463 (cornutum); 464, 464-3 (villosum); RU72-99 (chinense); RU72-118, RU72-140 (frutescens); RU72-175 (chinense); RU72-244, RU72-357 (frutescens); RU72–366 (parvifolium)
Pifano, D.S. & A.S.M. Valente 266 (schottianum)
Pimentel, J. & R. García 390 (annuum var. glabriusculum)
Pinell R., N. 27, 61 (rhomboideum)
Pineschi, R.B. 42 (schottianum)
Pinheiro, M. 253 (longidentatum)
Pinkley, H.V. 230, 256, 257, 258, 259 (chinense); 359 (pubescens); 544 (chinense)
Pinto, D. et al. 48 (frutescens)
Pinto, G.C.P. & H.P. Bautista 103, 104 (caatingae)
Pinto, L.J.S. et al. 92 (cornutum)
Pinto, P. & M. Dumont 563 (annuum var. glabriusculum)
Pinto E., P. et al. 6185 (annuum var. annuum)
Piper, C.V. 5648 (annuum var. glabriusculum)
Pipoly, J.J. 1602 (annuum var. annuum)
Pirani, J.R. & R. García 3105 (recurvatum)
Pire, E.F. 520 (chacoense)
Pires, M.J. 392, 433 (rabenii)
Pirondo, A. et al. 160 (baccatum var. pendulum)
Pittier, H. 105 (rhomboideum); 614 (annuum var. glabriusculum); 737 (dimorphum); 872 (lycianthoides); 1372, 3810, 4299 (annuum var. glabriusculum); 7124 (rhomboideum); 7909 (frutescens); 9623, 10399, 11542 (rhomboideum); 11561 (annuum var. glabriusculum); 11905 (rhomboideum); 12199 (frutescens); 12585 (rhomboideum); 12707 (pubescens); 12709 (annuum var. annuum); 12837 (annuum var. glabriusculum); 13132, 13366 (rhomboideum); 13543 (annuum var. glabriusculum); 14032 (parvifolium); 14710 (annuum var. glabriusculum)
Pivetta 938 (flexuosum)
Plaumann, F. 229 (flexuosum)
Plotkin, M.J. 139 (chinense)
Plowman, T.C. 2457, 2458 (chinense); 3205 (frutescens); 3223 (annuum var. glabriusculum); 5439 (hookerianum); 5566 (rhomboideum); 5821 (coccineum); 5822 (chinense); 6048 (annuum var. glabriusculum); 11896 (annuum var. annuum); 14539 (frutescens)
Plowman, T.C. & P.W. Alcorn 14335 (hookerianum)
Plowman, T. & E.W. Davis 4164 (annuum var. glabriusculum); 4572 (rhomboideum); 4788 (baccatum var. baccatum); 4809 (pubescens); 7407 (baccatum var. baccatum)
Plowman, T. & J. Schunke V. 11497 (chinense); 11523 (coccineum); 11694 (frutescens); 11695 (chinense)
Plowman, T. & D. Vaughan 5366-A (annuum var. glabriusculum); 5370 (annuum var. annuum)
Plowman, T. et al. 4054, 4055 (chinense); 6391 (annuum var. annuum); 6418 (frutescens); 6419, 6599 (annuum var. glabriusculum); 6902 (chinense); 7075 (annuum var. glabriusculum); 7077, 7138, 8812 (chinense)
Poeppig, E.F. 1799, 1845 (coccineum); 2220 (chinense)
Pohl, J.B. 3674 (villosum); 5173 (rabenii)
Poortman, H.A.-C. 520 (geminifolium)
Poppleton, J.E. 933 (annuum var. glabriusculum)
Portal, E. et al. 108 (neei); 495 (baccatum var. baccatum)
Porter, D.M. 1299 (annuum var. glabriusculum)
Posada, A. 2581 (annuum var. annuum); 2603, 2606 (frutescens)
Pott, A. et al. 5562 (frutescens)
Poveda, K. et al. 19 (dimorphum)
Pozner, R. 207 (chacoense)
Prado, D. 10 (chacoense)
Prance et al., G.T. 9295 (annuum var. glabriusculum); 25919 (recurvatum); 26505 (annuum var. glabriusculum)
Premauer, J. & M.I. Moreno 57 (rhomboideum)
Prieto, F. 21 (chinense)
Pringle, C.G. 1912 (annuum var. glabriusculum); 2544 (rhomboideum); 2706 (annuum var. glabriusculum); 4057, 6505, 15620 (rhomboideum)
Proctor, G.R. 7507, 44888 (annuum var. glabriusculum)
Profice, J.R. et al. 22 (campylopodium)
Puch, A. 1089 (annuum var. glabriusculum)
Puiggari, G.L.T. 3577 (recurvatum)
Pujupet, J.O. RBAE 1005 (chinense)
Pulido, M.T. 511 (pubescens)
Purkayastha & Singh, DRLT 12 (chinense)
Purpus, C.A. 124 (rhomboideum); 2015 (annuum var. glabriusculum); 2294, 2709, 3362, 3993, 4893, 5051 (rhomboideum); 6976 (lanceolatum); 7562, 8424 (rhomboideum); 8499 (annuum var. glabriusculum)
Purseglove, J.W. 6314 (annuum var. glabriusculum)
Queiroz, A.S. & F. França 34 (caatingae)
Queiroz, A.S. et al. 55 (caatingae)
Quevedo, F.L. et al. 1816 (chinense)
Quinet, A. et al. 231 (villosum)
Quintana, C. & S. Valencia 441, 520 ½, 669 (rhomboideum)
Quintana, C. et al. 3030 (annuum var. glabriusculum); 843 (rhomboideum)
Quipuscoa S., V. & L. Bardales 979 (pubescens)
Quipuscoa S., V. et al. 4374 (longifolium)
Raben, F.C. 7 (baccatum var. baccatum); 301 (rabenii)
Ragonese, A.E. 2227 (chacoense)
Ragonese, A.E. & J.A. Castiglioni 7991, 8036, 8039 (chacoense)
Ramalho, F.B. 132 (caatingae)
Ramírez A., S. 56 (annuum var. annuum) Ramírez A., S. 56 (chinense)
Ramírez Delgadillo, R. & L. Portillo M. 1497 (rhomboideum)
Ramírez P., B.R. 295, 1399, 1905, 2174, 5891, 7476 (rhomboideum)
Ramírez P., B.R. 5639 (pubescens)
Ramírez, F. & M. García 526 (annuum var. glabriusculum)
Ramírez, N. 1067 (rhomboideum); 2548 (parvifolium)
Ramírez, N. & M. López 3293 & 3294 (rhomboideum)
Ramos, H.J. 164 (frutescens)
Ramos, J.E. 418 (rhomboideum); 440 (annuum var. glabriusculum); 476 (rhomboideum); 1794 (lycianthoides)
Ramos, J.E. & N. Paz 915 (annuum var. glabriusculum)
Ramos, J.E. & P. Silverstone-Sopkin 846 (annuum var. glabriusculum)
Ramos, J.E. et al. 6826, 6909, 7172 (geminifolium); 7438 (rhomboideum)
Ramos Núñez, G. et al. 18V.C.031 (annuum var. glabriusculum)
Rangel Ch., O. et al. 5336, 5511, 5539 (dimorphum)
Reales, A. 65 (chacoense)
Reed, H.R. 432 (annuum var. glabriusculum)
Regnell, A.F. Ser. III, 1001 (flexuosum); ser. III, 1002 (rabenii)
Reiche, K. 908 (annuum var. glabriusculum)
Reina, A.L. et al. 537, 1012, 1123 (annuum var. glabriusculum)
Reitz, R. C 924 (recurvatum); 2776, 4543 (flexuosum); 4749 (rabenii); 4750 (annuum var. annuum); 5484 (flexuosum)
Reitz, R. & R.M. Klein 3760 (rabenii); 5766, 5943 (recurvatum); 7542, 11724, 12487, 14102, 16945 (flexuosum); 17345 (recurvatum)
Reko, B.P. 41 (pubescens)
Renjifo, L.M. 253 (dimorphum)
Rennó, L. 3027 (rabenii)
Renolfi, R. 137, 422 (chacoense)
Rente, J.A. 31 (campylopodium)
Rentería A., E. et al. 601 (dimorphum)
Repizzo, A. & Z. Calle 217 (dimorphum)
Reyes, D. & L. Carrillo 502, 773 (chinense); 1048 (annuum var. annuum); 1049 (frutescens)
Reyes, D. & G. Moya 234 (chinense)
Reyes, J. & L.E. Arévalo 32 (annuum var. glabriusculum)
Reyes, S.J. 1782, 1782a, 309, 972 (rhomboideum)
Reyes García, A. 237 (annuum var. glabriusculum)
Reyes-García, A. & C. Chavarria 5066 (rhomboideum)
Reyes García, A. & R. Hampshire 1933 (rhomboideum); 1936 (annuum var. glabriusculum)
Reyes García, A. & G. Urquijo 753 (rhomboideum); 833 (annuum var. glabriusculum); 981 (annuum var. glabriusculum); 982, 1237 (rhomboideum)
Reyes García, A. et al. 1828, 1844 (rhomboideum); 5319, 5867 (annuum var. glabriusculum)
Reyes Santiago, J. & F. Reyes S. 594 (rhomboideum)
Reynel, C. et al. 5235 (chinense)
Rezende, M.G. et al. 100 (pereirae)
Ribas, O.S. et al. 4624 (villosum); 6004, 7150 (recurvatum)
Ribeiro, R. 563 (schottianum)
