Research Article |
|
Corresponding author: Daniel Santamaría-Aguilar ( daniel.santamaria366@gmail.com ) Academic editor: Thomas L.P. Couvreur
© 2019 Daniel Santamaría-Aguilar, Reinaldo Aguilar, Laura P. Lagomarsino.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Santamaría-Aguilar D, Aguilar R, Lagomarsino LP (2019) A taxonomic synopsis of Virola (Myristicaceae) in Mesoamerica, including six new species. PhytoKeys 134: 1-82. https://doi.org/10.3897/phytokeys.134.37979
|
Abstract
A taxonomic synopsis of Virola (Myristicaceae) is presented for Mesoamerica. Fourteen species are recognised, amongst them six are described and published as new, based on morphology: V. allenii D.Santam. & Aguilar, sp. nov. from Costa Rica, V. otobifolia D.Santam., sp. nov. from Panama and V. amistadensis D.Santam., sp. nov., V. chrysocarpa D.Santam. & Aguilar, sp. nov., V. fosteri D.Santam., sp. nov. and V. montana D.Santam., sp. nov. from both Costa Rica and Panama. Additionally, a lectotype is designated for V. koschnyi, accompanied by an epitype in view of the fragmentary material. Finally, we recognise V. laevigata and V. nobilis as morphologically distinct species, though these are frequently considered synonymys of V. guatemalensis and V. surinamensis, respectively. Of the fourteen accepted species, twelve of them are endemic to Mesoamerica, while the remaining two species (V. elongata and V. sebifera) extend into South America. Illustrations, species diagnoses and distribution maps for each species are provided, as is an identification key to all species.
Keywords
Costa Rica, Flora Mesoamericana, hallucinogenic, Magnoliales, nutmeg, Panama, taxonomy
Introduction
Myristicaceae, a member of the magnoliid order Magnoliales (
Virola is the largest genus of Myristicaceae in America and, with about 60 species, it is the fourth largest genus in the family. The plants have two particularly distinctive characteristics: their growth form and the mature fruit. The growth form, sometimes called myristicaceous branching (e.g.
Morphology
As with many magnoliids, Virola has aromatic tissues. Unlike other magnoliids, though, when the bark is cut or a twig is broken, it yields an exudate that is initially watery and clear and quickly changes to red or pinkish. The leaves, which are exstipulate and simple with entire margins and occasionally have pellucid punctuation, are distichous. Most surfaces of the plant, including leaves, are usually covered with stellate or dendritic trichomes. Staminate inflorescences are paniculate, with few to many branches. Flowers, sometimes reported as fragrant, are unisexual, ebracteolate and inserted on a receptacle; they are produced in lax to dense clusters. Their yellow or brown perianth is connate to varying degrees and uniseriate, usually with 3-lobes. The androecium is compound, with filaments fused into a column, with 3– (4–6) anthers fused to the column. The pistillate inflorescences are shorter than staminate inflorescences. Pistillate flowers have a perianth that is more carnose than those of the staminate flowers and their gynoecia are pubescent and sessile or short- stipitate, topped by a stigma that is usually bilobed (
In Mesoamerica, where five of the six native genera of American Myristicaceae occur (Fig.