Richard 528 (campylopodium)
Richey, L.R. 98-355, 99-423 (annuum var. glabriusculum)
Ricinel 48 (baccatum var. baccatum)
Ricksecker, A.E. 232 (annuum var. glabriusculum)
Rico-Gray, V. & C. Chan 429 (annuum var. glabriusculum)
Ridoutt, C.A. 11714 (annuum var. annuum)
Riedel, L. 787 (baccatum var. baccatum); 1073 (flexuosum); 1074 (baccatum var. baccatum); 1080 (schottianum)
Riedlé, A. 1798 (annuum var. glabriusculum)
Rimbach, A. 170, 391 (rhomboideum)
Ríos, M. & A. Oña 429, 430 (chinense)
Rios, M. & F. Ghia 131 (annuum var. annuum)
Rios, M. & E. Vivanco 383 (annuum var. annuum)
Ritter, N. 989 (pubescens)
Rivas, V.M. & E. Guanín A. 113 (geminifolium)
Rivera C., J. L-166 (rhomboideum)
Rivera Díaz, O. 102 (rhomboideum)
Rivera Díaz, O. et al. 4217, 4270 (dimorphum)
Rivera H., J. et al. 371 (annuum var. glabriusculum)
Rivero, E. 67 (chinense); 218 (baccatum var. baccatum); 428 (chinense)
Robayo, C.X. 1 (annuum var. annuum)
Robles G., R. 886 (annuum var. glabriusculum)
Robleto, W. 902, 907 (annuum var. annuum)
Roca, A. 0689 (baccatum var. pendulum)
Rodrigo, A.P. 2637 (chacoense)
Rodrigues et al. 12335 (frutescens)
Rodrigues, C. et al. 03 (baccatum var. pendulum)
Rodrigues, F.M. & J.G. Oliveira 302 (baccatum var. baccatum)
Rodrigues, I.M.C. & M.M. Vieira 446, 447 (pereirae)
Rodrigues, I.M.C. et al. 473 (campylopodium); 502 (mirabile); 570 (annuum var. glabriusculum)
Rodrigues, S.T. & A.V. Carvalho 483, 490 (baccatum var. umbilicatum)
Rodrigues Vidal, R. 03 (baccatum var. pendulum)
Rodríguez, A. & L.D. Vargas 2524 (annuum var. glabriusculum)
Rodríguez, F. 2, 48, 49, 53, 59, 68, 71 (frutescens); 79 (chinense); 82, 112, 118, 120 (frutescens); 164 (baccatum var. baccatum); 165 (annuum var. annuum); 505 (frutescens); 1196 (chacoense)
Rodríguez, I. 104 (annuum var. glabriusculum)
Rodríguez, I. & R. Lira 63, 63a & 63b (annuum var. annuum)
Rodríguez, L. 461 (annuum var. glabriusculum)
Rodríguez, M. 168 (baccatum var. baccatum)
Rodríguez, M.E. & R.D. Aranda 171 (flexuosum)
Rodríguez, W. et al. 589 (dimorphum)
Rodríguez-M, G.M. & L. Olivares 272 (annuum var. glabriusculum)
Rodríguez R., E. 1928 (geminifolium)
Rodríguez R., E. & J. Campos de la Cruz 1811 (geminifolium)
Rodríguez R., E. & R. Cruz A. 2046 (geminifolium)
Rodschied, E.K. 29 (annuum var. annuum); 30 (frutescens)
Roe, K.E. 1331 (annuum var. glabriusculum)
Roe, K.E. & E. Roe 2223 (rhomboideum)
Roe, K.E. et al. 789 (rhomboideum)
Roic, L.D. 492 (chacoense)
Rojas, N. et al. 13 (annuum var. glabriusculum)
Rojas, R. et al. 886 (annuum var. glabriusculum); 1642 (geminifolium); 1649, 1744 (dimorphum);
Rojas, T. 2434 (chacoense); 2435 a, 2436, 2436 A (baccatum var. baccatum); 2440, 2605 (chacoense); 3356, 3363 (flexuosum); 3368 (baccatum var. baccatum); 6019 (flexuosum); 9529, 12452 (baccatum var. baccatum); 13815, 14325 (chacoense); 14415 (flexuosum)
Rojas G., R.D. 745 (annuum var. glabriusculum)
Roldán, F.J. & C. Cuartas 1853 (annuum var. glabriusculum)
Roldán, F.J. et al. 1626 (rhomboideum); 2332, 2350 (dimorphum)
Rombouts, H. 735 (annuum var. glabriusculum)
Romero Castañeda, R. 2101 (annuum var. glabriusculum); 2250 (rhomboideum); 2364 (annuum var. glabriusculum); 3797 (chinense); 6674 (pubescens); 9255, 10067 (annuum var. glabriusculum)
Romero, E. 64 (annuum var. glabriusculum)
Romero, G. et al. 1722 (annuum var. glabriusculum)
Romero, M.V. 1, 2, 3 (baccatum var. baccatum), 9 (chinense)
Roponen, S. & Å. Johannessen 85, 229 (lycianthoides)
Roque, A.A. 885, 1061 (parvifolium)
Rosa, E. 1697 (chacoense)
Rosa, M. 75 (schottianum); 107 (campylopodium); 111 (campylopodium); 118 (schottianum)
Rosa, S. 366 (baccatum var. baccatum)
Rosado, N. 01 (chinense)
Rosales, J.M. 1044 (rhomboideum)
Rosas R., M. 1320 (annuum var. glabriusculum)
Rose, J.N. 21950 (annuum var. glabriusculum)
Rose, J.N. & G. Rose 22258 (geminifolium); 23566 (rhomboideum)
Rosenthal, J.P. 101, 102, 104, 109 (annuum var. glabriusculum)
Rossi, L. & E.L.M. Catharino 1605 (schottianum)
Rossi, L. et al. 1474 (recurvatum); 2037 (cornutum)
Roybal, J.J. 539 (annuum var. glabriusculum)
Rubio, D. & G.A. Tipaz 2361 (hookerianum)
Rubio, H. 328 (annuum var. glabriusculum); 949, 1037, 2026 (rhomboideum)
Rueda, R.M. 12112 (rhomboideum)
Ruenes, R.M. 22 (annuum var. glabriusculum)
Rugama, R.A. 19 (rhomboideum)
Rugel, F. 87 (annuum var. glabriusculum)
Ruiz de Huidobro, M. 125 (chacoense)
Ruiz Núñez, N. 6, 7 (annuum var. glabriusculum)
Ruiz Terán, L.E. et al. 12210 (rhomboideum)
Ruiz, D. 185 (rhomboideum)
Ruiz, M. et al. 139 (dimorphum)
Ruiz, R. & E. Correa 929 (dimorphum)
Runyon, R. 285 (annuum var. glabriusculum); 948 (rhomboideum); 5449 (annuum var. glabriusculum)
Rusby, H.H. 680 (minutiflorum)
Rusby, H.H. & F.W. Pennell 503 (annuum var. glabriusculum); 111 (rhomboideum)
Russel, Padre A. 6140 (baccatum var. pendulum)
Rutten-Peckel Harig, C.J. 4 (rhomboideum)
Rzedowski, J. 6849, 7663, 10641, 10668 (rhomboideum); 16094 (annuum var. glabriusculum); 24373, 24631, 32523, 49555 (rhomboideum)
Saer, J. 109, 627 (rhomboideum)
Sagástegui A., A. 5846, 13036, 15559, 15612 (rhomboideum)
Sagastegui A., A. & S. Leiva González 12515 (rhomboideum)
Sagástegui A., A. et al. 12502 (rhomboideum); 12998 (geminifolium)
Saint Hilaire, A. 764 (rabenii)
Salas A., A. et al. 90 (annuum var. glabriusculum)
Salas M., S.H. et al. 185, 4061 (annuum var. glabriusculum)
Salaün 177 (frutescens); 185 (annuum var. glabriusculum)
Salazar et al., G. 8074 (annuum var. glabriusculum)
Saldías P., M. 110 (coccineum); 563, 606 (pubescens); 759 (annuum var. annuum); 760 (baccatum var. pendulum); 4977 (caballeroi)
Sales, M.F. et al. 604 (caatingae)
Salinas, C. & S. Benítez 240 (annuum var. annuum)
Salinas, N. et al. 7249 (geminifolium)
Salinas T., A. & E. Martínez-Correa 7357 (rhomboideum)
Salino, A. 3850 (campylopodium)
Sampaio, A. et al. 2363 (schottianum)
Sánchez, C. 981 (dimorphum)
Sánchez, D. et al. 125, 1457, 3176 (dimorphum)
Sánchez, F. et al. 954 (annuum var. glabriusculum)
Sánchez, H. et al. 579 (annuum var. glabriusculum)
Sánchez, M.E. 002, 7 (frutescens)
Sánchez, O. & C. Aguirre 358, 373 (rhomboideum)
Sánchez, R. & E. Linares 1479 (dimorphum)
Sánchez, R. et al. 1323 (dimorphum)
Sánchez C., J. 12 (annuum var. glabriusculum)
Sánchez L., F. 317 (annuum var. glabriusculum)
Sánchez L., F. & P. Trujillo V. 874 (annuum var. glabriusculum)
Sánchez L., F. et al. 258 (annuum var. glabriusculum); 953 (frutescens); 1190 (annuum var. glabriusculum)
Sánchez León, V.M. 1139 (pubescens)
Sánchez Martínez, A. & A. Nava 230, 247, 548 (annuum var. glabriusculum)
Sánchez Martínez, A. et al. 983 (annuum var. glabriusculum); 5138 (rhomboideum); 12745 (frutescens)