| 1 | Leaf blades with tertiary veins conspicuous and more or less parallel and perpendicular to the lateral veins |
Compsoneura
(Fig. |
| – | Leaf blades with tertiary veins inconspicuous or, when conspicuous, not as above | 2 |
| 2 | Leaf blades with malpighiaceous trichomes on abaxial surface | 3 |
| – | Leaf blades with stellate, dendritic, or irregularly stellate trichomes on abaxial surface | 4 |
| 3 | Vernation convolute; abaxial surface of leaf blades without hyaline crystals; flowers with bracteoles; fruits transversally ellipsoid, if globose, the pericarp 7–8 mm thick; seeds not gibbose at the apex |
Iryanthera
(Fig. |
| – | Vernation conduplicate; abaxial surface of leaf blades with hyaline crystals; flowers without bracteoles (in Mesoamerica); fruits longitudinally globose to subglobose; seeds usually gibbose near the apex |
Otoba
(Fig. |
| 4 | Trichomes on abaxial leaf surface stellate, concolorous; inflorescences sessile, with 1–2 (–3) main branches; flowers with bracteoles; stamens 10 (in Mesoamerica); fruits transversally ellipsoid |
Osteophloeum
(Fig. |
| – | Trichomes on abaxial leaf surface dendritic, irregularly stellate or if stellate, the central part of the trichome reddish to reddish-clear, constrasting in colour with the hyaline branches; inflorescences distinctly pedunculate, with 1 main branch; flowers without bracteoles; stamens 3–7; fruits ellipsoid, globose, ovoid or subglobose | Virola |
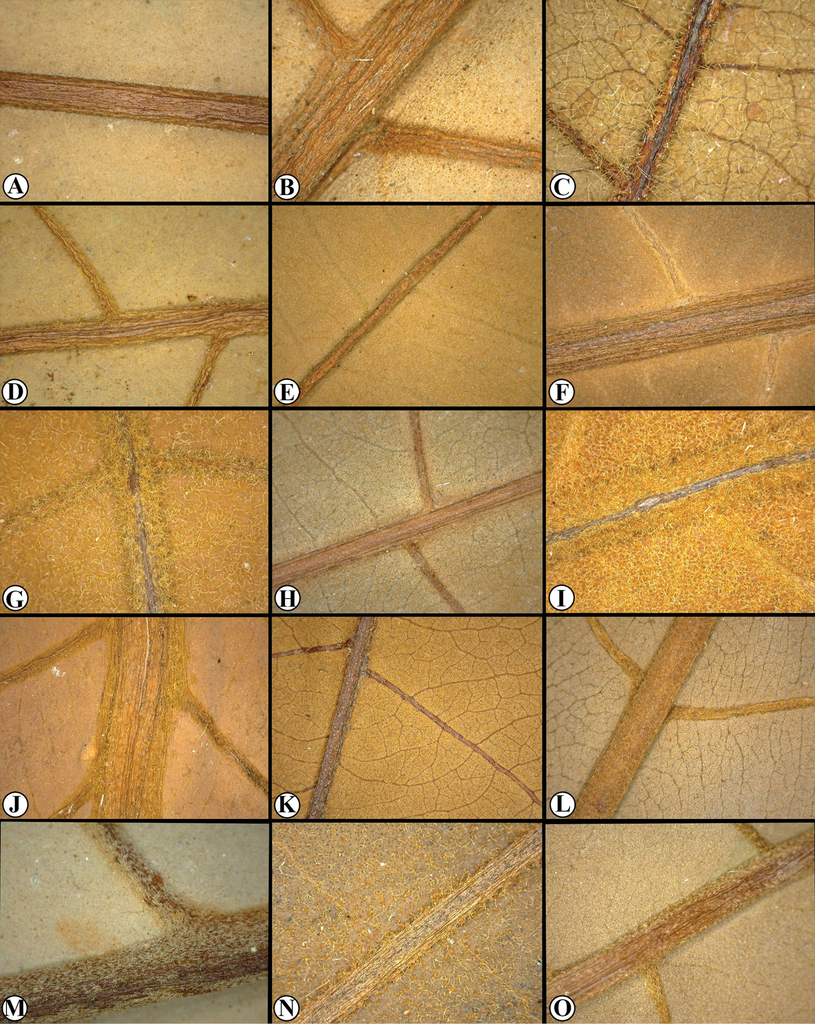
Genera of Myristicaceae present in Mesoamerica A–C Compsoneura sect. Compsoneura (C. excelsa) D–F Compsoneura sect. Hadrocarpa (C. capitellata) G–J Iryanthera (G–I Iryanthera spp; J I. megistocarpa) K–L Osteophloeum platyspermum M–O Otoba novogranatensis. Photos by Reinaldo Aguilar (A–C, M–O), Benjamin Chambi (E), Jason D. Wells (D, F from http://atrium.andesamazon.org/); Robin Foster (G–I, K from https://plantidtools.fieldmuseum.org/en/nlp); Alwyn H. Gentry (J from http://www.tropicos.org); and David A. Neill (L).
Biogeography
Virola is found throughout tropical America. Species have been collected from Mexico to southern Brazil, though are notably absent in El Salvador. Occurrence in the Caribbean is limited: one species is found in the West Indies (V. surinamensis [Rol. ex Rottb.] Warb.), though a fossil Virola flower, recently described from Dominican Republic (
Within Mesoamerica, the greatest concentration of Virola species occurs in Costa Rica and Panama. Together, these two countries contain 10 and 11, respectively, of the 14 Mesoamerican species (Fig.
List of the 14 accepted species of Virola in Mesoamerica with countries of occurrence, slope preference and elevation within Mesoamerica.
| Species | Distribution | Slope | Elevations (m) |
|---|---|---|---|
| V. allenii D. Santam. & Aguilar. | Costa Rica | Pacific | 0–350 (–1350) |
| V. amistadensis D. Santam. | Costa Rica, Panama | Caribbean | 650–1200 |
| V. chrysocarpa D. Santam. & Aguilar. | Costa Rica, Panama | Pacific | 0–700 |
| V. elongata (Benth.) Warb. | Panama | Caribbean | 50–450 |
| V. fosteri D. Santam. | Costa Rica, Panama | Caribbean | 0–350 (–800) |
| V. guatemalensis (Hemsl.) Warb. | Mexico, Guatemala, Honduras | Both slopes | 150–1250 |
| V. koschnyi Warb. | Mexico, Guatemala, Belize, Honduras, Nicaragua, Costa Rica, Panama | Both slopes, mainly on the Caribbean side | 10–1000 (–1700) |
| V. laevigata Standl. | Costa Rica, Panama | Pacific | 0–500 (1600?) |
| V. megacarpa A. H. Gentry. | Panama | Caribbean | 50–550 |
| V. montana D. Santam. | Costa Rica, Panama | Both slopes | 700–2000 |
| V. multiflora (Standl.) A. C. Sm. | Belize, Honduras, Nicaragua, Costa Rica | Caribbean | 0–650 (–1400) |
| V. nobilis A. C. Sm. | Costa Rica, Panama | Both slopes | 0–400 (–1300) |
| V. otobifolia D. Santam. | Panama | Caribbean | 50–850 |
| V. sebifera Aubl. | Honduras, Nicaragua, Costa Rica, Panama | Both slopes | 0–1000 |
Ethnobotany
Virola has diverse ethnobotanical uses and there are many reports of local use of several species of Virola for many non-medicinal purposes. For example, the oils from the seeds of Virola (e.g. V. guatemalensis, V. sebifera) are used as lubricant in machinery and to make soap and candles that emit intense light, produce little smoke and smell pleasant;
Virola also produces many chemicals that are biologically active in humans. For example, Virola is perhaps best known for its hallucinogenic properties, which are often incorporated into indigenous cultural practices of South America. The hallucinogen is usually obtained from the exudate of the inner bark of several species, including: Virola calophylla Warb., V. calophylloidea Markgr., V. duckei A. C. Sm., V. elongata, V. sebifera, V. surinamensis and V. theiodora Warb.; it has also been documented in Iryanthera and Osteophloeum platyspermum (Spruce ex A. DC.) Warb. (
Taxonomic history
Virola was first described by Aublet in “Histoire des plantes de la Guiane Françoise”, based on V. sebifera Aublet (
There is a long taxonomic history to the American species of Myristicaceae. This began with George
Since then, 17 new species of Virola have been published from South America (
Virola has been the subject of limited phylogenetic analysis. The first molecular phylogenetic study of Virola– and the only to date– demonstrated that the genus is divided into two large subclades, informally called “Multinervae” and “Sebiferae” (
Materials and methods
This work was undertaken as part of the “Flora Mesoamericana” project (http://www.tropicos.org/Project/FM) by the first author. However, our definition of “Mesoamerica” is different than that used in the Flora; here, it refers to the portion of Central America and southern Mexico from the Isthmus of Tehuantepec in Mexico in the north to the Panamanian-Colombian border in the south.
Approximately 500 physical herbarium specimens of Mesoamerican Virola were examined for this study from the following herbaria:
Species descriptions are based primarily on herbarium specimens, though observations during fieldwork by the first and second authors, especially in the Osa Peninsula of Costa Rica, were also important. If necessary and material permitted, flowers from herbarium specimens were rehydrated before measurement. A ruler was used to measure leaves and inflorescences; a digital Neiko caliper was used to measure fruits and seeds, as well as the thickness of the twigs, petioles and peduncles; and, finally, flowers, trichomes and thickness of the pericarp were measured with a micrometer calibration tool (1div = 1mm) under a dissecting stereoscope (Bausch & Lomb).