Sandeman, C. 2038 (cornutum)
Sanders, A.C. et al. 17496 (annuum var. glabriusculum)
Sandino, J.C. 253, 306 (annuum var. glabriusculum); 2975 (annuum var. annuum)
Sandoval, E.A. 308, 1743 (rhomboideum)
Sandoval, E.A. et al. 550 (rhomboideum)
Sanín, D. 1048 (dimorphum); 6236 (regale)
Santa et al., J. 155 (baccatum var. baccatum)
Santa María, E. 733 (annuum var. glabriusculum)
Santacruz, P. 20 (frutescens)
Santín, F. et al. 100 (annuum var. glabriusculum); 102 (chinense)
Santos, A.A. et al. 2663, 2829 (flexuosum)
Santos, E. et al. 2869 (flexuosum)
Santos, U.S. et al. 5 (caatingae)
Santos, L.A.S. 1237 (parvifolium)
Santos, L.B. 160 (rabenii)
Santos, R.M. et al. 1574 (caatingae)
Saravia, C. et al. 11572 (baccatum var. baccatum)
Saravia Toledo, C. 103, 828, 1197 (chacoense); 1404 (annuum var. glabriusculum); 1421, 4641 (rhomboideum); 10325 (eximium); 10706 (chacoense); 11607 (baccatum var. baccatum); 12126, 12245 (eximium); 12812, 13472, 13484, 13490 (chacoense)
Saravia Toledo, C. et al. 11372, 11392 (baccatum var. baccatum); 11495, 11496, 11544 (chacoense)
Saravia Toledo, C. & R. Jaramillo M. 1978 (annuum var. glabriusculum); 1806 (rhomboideum)
Saravia Toledo, C. & J. Nelson 10343, 10815 (chacoense)
Sargen, F.H. B69 (frutescens)
Särkinen, T. et al. 4805, 4813 (longifolium)
Sarma, J. & G. Dutta 394 (annuum var. annuum)
Sarukhán, J. et al. 3581 (annuum var. glabriusculum)
Sastre, C. 3298 (frutescens)
Sastre, C. et al. 2708 (annuum var. glabriusculum)
Sauleda, R.P. & D.S. Correll 2416 (annuum var. glabriusculum)
Saunes V., A. et al. 5641, 5676 (annuum var. glabriusculum)
Sawyer, N.W. 720 (geminifolium)
Sawyer, N.W. & J. Burneo 694 (geminifolium)
Sayago, M. B-113 (chacoense)
Saynes Vásquez, A. 657 (rhomboideum)
Saynes Vásquez, A. et al. 3263, 5601 (annuum var. glabriusculum)
Scaldaferro, M. 9 (annuum var. glabriusculum); 13 (frutescens); 19, 20 (baccatum var. pendulum); 22, 23, 24, 27, 28, 29, 32, 33 (chacoense); 34 (annuum var. annuum); 36 (chacoense); 37, 38, 39, 40 (baccatum var. pendulum); 41 (annuum var. glabriusculum); 42, 43 (baccatum var. pendulum); 44 (chacoense); 45, 49 (baccatum var. pendulum); 50 (baccatum var. baccatum); 57, 63 (baccatum var. umbilicatum); 65 (chacoense); 73 (rhomboideum)
Scalon, V.R. et al. 628 (mirabile)
Scariot, A.O. et al. 340 (rabenii); 381 (mirabile)
Scarpa, G.F. 649 (chacoense)
Schenkel, E.P. et al. 447 (baccatum var. baccatum)
Schickendantz, F. 74, 158, 187, 211, 299 (chacoense)
Schinini, A. 4140 (baccatum var. baccatum); 4248 (chacoense); 4901 (flexuosum); 13279 (annuum var. annuum); 14061 (baccatum var. baccatum); 19988 (chacoense); 23074 (flexuosum); 34466 (baccatum var. baccatum); 35123 (baccatum var. umbilicatum); 36578 (baccatum var. baccatum); 36777 (flexuosum)
Schinini, A. & E. Bordas 14930, 15041, 16524 (chacoense); 20626 (baccatum var. baccatum); 24511 (frutescens)
Schinini, A. & G. Caballero Marmori 26990 (flexuosum)
Schinini, A. & M. Dematteis 33242 (flexuosum); 33747 (baccatum var. baccatum)
Schinini, A. & A. Fernández 5952, 5967, 6058 (flexuosum)
Schinini, A. & R. Palacios 25565 (baccatum var. baccatum); 25726 (chacoense)
Schinini, A. & S.N. Pire 24216 (chacoense)
Schinini, A. & C.L. Quarín 11524 (chacoense); 11542 (baccatum var. baccatum)
Schinini, A. & M. Urbani 35782 (chacoense)
Schinini, A. et al. 11282, 17024 (baccatum var. baccatum); 17088 (chacoense); 27787 (rabenii); 28711, 28756 (flexuosum); 34778 (eximium); 35295 (chacoense)
Schipp, W.A. 1076 (annuum var. glabriusculum)
Schmalzel, R.J. 1211 (annuum var. glabriusculum)
Schmeda, C. 167, 863, 1301 (chacoense); 1584 (baccatum var. baccatum)
Schnee, L. 1183 (annuum var. glabriusculum)
Schneider, M. 216 (rhomboideum); 893 (geminifolium); 912 (rhomboideum);
Schott, H.W. 5409 (campylopodium); 5416 (muticum); 5425, 5426 (schottianum)
Schreiter, R. 1927, 3791, 10101 (chacoense)
Schultes, E.E. 3794 (frutescens)
Schultes, E.E. & Cabrera 13046 (frutescens)
Schultes, R.E. 3269 (pubescens); 3497, 5518, 6620 (chinense)
Schultes, R.E. & I. Cabrera 13138 (chinense); 13752 (frutescens)
Schultes, R.E. & M. Villareal 7598, 7958 (pubescens); 7863 (rhomboideum)
Schulz, A.F. 7155, 7204 (flexuosum)
Schulz, A.G. 1, 2, 87, 90 (chacoense); 2028 (baccatum var. baccatum); 2030, 2032 (chacoense); 2050, 2103 (baccatum var. baccatum); 3814, 4272, 5222 (chacoense); 6714 (baccatum var. baccatum); 6719 (chacoense); 7155 (baccatum var. baccatum); 7361, 7363 (chacoense); 8049 (baccatum var. baccatum); 8178, 8284, 8930, 9032, 9064, 10649, 15632, 15801 (chacoense); 15929 (baccatum var. baccatum); 17367, 17721, 17958, 18190 (chacoense)
Schulz, C.L. 845, 932, 1082, 1141, 1180 (chacoense)
Schunck 9711 (chacoense)
Schunke V., J. 821 (coccineum); 1397 (frutescens); 3415, 5199, 6442, 6554, 9984, 10620, 10777, 12469 (coccineum); 14492 (annuum var. annuum); 14518 (chinense)
Schuncke V., J. & J.G. Graham 15004, 16612 (coccineum)
Schwabe, H. 650 (chacoense)
Schwarz, G.J. 2875, 2938, 2939, 2986, 3414, 4023, 4160, 4389, 4458, 4690, 5763, 5991, 6900, 7037, 7038, 7589, 7755, 7882, 11868 (flexuosum)
Schwindt, E. 305, 947 (flexuosum); 996, 1362 (chacoense); 1548 (baccatum var. baccatum); 1871, 1911, 1948, 1966, 4217, 4310, 4581, 4790 (flexuosum)
Schwirkowski, P. 139 (recurvatum); 885 (rabenii); 1425, 1433, 1470 (recurvatum); 1519 (flexuosum); 2534 (recurvatum); 2713 (rabenii);
Scolnik, R. 821, 822 (pubescens); 1389 (geminifolium); 1594 (rhomboideum)
Scolnik, R. & J. Correa V. 458 (annuum var. glabriusculum)
Scolnik, R. & R. Luti 526 (pubescens); 605 (baccatum var. pendulum); 637 (annuum var. annuum); 672 (frutescens); 703 (annuum var. annuum)
Scolnik, R. et al. 19An322 (rhomboideum); 19An329, 19S022 (annuum var. glabriusculum)
Scott de Birabén, M.I. & M. Birabén 1143 (chacoense)
Sehnem, A. 3824, 3828, 3927 (flexuosum)
Seidel, R. & S. Beck 59, 361 (baccatum var. baccatum)
Seidel, R. & I. Hinojosa 1267 (ceratocalyx)
Seidel, R. & D. Vaquiata 7689 (coccineum)
Seigler, D. et al. 13144, 13353 (rhomboideum)
Seijo, G.J. 1833 (chacoense)
Seijo, J.G. et al. 3351, 3356 (chacoense); 3448, 3580, 3812 (baccatum var. baccatum); 3820, 3864, 3869 (chacoense)
Seler, E. 253, 610 (annuum var. glabriusculum)
Sellow, F. 6 (campylopodium); 79 (muticum); 209 (mirabile); 221 (baccatum var. baccatum)
Serberg, O. 1753 (frutescens)
Serralta P., L. 104 (annuum var. glabriusculum)
Serrano, M. 1566, 1903 (baccatum var. baccatum)
Serrano, M. et al. 5482 (neei); 6882 (caballeroi); 7297 (baccatum var. baccatum)
Sessé, M. & J.M. Mociño 1508 (rhomboideum); 1546 (annuum var. glabriusculum)
Sevilha, A.C. & A.O. Scariot 1945, 2137 (rabenii)