The preliminary conservation status of each new species was assessed using quantitative criteria recommended by the IUCN Red List (
Specimens cited are listed first by country in a north to south sequence. Within country, specimens are listed alphabetically by major division and then alphabetically by province or department and, finally, in alphabetical order by the collector’s surname. When the coordinates and/or elevation were not included on the herbarium label, but were present in the TROPICOS database, the values from TROPICOS are included. Dot-distribution maps were compiled from studied specimens and generated with SimpleMappr (
In the nomenclatural section for each new species, we cite both accession numbers and barcodes when present. Barcodes are included in square brackets and follow the format of a series of numbers preceded by a herbarium acronym (e.g. [GH00039891]); all other numbers correspond to accession numbers.
Knoweldge of the group was improved by numerous field trips since 1991 throughout Costa Rica, especially in the Osa Peninsula and La Selva Biological Station, by the second author and, more recently, by the first author in the same region.
Key to the species of Virola in Mesoamerica
| 1 | Abaxial leaf surface with pediculate trichomes (sometimes sessile in V. sebifera and with short pedicle in V. elongata) (Fig. |
2 |
| – | Abaxial leaf surface with sessile trichomes (Fig. |
5 |
| 2 | Leaf blades with 9–17 lateral veins per side, free or slightly anastomosing near the margin and without forming a very marked intramarginal vein, indument on abaxial surface caducous; petiole slightly canaliculate; staminate flower with the filament column 0.1–0.4 mm long, anthers with apiculus 0.1–0.2 mm long; fruits 1.1–1.7 (–2.3) × 0.8–1.3 (–1.6) cm, globose to subglobose (Fig. |
V. sebifera |
| – | Leaf blades with (16–) 20–50 lateral veins per side, anastomosing near the margin and forming a conspicuous intramarginal vein, indument on abaxial surface persistent; petiole markedly canaliculate; staminate flower with the filament column 0.7–1.5 mm long, anthers without apiculus or this very inconspicuous; fruits 1.9–5.7 × 1.5–2.9 cm, ellipsoid, ovoid-ellipsoid or sometimes subglobose (Fig. |
3 |
| 3 | Leaf blades with (32–) 40–50 lateral veins per side; fruits 4–5.7 × 2–2.9 cm, the apex acuminate to rostrate (Fig. |
V. megacarpa |
| – | Leaf blades with (16–) 20–35 lateral veins per side; fruits 1.9–3.1 × 1.5–1.9 cm, the apex acute to apiculate or obtuse; pericarp 1.2–3.1 mm thick | 4 |
| 4 | Mature leaf blades on adaxial surface pubescent and asperous to the touch in dry specimens, abaxial surface densely hirsute to hirsutulous, trichomes with 3–6 branches, the branches 0.2–0.6 mm long; staminate flowers with filament column 1.3–1.5 mm long | V. chrysocarpa |
| – | Mature leaf blades on adaxial surface glabrous to glabrescent and smooth to the touch in dry specimens, abaxial surface densely tomentose, trichomes with 4–10 branches, the branches 0.1–0.2 mm long; staminate flowers with filament column 0.7–0.9 (–1.4) mm long | V. koschnyi |
| 5 | Leaf blades with 9–20 lateral veins per side, (0.8–) 1–3 cm apart; staminate flowers with perianth infundibuliform, the filament column 0.2–0.9 (–1) mm long; fruits green when mature; aril laciniate in very narrow bands distally when dry, membranaceous | 6 |
| – | Leaf blades with 10–34 lateral veins per side, 0.2–1.1 (–1.5) cm apart; staminate flowers with perianth campanulate, globose or subglobose, the filament column 0.6–1.3 mm long; fruits yellow, orange or a combination of these colours when mature; aril laciniate in narrow bands distally when dry, coriaceous | 9 |
| 6 | Abaxial leaf surface sparsely pubescent, trichomes concolorous, the trichome branches 0.1–0.2 mm long; peduncle of staminate inflorescences 0.07–0.15 cm thick; staminate flower with the perianth 1.5–1.9 mm long; fruits 1.6–1.9 × 0.9–1.1 cm (Fig. |
V. elongata |