Shafer, J.A. 45, 169, 330, 1458, 2361 (annuum var. glabriusculum)
Shannon, W.C. 206 (annuum var. glabriusculum)
Shonle et al., I. 23 (annuum var. glabriusculum)
Sidwell, K.J. et al. 695 (rhomboideum)
Siles G., E.L. 30, 58, 68 (rhomboideum)
Silva, A.B. 1402 (caatingae)
Silva, A.C.C. 244, 259, 287 (caatingae)
Silva, A.F.B. 139 (parvifolium)
Silva, E.L. et al. 1 (caatingae); 40 (recurvatum)
Silva, F. et al. 19An404 (pubescens)
Silva, H.S.J. & M. Hernández 343 (rhomboideum)
Silva, J.M. & L.M. Abe 4553 (recurvatum)
Silva, J.M. & J. Cordeiro 6187 (recurvatum)
Silva, J.M. et al. 2264 (recurvatum); 3559 (flexuosum); 4218 (recurvatum)
Silva, J.S. 272 (recurvatum)
Silva, R.A. 1506, 1793 (caatingae)
Silva, R.A. & D. Moura 1586, 1632 (caatingae)
Silva, S.M. & R.M. Britez 1714 (flexuosum)
Silva Neto, S.J. et al. 736, 1795 (villosum)
Silva-Santos, A. et al. 1990 (flexuosum)
Silveira, M. 1080 (coccineum)
Silveira, M. et al. 551, 650 (coccineum)
Silverstone-Sopkin, P.A. 556 (lycianthoides); 1451, 3689, 6108 (rhomboideum)
Silverstone-Sopkin, P. & N. Paz 6614 (annuum var. glabriusculum)
Silverstone-Sopkin, P.A. et al. 2322 (rhomboideum); 5884 (annuum var. glabriusculum); 6641, 6643 (dimorphum); 9069, 9249, 9377, 9790, 10070 (lycianthoides)
Simá, P. 517 (annuum var. glabriusculum); 606, 610 (annuum var. annuum)
Simá, P. et al. 1552 (annuum var. glabriusculum)
Simonis, J.E. et al. 207 (baccatum var. baccatum); 216 (rabenii)
Simpson, D.R. & J. Schunke V. 469 (hookerianum)
Simpson, J.H. 86 (annuum var. glabriusculum)
Singleton W.R. 611 (annuum var. glabriusculum)
Sintenis, P. 461 (annuum var. glabriusculum); 865 (annuum var. annuum); 866 b (chinense); 3685 (annuum var. glabriusculum)
Siquihua, L. 4 (frutescens)
Skorupa, L.A. 330 (baccatum var. pendulum)
Skorupa, L.A. & J.N. da Silveira 328 (frutescens); 329, 378 (chinense)
Skutch, A.F. 1450 (lanceolatum); 1936 (rhomboideum)
Slanis, A.C. 72 (eximium)
Slanis, A.C. et al. 116, 194 (chacoense)
Sleumer, H. & F. Vervoorst 2349 (chacoense)
Small, J.K. 2121, 2772, 3867, 4604, 7468, 7686, 8740 (annuum var. glabriusculum)
Small, J.K. & J.J. Carter 793, 2673, 2676, 8553 (annuum var. glabriusculum)
Small, J.K. & C.A. Mosier 5695 (annuum var. glabriusculum)
Small, J.K. & E.W. Small 4864 (annuum var. glabriusculum)
Small, J.K. & G.K. Small 4983 (annuum var. glabriusculum)
Small, J.K. & P. Wilson 1773, 1892 (annuum var. glabriusculum)
Smith, D.N. et al. 13470 (caballeroi)
Smith, H.H. 1164, 1169 (baccatum var. baccatum); 1176, 1183 (parvifolium); 1479 (baccatum var. baccatum)
Smith, J.D. 424 (annuum var. glabriusculum)
Smith, L.B. 7974 (recurvatum)
Smith, L.B. & R.M. Klein 9108, 9968, 13238 (flexuosum)
Smith, L.B. & R. Reitz 8603, 8833 (flexuosum)
Smith, L.B. et al. 11764 (flexuosum)
Smith, L.C. 659 (rhomboideum)
Smith, P.G. SA 272, SA 276 (eximium); SA 281 (eshbaughii); SA 325, SA 351 (eximium); SA 374 (frutescens); SA 393 (coccineum); 406 (cardenasii); Ac 689 (chacoense); Ac. 771 (baccatum var. pendulum); Ac 1256 (chacoense); Ac. 1425 (pubescens); 1519 (baccatum var. pendulum); Ac 2017 (tovarii); PS 350 (eximium);
Smith, P.S. 149 (pubescens)
Smith, S.D. 203 (lycianthoides)
Smith, S.G. 1133 (rhomboideum)
Smith AC, P. 1905 (chinense)
Smith V., R.F. 8617 (rhomboideum); 9349 (parvifolium)
Sneidern, K. 4528 (annuum var. glabriusculum)
Soares, E.L.C. 83, 84 (flexuosum)
Sobral, M. 2989 (flexuosum)
Sobral, M. & I.S. Almeida 7926 (flexuosum)
Sobral, M. & Ganev 7646 (caatingae)
Sobral, M. & J.A. Jarenkow 8021 (flexuosum)
Sobral, M. et al. 961, 5161, 1321, 2640, 3003, 3103 (flexuosum); 13870 (rabenii)
Sodiro, L. 114/82, 114/84 (geminifolium)
Soejarto, D.D. & H. Cardozo 813 (chinense)
Soejarto, D.D. & E. Hernández 1393 (rhomboideum)
Soejarto, D.D. et al. 2539 (frutescens)
Sohn, S. 98 (recurvatum)
Sohns, E.R. 1203, 1384 (annuum var. glabriusculum)
Sol S., A. 134 (frutescens)
Solange, P. 8 (chinense)
Solbrig, O. & G.F. Pabst 6884 (recurvatum)
Solheim, S.L. 1654 (rhomboideum)
Solomon, J.C. 2417 (annuum var. glabriusculum); 5304 (cardenasii); 5305 (pubescens); 5306 (annuum var. annuum); 8844, 12086 (ceratocalyx); 12991, 13104 (pubescens); 14672 (chinense); 15508 (pubescens); 19186 (annuum var. glabriusculum)
Solomon, J.C. & M. Nee 18181 (pubescens)
Solomon, J.C. et al. 6778, 6966 (baccatum var. baccatum)
Solomón Maya, J. 1689, 1734 (rhomboideum); 2570 (lanceolatum); 3039, 3430 (rhomboideum); 3521 (lanceolatum); 3551 (rhomboideum); 3704, 3867 (lanceolatum)
Soria, N. 2901, 3890 (flexuosum); 3900 (chacoense)
Soriano, A. 900 (chacoense)
Soriano, A. & W. Barrett 3592 (chacoense)
Soto, A. et al. 1472 (rhomboideum)
Soto, C. et al. 4025 (annuum var. glabriusculum)
Soto, D. et al. 95 A (minutiflorum)
Soto, J.C. et al. 13288 (annuum var. glabriusculum)
Soto Núñez, J.C. 11189 (annuum var. glabriusculum); 14047, 14048, 14302 (annuum var. annuum); 14302 (annuum var. glabriusculum)
Soto Núñez, J.C. & S. Aureoles C. 7889 (annuum var. glabriusculum)
Soto Núñez, J.C. & G. Silva R. 3842 (chinense); 14103 (annuum var. annuum)
Soto Núñez, J.C. & F. Solórzano G. 12592 (pubescens)
Soto Núñez, J.C. & R. Torres C. 2764 (annuum var. glabriusculum)
Soto Núñez, J.C. et al. 5442, 5443 (annuum var. annuum); 6021 (annuum var. glabriusculum); 6369 (pubescens)
Soukup, J. 1851 (annuum var. glabriusculum)
Sousa, F.F.S. 17 (frutescens)
Souza, E.B. 3924 (parvifolium)
Souza, F.M. et al. 1073 (flexuosum)
Souza, G.R. et al. 1680 (baccatum var. baccatum)
Souza, H.O. et al. 9081 (pereirae)
Souza, J.P. et al. 30 (flexuosum); 60 (recurvatum); 793 (villosum); 970 (hunzikerianum); 11012, 11029 (parvifolium)
Souza, P.T. 21 (pubescens)
Souza, V.C. 2140 (rabenii); 11168 (mirabile); 40126 (rabenii)
Souza, V.C. & T.B. Flores 35055 (recurvatum)
Souza, V.C. & M.O. Pedraz 1116 (flexuosum)
Souza, V.C. et al. 18426 (annuum var. glabriusculum); 26687 (caatingae); 32883 (baccatum var. baccatum)
Souza Pedroza, D.S. et al. 658 (campylopodium)
Soza, D. et al. 141 (rhomboideum)
Sparre, B. & F. Vervoost 552 (rabenii)
Sparre, B. 14042, 14365, 14591 (lycianthoides); 14653 (rhomboideum); 16714, 16984 (lycianthoides); 19319 (geminifolium)
Spooner, D. et al. 5036 (geminifolium); 6657 (baccatum var. baccatum)
Spruce, R. 5050 (rhomboideum)
St. John, H. 20655 (dimorphum)
Ståhl, B. & S. Báez 7061 (lycianthoides)
Ståhl, B. & J.T. Knudsen 1065, 1090, 1224 (lycianthoides)
Standford, L.R. et al. 955, 2112 (annuum var. glabriusculum)