| – | Abaxial leaf surface densely pubescent, trichomes dicolorous (the central part usually reddish, contrasting in colour with the hyaline to pale reddish branches), the trichome branches ± 0.01–0.05 mm long; peduncle of staminate inflorescences 0.09–0.34 cm thick; staminate flower with the perianth (1.5–) 2–2.8 mm long; fruits 2.1–4.5 × 1.5–2.9 cm (Fig. |
7 |
| 7 | Leaf blades elliptic to widely elliptic; staminate flowers with the filament column 0.2–0.4 mm long; fruits with pericarp 1–2 mm thick | V. amistadensis |
| – | Leaf blades oblong-elliptical or rarely elliptical; staminate flowers with the filament column 0.5–1 mm long; fruits with pericarp (2.7–) 3–4.7 mm thick | 8 |
| 8 | Leaf blades 3.2–7.3 cm wide; lateral veins 15–20 per side, with 4–5 per 5 cm, 1.2–1.8 cm apart; staminate flowers in dense terminal fascicles; staminate perianth lobes glabrous or sparsely pubescent on the adaxial margin; filament column 0.5–0.6 mm long, thin throughout its length (sometimes slightly thickened at the base), not constricted at the apex; mature fruits with trichomes that fall like dust | V. allenii |
| – | Leaf blades (4.1–) 7.3–14.2 cm wide; lateral veins 10–16 per side, with 2–4 per 5 cm, 1.7–3 cm apart; staminate flowers in lax terminal fascicles; staminate perianth lobes pubescent adaxially; filament column 0.9–1 mm long, conspicuously thickened throughout its length, except constricted at the apex; mature fruits with persistent trichomes | V. otobifolia |
| 9 | Abaxial leaf surface with caducous trichomes or practically glabrous or, if some persistent trichomes, then along the veins, never on the entire surface | 10 |
| – | Abaxial leaf surface with trichomes persistent throughout | 11 |
| 10 | Twigs and petioles glabrous; leaf blades with 12–20 lateral veins per side; staminate flowers with the filament column 0.8–1.3 mm long; fruits 1.8–2.9 × 1.5–1.8 cm (Fig. |
V. laevigata |
| – | Twigs and petioles pubescent; leaf blades with (15–) 18–30 lateral veins per side; staminate flowers with the filament column 0.6–0.9 mm long; fruits (2.8–) 3–3.6 × 2–2.5 cm (Fig. |
V. montana |
| 11 | Leaf blades 1.4–3.6 (–4.8) cm wide | 12 |
| – | Leaf blades (2.4–) 3.8–7.1 (–8.9) cm wide | 13 |
| 12 | Leaf blades with the base revolute; lateral veins 16–24 per side, with 10–15 veins per 5 cm, 0.2–0.5 (–0.7) cm apart; staminate flowers with the filament column 0.9–1.3 mm long, anthers 0.6–0.9 mm long; fruits 1.5–2.3 × 1.2–1.8 cm (Fig. |
V. fosteri |
| – | Leaf blades with the base not revolute; lateral veins 10–18 per side, with (6–) 8–10 veins per 5 cm, 0.4–0.8 (–1.2) cm apart; staminate flowers with the filament column 0.7–1 mm long, anthers 0.3–0.6 mm long; fruits 1.3–1.9 × 0.9–1.2 (–1.4) cm (Fig. |
V. multiflora |
| 13 | Lateral veins 13–21 per side, slightly elevated or flat on the abaxial leaf surface, tertiary veins almost indistinct or very slightly visible on both surfaces (Fig. |
V. guatemalensis |
| – | Lateral veins 20–30 (–32) per side, markedly elevated on the abaxial leaf surface, tertiary veins usually distinct on both surfaces (especially abaxially) (Fig. |
V. nobilis |
Trichomes in Mesoamerican Virola A Virola allenii (R. Aguilar 2224,
Fruits of Mesoameican Virola, organised in groups of morphologically similar species A V. allenii (P. H. Allen 6727, GH) B V. amistadensis (G. McPherson 9715,
Taxonomy
Virola allenii , sp. nov.
Diagnosis
Species resembling Virola macrocarpa in its leaf blades that are whitish on the abaxial side and covered with stellate, sessile trichomes with the centre reddish and contrasting with the hyaline branches to reddish-clear in colour and lateral veins that are not densely arranged, as well as large fruits that are covered with ferruginous trichomes. It differs in its narrow leaf blades (3.2–7.3 cm vs. 7–11 cm wide) with acute or obtuse to rounded bases (vs. broadly obtuse), fruits with thick pericarp (3.2–3.8 mm vs. 1.8–3 mm thick) and preference for humid lowland forests at 0–350 (–1350) m elevation (vs. montane forests in Andes of Colombia at around 1100 m elevation).
Type
Costa Rica. Puntarenas: Esquinas forest preserve, 0 m, 10 Jan 1951 (♂ fl), P. H. Allen 5763 (holotype: F-2 sheets* [1394346!, 1679106!]; isotype: USJ [9016]).
Virola allenii A treetop B branching C lower trunk and buttress D branch with leaves, showing the adaxial surface E leaf blades on abaxial surface F exudate of the trunk G leaf blade surfaces, adaxial (above) and abaxial (below) H petiole and leaf base I mature fruit, inset left and arrow showing the galls on leaves and branches, respectively J fruits K fruit close-up. Photos by Reinaldo Aguilar.
Comparisons of Virola macrocarpa with similar species in Mesoamerica A V. allenii (P. H. Allen 5763, F; inset fruits, from P. H. Allen 6727, GH) B V. amistadensis (G. McPherson 9717,
Description
Tree
13–30 m × 10.4–50 cm DBH; bark sometimes described as smooth and reddish or dark brown. Exudate sometimes described as abundant and reddish or watery, but without specifying from where or red in the trunk. Twigs 0.16–0.22 cm thick, terete to slightly flattened laterally, puberulent, trichomes stellate to irregularly stellate, ferruginous. Leaves: petiole 0.5–1.4 × 0.13–0.24 cm, slightly canaliculate, tomentose, the trichomes stellate to irregularly stellate; leaf blades 16.2–29.2 × 3.2–7.3 cm, oblong-elliptic or rarely elliptic; adaxial surface of mature leaves olive or light brown (sometimes shining) when dry, glabrous or with scattered stellate trichomes, the surface smooth; abaxial surface pale brown to whitish when dry, densely but inconspicuously pubescent, trichomes stellate, sessile, the central part of the trichome reddish and contrasting in colour with the hyaline branches to reddish-clear, with 4–10 branches, the branches ± 0.01–0.05 mm long, persistent; lateral veins 15–20 per side, 4–5 veins per 5 cm, 1.2–1.8 cm apart, the same colour as the adaxial surface or slightly transparent, on adaxial side flat to sunken, on abaxial side slightly elevated, arcuate-ascending, slightly anastomosing near the margin and without forming a very marked intramarginal vein; tertiary veins barely visible on both sides; midvein adaxially slightly elevated (sometimes flat, distally), abaxially raised, rounded to somewhat triangular, tomentose to glabrate; base acute or obtuse to rounded, not revolute, flat; margin flat; apex acuminate or rarely rounded. Staminate inflorescences 3.5–5.5 cm long, axillary, axes flattened, tomentose, with trichomes irregularly stellate, ferruginous; peduncle 1.2–1.9 × ca. 0.1 cm long; bracts not seen; terminal fascicles dense, with 15—40+ flowers. Staminate flowers with the pedicel 0.3–1.2 mm long; receptacle 1.2—2 mm wide; perianth 2–2.8 mm long, infundibuliform, yellow when fresh, connate by 1.1–1.7 (–2.3) mm long, external surface pubescent, with brown trichomes, internal surface glabrous or with few trichomes close to the margin of the lobes; lobes 3 (4), 0.8–1.5 × 0.6–0.9 (–1.2) mm; stamens 3, the filament column 0.5–0.6 mm long, glabrous, straight, thin, sometimes slightly thickened at the base, not constricted at the apex; anthers 0.6–0.9 mm long; apiculus 0.06–0.1 mm long, acute to apiculate, connate. Pistillate inflorescences and pistillate flowers not seen. Infructescence 2.5–7.5 cm long, with 2–13 fruits, peduncle 2–3.5 × ca. 0.47 cm. Fruits 2.7–3.5 × 1.5–2.5 cm, usually ellipsoid or rarely ovoid, stipitate, densely tomentose, the trichomes dendritic, ferruginous and falling very easily to the touch (as dust), the surface rugose or smooth when dry, the line of dehiscence usually carinate, but not very conspicuous, the base obtuse, the apex acute to obtuse, green or golden and ferruginous by the pubescence when fresh; pericarp 3.2–3.8 mm thick; pedicel 0.4–0.7 cm long; seed ca. 2.5 × 1.3 cm, the testa when dry whitish-greyish, markedly grooved; aril usually described as red when fresh, pale brown when dry, membranaceous, the texture dry and thin, laciniate in narrow bands distally. Germination epigeal, seedling cryptocotylar, the first pair of leaves (sub)opposite (
Distinctive characters
Virola allenii is recognised by its narrow leaf blades with lateral veins that are well separated (Fig.