Standley, P.C. 9714, 9810, 10058 (rhomboideum); 14077 (lanceolatum); 14190 (rhomboideum); 19872, 20689, 20903, 21308 (annuum var. glabriusculum); 22237 (frutescens); 23453 (annuum var. glabriusculum); 23755 (frutescens); 24095 (rhomboideum); 24297, 25055, 25504 (frutescens); 25825, 25887 (annuum var. glabriusculum); 26247 (rhomboideum); 26254 (annuum var. glabriusculum); 26493 (frutescens); 26862 (annuum var. glabriusculum); 28523 (annuum var. annuum); 29336, 29640 (annuum var. glabriusculum); 29880 (annuum var. annuum); 30539 (annuum var. glabriusculum); 31479 (annuum var. annuum); 31543, 31921 (annuum var. glabriusculum); 32004 (frutescens); 36044 (annuum var. glabriusculum); 53392 (frutescens); 53628, 53913, 54485, 55021, 55721 (annuum var. glabriusculum); 59930 (rhomboideum); 60647 (annuum var. glabriusculum); 63153 (lanceolatum); 64208 (annuum var. glabriusculum); 64892 (rhomboideum); 68235, 68244, 68294 (lanceolatum); 74361, 78366 (annuum var. glabriusculum); 80793 (pubescens); 86930 (lanceolatum); 88022, 90936 (annuum var. glabriusculum); 91227 (pubescens); 91469 (lanceolatum)
Stearn, W.T. 588, 616 (annuum var. glabriusculum)
Steere, W.C. 1314 (annuum var. glabriusculum); 1579 (annuum var. annuum); 1813 (annuum var. glabriusculum); 7404 (frutescens)
Stehlé, H. 2951 (annuum var. glabriusculum)
Stehmann, J.R. & F.S. Faria 6269 (carassense)
Stehmann, J.R. & A. Ippolito 1694 (flexuosum)
Stehmann, J.R. et al. 1520 (flexuosum); 2710 (mirabile); 4813 (schottianum); 4830, 4831 (hunzikerianum); 5037 (mirabile); 6344, 6347 (carassense); 6467 (flexuosum); 6474 (mirum)
Steibel, P.E. & H.O. Troiani 3835 (chacoense)
Steinbach, J. 2044 (baccatum var. baccatum); 2625, 3038, 3475 (coccineum), 5373, 5484 (frutescens); 5550 (coccineum); 8288 (pubescens)
Stellfeld, C. 1677 (baccatum var. pendulum)
Stergios, B. & L. Delgado 13640 (annuum var. glabriusculum)
Stergios, B. et al. 13316 (frutescens)
Stern, W.L. 263, 1901 (annuum var. glabriusculum)
Stern, W.L. et al. 134 (rhomboideum); 798 (annuum var. glabriusculum)
Stevens, W.D. 2604, 2701, 2805, 2888, 3013, 3360, 3431, 3774, 3854, 4223, 4688, 9348, 13617 (annuum var. glabriusculum); 31295 (baccatum var. baccatum)
Stevens, W.D. & M. Araquistain 13242 (annuum var. annuum)
Stevens, W.D. & E. Duarte M. 29871 (rhomboideum)
Stevens, W.D. & O.M. Montiel 16042, 17325, 18532, 22220, 29552, 31749 (rhomboideum); 35819 (annuum var. glabriusculum)
Stevens, W.D. et al. 14899 (annuum var. glabriusculum); 20300 (frutescens); 27284, 30086 (rhomboideum)
Stevensen, J.A. 2464 (annuum var. glabriusculum)
Stewart, A. 3352 (galapagoense); 3354, 3355, 3356, 3357 (frutescens)
Stewart, R.M. 1522 (annuum var. glabriusculum)
Steyermark, J.A. 29933 (lanceolatum); 30089 (annuum var. glabriusculum); 33357, 33429 (lanceolatum); 34462 (annuum var. annuum); 35237, 35422 (lanceolatum); 36930 (pubescens); 38343 (annuum var. glabriusculum); 46797, 47328, 47911, 48958 (lanceolatum); 50903 (rhomboideum); 51277, 51364 (annuum var. glabriusculum); 52093 (lanceolatum); 52482 (geminifolium); 52690 (pubescens); 52914 (lycianthoides); 54146, 54149 (geminifolium); 56884, 62772, 62814 (rhomboideum); 76028, 76029 (chinense); 76030, 88191, 91845, 99428 (annuum var. glabriusculum); 108787, 111696 (rhomboideum); 123436 (annuum var. glabriusculum)
Steyermark, J. & G. Agostini 91269 (annuum var. glabriusculum)
Steyermark, J.A. & P. Berry 111788 (rhomboideum)
Steyermark, J. & G. Bunting 102309 (annuum var. glabriusculum)
Steyermark, J.A. & V. Carreño Espinoza 107636 (rhomboideum)
Steyermark, J.A. & P. Colvée 121161 (rhomboideum)
Steyermark, J.A. & B.J. Manara 107966 (rhomboideum)
Steyermark, J. et al. 108086, 108194 (parvifolium); 114061, 118828, 120128 (rhomboideum); 123045 (annuum var. glabriusculum);
Stimson, W.R. 3049, 3922 (annuum var. glabriusculum)
Stoffers, A.L. 1186 (annuum var. glabriusculum)
Stork, H.E. & O.B. Horton 9462, 9463 (chinense); 9852 (geminifolium)
Strong, M.T. & F. Strong 4071 (annuum var. glabriusculum)
Stuckert, T. 917, 1008, 1120, 1320, 2405, 1166, 4238, 4247, 5557, 5614, 6007, 9472, 9913, 11559, 12551, 13092, 13210, 13644, 14330, 15477 (chacoense); 19345 (baccatum var. baccatum); 19510, 20267, 21449, 22168 (chacoense)
Stutz, L.C. 364, 1348, 1720, 2095 (flexuosum)
Suárez M., A. & J. Jácome 32 (rhomboideum)
Subieta, M. 56 (chacoense)
Subils, R. 1437 (chacoense); 2064 (annuum var. annuum); 2107, 2335, 3876 (chacoense); 4569 (baccatum var. pendulum); 4673 (annuum var. annuum); 4680 (frutescens)
Subils, R. & L. Articó 6 (chacoense)
Subils, R. & G. Barboza 4611 (annuum var. annuum); 4612 (pubescens); 4645 (annuum var. glabriusculum)
Subils, R. & E. Moscone 4133 (flexuosum); 4142 (annuum var. annuum); 4154 (flexuosum); 4166 (annuum var. annuum); 4265 (baccatum var. pendulum); 4271 (flexuosum); 4273 (baccatum var. baccatum)
Subils, R. et al. 1229, 3564 (chacoense)
Sucre, D. 6114, 6191, 7387, 8156, 8238 (campylopodium); 8934 (frutescens)
Sucre, D. & P.I. Braga 6402 (campylopodium); 6511 (schottianum)
Sucre, D. & Pe. L. Krieger 6863 (pereirae)
Sucre, D. & T. Soderstron 8987 (rabenii)
Sucre, D. et al. 2907 (villosum)
Sugiyama, M. & S.A. Chiea 1094 (cornutum)
Sugiyama, M. et al. 230 (cornutum); 1293 (schottianum); 1399 (cornutum)
Sullivan, V.I. 1067 (annuum var. glabriusculum)
Svolenski, A.C. & G. Tiepolo 236 (flexuosum)
Tabosa, F.R.S. et al. 55 (longidentatum)
Tafur, V. 219 (chinense); 237 (rhomboideum)
Tamayo, F. 281, 2006 (rhomboideum)
Tamayo, T. 2618 (annuum var. glabriusculum)
Tavares, B.V. et al. 17, 23 (rabenii)
Taylor 9662 (annuum var. glabriusculum)
Taylor E., M. 108 (rhomboideum)
Taylor G., R.G. 9 (frutescens)
Taylor, K. 385 (annuum var. glabriusculum)
Taylor, N. 3 (annuum var. glabriusculum)
Taylor Jr., R.J. 7249 (annuum var. glabriusculum)
Tejero-Díez, D. 6174, 6190 (annuum var. glabriusculum)
Teles Walter, B.M. et al. 567, 572, 573, 576, 579 (chinense)
Téllex, O. 7154 (lanceolatum)
Téllez, O. & L. Rico 3442 (annuum var. glabriusculum)
Téllez V., O. et al. 3770, 8073 (annuum var. glabriusculum)
Tenorio L., P. 9414 (rhomboideum)
Tenorio L., P. & R. Hernández M. 327 (rhomboideum)
Tenorio L., P. & C. Romero de T. 2229, 2279 (rhomboideum); 2389, 6591 (annuum var. glabriusculum); 6631, 6821 (rhomboideum)
Tenorio L., P. & M. Sousa S. 19436 (annuum var. glabriusculum)
Tenorio L., P. et al. 4204 (rhomboideum); 8305, 10513 (annuum var. glabriusculum); 11156, 14756, 17209 (rhomboideum)
Tepe, E.J. & M.P. Moreno 3014 (rhomboideum)
Tepe, E. et al. 2318 (geminifolium)
Terrazas, J. 1, 2, 4, 5, 6 (pubescens)
Terribile, M. 407 (chacoense)
Tessmann, G. 3432 (recurvatum)
Tewksbury, J. 1007, 1009 (baccatum var. baccatum); 1020 (chacoense)
Tharp, B.C. 256 (annuum var. glabriusculum)
Tharp, B.C. & F.A. Barkley 13A136 (annuum var. glabriusculum)
Thode, V. 277, 298 (flexuosum)
Thomas, E. 291 (eximium); 705, 1092, 1093 (chinense)
Thomas, E. & R. Berdeja 1428 (coccineum)
Thomas, R.D. et al. 87043 (annuum var. glabriusculum)
Thomas, W.W. & S. Sant’Ana 12496 (pereirae)
Thomas, W.W. et al. 9080 (frutescens); 13660 (villosum)
Thompson, S.A. et al. 7626, 10651, 10775 (annuum var. glabriusculum)
Thorne, R.F. & E. Lathrop 40532 (annuum var. glabriusculum); 41699 (lanceolatum)
Timaná, M. 758, 818, 1007, 1930, 3670, 3673 (coccineum)
Timaná, M. & N. Jaramillo 2878, 3224 (coccineum)
Ticona, J.P. & F. Saravia May 10 (annuum var. annuum)
Tillett, S.S. & C.L. Tillett 45424, 45627 (chinense)
Tipaz, G.A. et al. 865 (hookerianum)
Tirado, M. et al. 1309 (lycianthoides)
Tolaba, J. 2843 (baccatum var. baccatum); 3918 (eximium)
Tolaba, J. et al. 1375, 4090 (chacoense)
Toledo, C.B. et al. 69 (flexuosum)
Ton, A.S. 125 (pubescens); 274, 275 (annuum var. annuum); 398 A (pubescens); 2744, 2956, 2996, 3158, 3261 (annuum var. glabriusculum); 4395 (rhomboideum); 7803 (lanceolatum)
Tonaco, A.C. & J.M. Lanna 137 (villosum)
Tonduz, A. 9409, 12890 (annuum var. glabriusculum)
Torrecillas, E. 75 (rhomboideum); 78, 103 (annuum var. glabriusculum)
Torres, C. & P. Morales 139 (annuum var. annuum)
Torres, M. & F. Rodríguez 2020, 2025 (frutescens); 2032 (chinense)
Torres, M. et al. 199 (annuum var. glabriusculum); 4015, 4055 (frutescens)
Torres, R. et al. 5457 (rhomboideum)
Torres, S. 127 (frutescens)
Torres C., R. 148709 (rhomboideum)
Torres C., R. & E.F. Cabrera C. 6173 (rhomboideum)
Torres C., R. & L. Cortés A. 8821 (annuum var. glabriusculum); 10360 (lanceolatum)
Torres C., R. & E. Martínez S. 11099 (annuum var. glabriusculum)
Torres C., R. & P. Tenorio L. 3751, 3903 (annuum var. glabriusculum)
Torres C., R. et al. 3549 (rhomboideum); 5424 (annuum var. glabriusculum); 9826 (rhomboideum)
Torres R., J.H. 965 (dimorphum)
Tovar, O. 1867, 3750, 4114, 5012, 5363, 7865 (tovarii)
Tovilla Hernández, C. & I. Escalona Luttig 480 (annuum var. glabriusculum)
Tracy, S.M. 6831, 8650 (annuum var. glabriusculum)
Traverse, A. 2523 (annuum var. glabriusculum)
Trécul, M. 1126 (annuum var. glabriusculum)
Trejo, I. 2319 (annuum var. glabriusculum); 2321 (rhomboideum); 2424 (rhomboideum); 2537 (annuum var. glabriusculum)
Tressens, S. et al. 3092, 3097 (flexuosum); 3116, 3873, 3991 (baccatum var. baccatum); 4694, 4977, 5131, 5802, 5881 (flexuosum); 6925 (chacoense)
Triana, G. 18 (frutescens)
Triana, J. 346, 2284 (rhomboideum); 3864 (geminifolium)
Troiani, H.O. & P.E. Steibel 4077 (chacoense)
Troll, C. 1292 (eximium)
Troncoso, N.S. 1741 (chacoense)
Troncoso, R. & M. Fabbroni 224 (chacoense)
Trujillo 6244, 6353, 6885 (rhomboideum)
Trujillo & Fernández 296 (rhomboideum)
Trujillo et al. 247 (rhomboideum)
Trujillo, B. 3711 (annuum var. glabriusculum); 4643 (frutescens); 6351 (annuum var. glabriusculum)
Trujillo, B. & Rodríguez 18026 (frutescens)
Trujillo, B. & M. Trujillo 388, 15605 (frutescens)
Trujillo, B. et al. 17177, 20576 (annuum var. glabriusculum)
Trujillo, W. 130 (chinense); 208 (frutescens)
Trujillo E., R. & S.D. Martínez y Day 75 (annuum var. glabriusculum)
Tucker, J.M. 511 (annuum var. glabriusculum)
Tyson, E.L. 5214, 522, 5588 (annuum var. glabriusculum)
Tyson, E.L. & K. Blum 1946 (annuum var. glabriusculum)
Ucan Ek, E. 3893, 4617 (annuum var. glabriusculum); 4652 (chinense); 5058 (annuum var. glabriusculum)
Ucan Ek, E. et al. 3445, 3527 (annuum var. glabriusculum); 3529 (annuum var. annuum)
Ule, E. 635 (villosum); 2416, 3273 (campylopodium); 3744 (villosum); 7475 (parvifolium); 9732, 9738 (coccineum)
Ulibarri, E.A. 456 (chacoense)
Urban, I. 308, 461, 461 b (annuum var. glabriusculum); 866b, 5055 (frutescens)
Urdampilleta, J.D. et al. 693 (chacoense)
Uribe U., L. 1387 (rhomboideum); 1655 (geminifolium); 2117 (dimorphum); 4762 (rhomboideum); 6059 (dimorphum)
Utley, J.F. & K. Burt-Utley 3934 (annuum var. glabriusculum)
v. Wettstein, R. 975 (flexuosum)
Valenzuela, L. 9902 (annuum var. glabriusculum); 20885 (dimorphum)
Valenzuela, L. & I. Huamantupa 1013 (coccineum)
Valenzuela, L. et al. 3117 (baccatum var. baccatum); 11575 (geminifolium); 13438 (dimorphum)
Valerio R., J. 3039, 3237 (pubescens)
Valerio, M. 151 (frutescens)
Valeur, E.J. 275, 476, 964 (annuum var. glabriusculum)
Valiente B., A. 98, 272 (rhomboideum)
Valiente B., A. & J.I. Solís 360 (rhomboideum)
Valiente B., A. & J.L. Viveros S. 179 (rhomboideum)
Valla, J.J. 10 (chacoense)
Valle, M.H. 90 (schottianum)
Valls, J.F.M. et al. 9014 (frutescens)
Valverde, F.M. 35, 392 (annuum var. annuum)
Valverde R., J.L. 136 (annuum var. glabriusculum)
van Asdall, W. 82-59 (frutescens)
van den Eynden, V. & E. Cueva 654 (frutescens)
van den Eynden, V. et al. 700 (annuum var. glabriusculum)
van der Berg, C. 986 (caatingae)
van der Werff, H.H. 909 (frutescens)
van der Werff, H. & E. Gudiño 10652 (rhomboideum); 11196 (regale)
van der Werff, H. & R.C. Wingfield 7444 (rhomboideum); 7446 (annuum var. glabriusculum)
van der Werff, H. et al. 14624, 15144 (annuum var. glabriusculum)
van Devender, T.R. et al. 1005, 1074, 1170 (annuum var. glabriusculum)
Van Ee, B.W. et al. 669 (chacoense)
van Hermann, H.A. 164, 682 (annuum var. glabriusculum)
van Proosdij A.S.J. et al. 1057 (annuum var. glabriusculum)
Vandebroek, I. 356 (annuum var. glabriusculum)
Vanni, R. & D. Kurtz 3708 (chacoense)
Vanni, R. & A. Schinini 3057 (flexuosum)
Vanni, R. et al. 299 (baccatum var. baccatum); 2081, 2282, 2561 (chacoense); 3289 (baccatum var. baccatum); 3945 (flexuosum)
Vara et al. 417 (annuum var. glabriusculum)
Varela E., L. 179 (annuum var. glabriusculum)
Vargas, J.M. 249 (rhomboideum)
Vargas, L. et al.1310 (coccineum)
Vargas, W.G. 3546 (lycianthoides)
Vargas, W.G. et al. 4238 (dimorphum)
Vargas C., C. 1686 (baccatum var. baccatum)
Vargas C., I.G. 30 (pubescens); 36 (eximium); 66 (annuum var. annuum); 79 (baccatum var. baccatum); 816, 906 (eximium); 932 (pubescens); 1382 (eximium); 2131 (minutiflorum)
Vargas C., I.G. & J.M. Camacho 3118 (caballeroi)
Vargas C., I.G. & C.G. Jordán 7000 (caballeroi)
Vargas C., I.G. & E. Prado 1282, 1286 (caballeroi)
Vargas C., I.G. et al. 1343 (caballeroi); 1979 (pubescens); 2173 (minutiflorum); 2666, 2667 (chinense); 4151, 7233 (caballeroi)
Vargas Contreras, M. 141 (baccatum var. baccatum); 254 (minutiflorum)
Vargas L., H. 5065, 6292 (lycianthoides)
Vasconcelo Neto, J. 05/15 (flexuosum); 05/16, 6707 (rabenii); 6813 (flexuosum); 20857, 20989 (recurvatum); 21594 (flexuosum)
Vásquez, B. T. 611 (annuum var. glabriusculum)
Vásquez, M.E. 002 (frutescens); 011, 012, 013 (annuum var. annuum)
Vásquez, R. & R. Francis 27853, 28032 (geminifolium)
Vásquez, R. & L. Valenzuela 35377 (geminifolium)
Vásquez, R. et al. 25940 (rhomboideum); 27256 (geminifolium); 28196, 30563, 31010 (dimorphum); 31019, 31021 (geminifolium)
Vattuone, J. 99 (chacoense)
Vauthier, A-C. 526 (muticum); 528 (campylopodium)
Vázquez, B. 673, 998 (annuum var. annuum)
Vázquez B., F. & O. Hernández 45 (lanceolatum)
Vega A., R. 1297, 4511 (annuum var. glabriusculum)
Vejarano, S. & G. Galeano 28 (rhomboideum)
Velarde Nuñez, O. 1, 2, 3 (baccatum var. pendulum); 4 (chinense); 5, 6 (baccatum var. pendulum); 7 (annuum var. annuum); 9 (chinense); 10, 11 (baccatum var. pendulum); 12 (chinense); 13, 15 (baccatum var. pendulum); 16 (chinense); 17 (baccatum var. pendulum); 18, 19, 20 (annuum var. annuum); 21 (baccatum var. pendulum); 22 (annuum var. annuum); 23, 24, 25 (baccatum var. pendulum); 26 (annuum var. annuum); 27 (baccatum var. pendulum); 326 (hookerianum)
Velasco, L.V. 8925 (frutescens)
Velasco G., K. et al. 5532 (annuum var. glabriusculum)
Velásquez Castillo, L.G. MA-4, MA-7 (frutescens); MA-8, MA-9 (chinense)
Velásquez D., D. 113 (annuum var. glabriusculum)
Velázquez L., C. 104 (pubescens)
Vélez, C. et al. 5014 (dimorphum)
Vélez, M.C. et al. 1008 (lycianthoides)
Vélez-Puerta, J.M. et al. 3164, 4181 (rhomboideum)
Véliz, M. 7079, 8126 (annuum var. glabriusculum); 14613 (frutescens); 97501 (rhomboideum)
Véliz, M. & F. Ramírez 12818 (annuum var. glabriusculum)
Velloso, H. 158 (schottianum); 556 (campylopodium)
Venda, A.K.L. et al. 54 (mirabile)
Ventura A., F. 798 (lanceolatum); 3117, 3945, 4367, 9378 (rhomboideum); 9739 (pubescens); 10619 (rhomboideum); 11600 (lanceolatum); 13411, 13678, 14331 (rhomboideum)
Ventura V., E. & E. López 4208, 4381, 5472 (lanceolatum); 6332, 7328, 8925, 9887 (rhomboideum)
Venturi, S. 737, 1066, 1598 (chacoense); 4226 (baccatum var. baccatum); 7860 (chacoense)
Vera-Sánchez, L.F. 987 (dimorphum)
Vervloet, R.R. & W. Pizziolo 2378 (baccatum var. baccatum)
Vervoorst, F. 3535 (chacoense)
Vianna 1509 (campylopodium)
Viana, G. 955 (caatingae)
Viana, G. et al. 1986 (caatingae)
Viana, P.L. & A.R. Barbosa 3500 (schottianum)
Viana, P.L. et al. 2253 (pereirae)
Vibrans, H. 2003 (annuum var. glabriusculum)
Vickers, W.T. 115, 178 (chinense); 200 (annuum var. annuum); 208 (frutescens); 211 (annuum var. annuum); 226 (frutescens); 227 (annuum var. annuum)
Vidal, C.V. & R.L. de Paula 1157 (carassense)
Vidal, J. II-308, II-5672 (mirabile); 01490 (flexuosum)
Vidal, W.N. et al. 633 (villosum)
Viegas, A.P. 3509 (schottianum)
Vieira, M.C.W. 117 (schottianum); 2177, 2179 (rabenii)
Vieira, M.F. 542 (campylopodium)
Vieira, M.F. et al. 475 (villosum)
Vieira, R.F. 700 (rabenii); 709 (frutescens)
Vieira, R.F.& I. Costa 683 (rabenii)
Viereck, H.W. 350, 1011 (rhomboideum)
Vignati, M.A. 337 (chacoense)
Vilar, T.S. & A.A. Santos 3 (rabenii)
Vilcapoma, G. 84, 85 (annuum var. annuum); 284, 327 (annuum var. glabriusculum)
Villa Carenzo, M. 424, 159, 202, 2789 (chacoense); 3373 (flexuosum); 3499 (chacoense)
Villa Carenzo, M. & P.R. Legname 1440 (chacoense); 3846 (baccatum var. baccatum)
Villa Kamel, A. 71, 106 (annuum var. annuum)
Villacorta, R. & J. Giammattei 2551 (annuum var. glabriusculum)
Villacorta, R. et al. 2819 (rhomboideum)
Villafañe, M. 348, 400 (chacoense)
Villalobos C., G. & E. Guerrero 182, 205 (annuum var. glabriusculum)
Villanueva, R. 591 (annuum var. annuum)
Villarreal, J.A. et al. 18036 (annuum var. glabriusculum)
Villarroel, D. 483 (baccatum var. baccatum); 1677, 1712 (minutiflorum)
Vimercat, J.M. 124 (baccatum var. baccatum)
Vincent, M.A. et al. 10583 (annuum var. glabriusculum)
Virillo, C.B. et al. 63 (recurvatum)
Vivar C., F. 4003 (frutescens)
Vivar C., F. & B. Merino 3027 (frutescens)
Vivar C., F. & Estudiantes 1066 (benoistii)
Vogel, S. 573 (flexuosum)
Vogl, C. 424 (annuum var. glabriusculum); 709 (rhomboideum)
Vogt, C. & F. Mereles 231 (chacoense)
von Eggers, H.F.A. 322, 322 b, 583, 1993, 2247, 2796, 4715 (annuum var. glabriusculum); 5302 (chinense); 13177 (rhomboideum); 15555 (hookerianum)
von Friedrichsthal, E. 201, 310 (annuum var. glabriusculum)
von Humboldt, A.J.A. & F.W. Bonpland 150 (baccatum var. baccatum); 3027 (pubescens); 4518 (annuum var. glabriusculum)
von Martius, C.F.P. 132 (baccatum var. baccatum); 3074 (schottianum)
von Rentzell de Atkinson, I. 11 (frutescens)
von Rozynski, H.W. 183, 211 (annuum var. glabriusculum); 467 (rhomboideum)
von S. Medeiros, E. et al. 176 (frutescens); 182, 196 (chinense); 198, 199 (frutescens); 200 (chinense)
von Sneidern, K. 3044, 3214 (dimorphum)
von Sohsten Medeiros, E. et al. 353 (pereirae)
von Wedel, H. 724, 1237, 2610 (annuum var. glabriusculum)
Voorhies, B. & A. Sanchez 61-12 (annuum var. glabriusculum)
Wagner, R. 1215, 1632 (annuum var. glabriusculum)
Wagenbreth, I. 130, 687 (annuum var. annuum)
Wallnöfer, B. & F.M. Tut-Tesucun 6028 (annuum var. glabriusculum)
Walter, B.M.T. 3628 (rabenii)
Walter, B.M.T. et al. 566 (baccatum var. pendulum); 570, 575, 581, 582, 3249, 3851 (frutescens); 6014 (rabenii)
Wanderley, M.G.L. 264 (villosum)
Wängler, M.S. 1569 (mirabile)
Wängler, M.S. & V.S. Ferreira 1027 (mirabile)
Ward, D.B. 2836, 5555 (annuum var. glabriusculum)
Warner, P. 376 (baccatum var. baccatum)
Warner, R.H. 215 (annuum var. glabriusculum)
Wasum, R. 783 (flexuosum)
Watson, K. et al. 39 (lanceolatum)
Wawra, H. & F. Maly 413 (muticum)
Weberbauer, A. 6059 (rhomboideum); 7684 (hookerianum)
Webster, G.L. & S. Lynch 17661 (annuum var. annuum)
Webster, G.L. & R. Rhode 29283 (lycianthoides)
Webster, G.L. & T. Thompson 27964 (lycianthoides)
Webster, G.L. & UREP participants 27868 (lycianthoides)
Webster, G.L. et al. 10450, 10791(annuum var. glabriusculum); 28749, 30044, 30501 (lycianthoides); 32023 (rhomboideum); 32907 (lycianthoides)
Webster, L. 27143 (lycianthoides)
Webster, L. & L. Hebert 27493 (lycianthoides)
Weigend, M. & B.R. Ramírez 3528 (rhomboideum)
Weigend, M. et al. 5285 (geminifolium); 7528 (rhomboideum)
Wendelberger, K. 170 (neei)
Wendt, T. & D. Riskind 1611 (annuum var. glabriusculum)
Wendt, T. et al. 4685, 5173 (lanceolatum)
Werling, L. & S. Leth-Nissen 371 (pubescens); 397 (lycianthoides)
Werner, F.A. 1548 (longifolium); 2594 (geminifolium)
West, J. 3739 (pubescens); 8248 (chacoense); 8389 (baccatum var. baccatum)
Weston, A.S. & J. Weston 4754 (annuum var. glabriusculum)
Weston, A.S. et al. 4173 (annuum var. glabriusculum)
Weyland Vieira, M.C. 117 (annuum var. glabriusculum)
White, D.A. 178 (annuum var. glabriusculum)
White, S.S. 1606, 3640 (annuum var. glabriusculum)
Whitefoord, C. 5629, 7246, 10238 (annuum var. glabriusculum)
Whitefoord, C. & V. Quiroz 10699 (annuum var. glabriusculum)
Whiting, M.G. 407 (annuum var. glabriusculum)
Wiggins, I.L. 7368 (annuum var. glabriusculum); 18643 (frutescens)
Wiggins, I.L. & R.C. Rollins 338 (annuum var. glabriusculum)
Wilke, B. 5 (annuum var. glabriusculum)
Williams, D.E. 696 (baccatum var. pendulum); 764 (frutescens); 772, 773 (chinense); 932 (baccatum var. baccatum); 933 (frutescens); 934, 1177 (chinense)
Williams, D.E. & A. Krapovickas 1122 (chacoense)
Williams, K.A. et al. 121 (baccatum var. pendulum); 122 (baccatum var. baccatum); 125 (flexuosum); 127 (baccatum var. pendulum); 129, 131 (baccatum var. baccatum); 133 (flexuosum); 134 (baccatum var. baccatum); 135 (baccatum var. pendulum); 136 (baccatum var. baccatum); 140 (chacoense); 210 (rhomboideum)
Williams, L. 1277 (annuum var. glabriusculum); 3405 (pubescens); 5224 (annuum var. glabriusculum); 5225 (chinense); 5785 (annuum var. glabriusculum); F-8202 (chinense); 9538 (frutescens); 10595 (rhomboideum); 10597 (annuum var. glabriusculum)
Williams, L.O. & A. Molina R. 14671 (annuum var. glabriusculum)
Williams, L.O. & R.P. Williams 18295 (annuum var. glabriusculum)
Williams, L.O. et al. 25674, 25950, 26924, 43125, 43612 (lanceolatum)
Williams, R.S. 623 (coccineum); 634 (coccineum); 715 (annuum var. glabriusculum)
Wilson, P. 1019, 1393, 7512, 9468 (annuum var. glabriusculum)
Wolff, R.A. 16 (baccatum var. baccatum)
Wood, C.E. et al. 9220 (annuum var. glabriusculum)
Wood, J.R.I. 8774 (eximium); 9060 (caballeroi); 9104 (neei); 11102 (caballeroi); 14892 (coccineum); 22267 (baccatum var. baccatum)
Wood, J.R.I. & M. Atahuachi 21581 (eximium)
Wood, J.R.I. & A. Carretero 19317 (chacoense)
Wood, J.R.I. & D.J. Goyder 15579 (baccatum var. baccatum); 16736 (eximium);
Wood, J.R.I. & J. Gutiérrez 20218 (eximium)
Wood, J.R.I. & H. Huaylla 21532 (eximium)
Wood, J.R.I. & M. Menacho 12642 (coccineum)
Wood, J.R.I. & M. Mendoza 19144 (chacoense); 19165, 19166, 21490 (eximium); 22105 (caballeroi)
Wood, J.R.I. & M. Serrano 13331 (eximium)
Wood, J.R.I. & D. Wasshausen 13893 (coccineum)
Wood, J.R.I. et al. 21229 (eximium); 21792 (chacoense); 22339 (baccatum var. baccatum); 22449 (eshbaughii); 22798 (baccatum var. baccatum); 22801 (coccineum)
Woodmansee, S.W. et al. 432 (annuum var. glabriusculum)
Woodson Jr., R.E. & R.W. Schery 840 (annuum var. glabriusculum)
Woolston, A. 165 (baccatum var. baccatum); 729 (flexuosum)
Wooton, E.O. 48, 49 (annuum var. annuum)
Woytkowski, F. 1176 (coccineum); 5678 (rhomboideum); 7516, (coccineum); 35161 (annuum var. glabriusculum)
Wright, C. 384, 537 (annuum var. glabriusculum)
Wullschlaegel, H.R. 396 (annuum var. glabriusculum)
Wunderlin, R.P. 5306 (annuum var. glabriusculum)
Wunderlin, R.P. et al. 6246 (annuum var. glabriusculum)
Wussow, J. & G. Landry 253 (rhomboideum)
Wydler, H. 264 bis (annuum var. glabriusculum)
Wynd, F.L. 687 (annuum var. glabriusculum)
Yáñez, A.P. & R. Foster 262 (lycianthoides)
Yañez, A.P. et al. 1407 (frutescens); 1485 (annuum var. annuum)
Yañez, M. 440, 572 (annuum var. glabriusculum); 783 (rhomboideum)
Yepes-Agredo, S. 1172 (rhomboideum)
Young, K. & G. Sullivan 713 (coccineum)
Yuncker, T. 17118, 17253, 18460 (annuum var. glabriusculum)
Zak, V. 836 (lycianthoides); 1423 (rhomboideum); 1433 (lycianthoides); 1752, 1759 (geminifolium); 1970 (rhomboideum); 9804 (lycianthoides)
Zak, V. & J.L. Jaramillo 2147, 2220, 3099 (lycianthoides)
Zamora C., P. 24, 25 (annuum var. annuum)
Zamora C., P. & M.G. Zola B. 4 (annuum var. annuum)
Zamora, V.H. 193 (eximium)
Zanoni, T. & M. Mejía 17002, 17077 A, 26168 (annuum var. glabriusculum)
Zanoni, T. & J. Pimentel 25313 (annuum var. glabriusculum)
Zanoni, T. et al. 12012, 21507, 22115, 30601, 32161, 42707 (annuum var. glabriusculum)
Zapata C., D.A. 827 (geminifolium)
Zardini, E.M. 1739 (baccatum var. pendulum); 1870 (eximium); 5771, 7166, 7200 (flexuosum); 10972 (rabenii); 11076 (flexuosum); 30803 (rabenii); 38869 (chacoense); 41733, 41772, 45402, 47751, 50492, 53141, 53151 (flexuosum)
Zardini, E.M. & A. Acosta 42322, 42328, 42346, 42450, 42445 (chacoense); 56060 (baccatum var. baccatum)
Zardini, E.M. & A. Aguayo 9508 (rabenii); 9522, 9549, 9729 (baccatum var. baccatum)
Zardini, E.M. & C. Balbuena 42915 (baccatum var. baccatum)
Zardini, E.M. & A. Benítez 47532 (flexuosum)
Zardini, E.M. & R. Franco 30370 (baccatum var. baccatum)
Zardini, E.M. & J. Godoy 50089 (chacoense)
Zardini, E.M. & L. Guerrero 33706 (baccatum var. baccatum); 37883, 37913, 37916 (chacoense)
Zardini, E.M. & D. Quintana 53786 (baccatum var. baccatum)
Zardini, E.M. & J.C. Rivas 58461 (chacoense)
Zardini, E.M. & T. Tillería 39351 (chacoense)
Zardini, E.M. & M. Velázquez 10019, 13104 (baccatum var. baccatum)
Zardini, E.M. & R. Velásquez 8922 (baccatum var. baccatum); 12460, 12920 (flexuosum); 13856 (baccatum var. pendulum); 17171 (baccatum var. baccatum)
Zardini, E.M. & U. Velásquez 15600 (flexuosum)
Zardini, E.M. et al. 1641, 1740 (eximium); 12774 (baccatum var. baccatum); 12744 (rabenii); 19653 (chacoense)
Zarucchi, J.L. 2173 (frutescens)
Zarucchi, J. & D. Cárdenas L. 4366 (dimorphum)
Zarucchi, J. et al. 4978 (annuum var. glabriusculum); 5476 (rhomboideum); 5478 (annuum var. glabriusculum); 5491 (rhomboideum)
Zelaya, L. 239 (annuum var. glabriusculum)
Zenteno, F. et al. 3608 (eximium); 12798 (baccatum var. baccatum)
Zizumbo, D. & P. Colunga 145 (annuum var. annuum); 220, 299 (annuum var. glabriusculum)
Zola B., M.G. 815 (annuum var. glabriusculum)
Zola B., M.G. & R.M. Baizabal 1459, 1467, 1496, 1500, 1503, 1506, 1511, 1514, 1548, 1550, 1599, 1620 (annuum var. annuum)
Zola B., M.G. & P. Zamora 1078, 1081, 1096, 1110, 1138, 1243, 1249, 1261, 1277 (annuum var. annuum)
Zollinger, H. 489 (annuum var. annuum)
Zorzanelli, J.P. 957, 968 (pereirae)
Zuchiwschi, E. 55 (rabenii)
Zuloaga, F. et al. 5171, 5431 (flexuosum); 6377 (baccatum var. baccatum); 6722 (flexuosum).
Supplementary materials
Appendix 1. Anatomical characters of Capsicum fruits
Data type: Morphological (excel file)
Explanation note: Description of fruit anatomical characters for each Capsicum species.
Appendix 2. Seed characters and seed coat features in Capsicum
Data type: Morphological (excel file)
Explanation note: Description of seed and seed coat characters for each Capsicum species.
Appendix 3. Comparative data on distribution, habitat, biomes and number of ecoregions inhabited by Capsicum taxa
Data type: Occurences (excel file)
Explanation note: Details of biomes and ecoregions are not applicable to domesticated taxa.
Appendix 4. Specimens examined
Data type: Occurences (PDF file)
Explanation note: Full information of the specimens examined for each Capsicum species.