Pattern of the lateral veins in Mesoamerican Virola A Virola allenii (K. Thomsen 1284, NO) B V. amistadensis (G. McPherson 8703,
Etymology
The specific epithet honours the collector of the type specimen, Paul H. Allen (1911–1963), who was probably the first person to collect this species 67 years ago (P. H. Allen 5763; 10 Jan 1951). During his five-year residency in Palmar Norte, Puntarenas, Costa Rica (
Distribution
Virola allenii is known only from Costa Rica (Puntarenas and San José) (Fig.
Preliminary conservation status
Virola allenii is Vulnerable following IUCN critera B1a and B2a. Justifying its status, it is known from seven localities and has an EOO of 3,424 km2 and an AOO of 40 km2. Specimens have been collected regularly since the 1990s during botanical expeditions in the Osa Peninsula of Costa Rica, though only 22 specimens have been verified.
Common names
None recorded.
Phenology
Flowering of Virola allenii has been recorded in January, March, April and December. Fruits have been observed in January, August to October. Pistillate flowers were not seen in the studied material.
Field characters
The bark is described as brown and smooth or as peeling in small pieces, sometimes with a strong, spicy scent. Twigs and leaves often have galls (e.g. Fig.
Discussion
Virola allenii is most similar to V. macrocarpa, a species from montane forests at 1100 m elevation in the Andes of Colombia (Boyacá) and this name has been previously applied to the species described here (e.g.
The comparison presented hereafter for Virola macrocarpa (Fig.
Based on a number of features listed in the diagnosis of V. alleni, including colour of the abaxial leaf blade, sessile trichomes, degree of separation of lateral veins and the length of the anther apiculus, V. allenii is similar to V. calophylla (Fig.
Virola calophylla A branch with leaves, showing both sides B branch with inflorescences. Virola elongata C leafy branch with inflorescences, inset fruit D inflorescences. Virola guatemalensis E, F leaf blades showing adaxial (E) and abaxial (F) surface. G young twigs and petioles. Photos by Robin Foster (A, B), from https://plantidtools.fieldmuseum.org/en/nlp; Steven Paton (C, D), Rolando Pérez (C inset), from https://stricollections.org/portal/index.php and Angela Rojas (E–G).
In Mesoamerica, Virola allenii can be confused with V. amistadensis and V. otobifolia (which are formally described as new below). All these species have lateral veins that are well spaced (Fig.
Some of the first specimens of this new species were confused with other taxa, though they differ based on their trichomes: Otoba (e.g. L. J. Poveda 887,
Comparison of Virola allenii with the morphologically most similar species.
| Character | V. allenii | V. calophylla | V. calophylloidea * | V. macrocarpa |
|---|---|---|---|---|
| Leaf blade | 16.2–29.2 × 3.2–7.3 cm; base acute or obtuse to rounded | (15–) 20–60 × 10–16 cm; base (usually) deeply cordate to truncate (obtuse) | 16–33 × 4.5–8 cm; base cordate to rounded or broadly obtuse | 20–40 × 7–11 cm; base broadly obtuse† |
| Length of staminate inflorescences | 3.5–5.5 cm | 6–30 cm | 1–4 cm | Unknown |
| Length of perianth of staminate flowers | 2–2.8 mm | 1–2.1 mm | 1.7–2 mm | Unknown |
| Filament column | 0.5–0.6 mm long; not constricted at apex | 0.2–0.6 mm long; constricted at apex | 0.7–0.8 mm long; abruptly narrowed at apex | Unknown |
| Anther length | 0.6–0.9 mm | 0.4–0.5 mm | 0.5–0.6 mm | Unknown |
| Fruit | 2.7–3.5 × 1.5–2.5 cm; base obtuse | 2.5–3 × 1.2–2.5 cm; base usually truncate | 1.8–2.1 ×0.8–1.1 cm; base obtuse | 2.7–3.3 × 2–2.3 cm; base obtuse† |
| Pericarp thickness | 3.2–3.8 mm | 0.5–5 mm | 0.5–0.8 mm | 1.8–3 mm |
Comparison of Virola allenii with the two other morphologically most similar species in Mesoamerica.
| Characters | V. allenii | V. amistadensis | V. otobifolia |
|---|---|---|---|
| Leaf blades | 16.2–29.2 × 3.2–7.3 cm | 12.3–22.8 (–27) × 4.4–9.5 (–12.5) cm | (14–) 18.2–42.5 × (4.1–) 7.3–14.2 cm |
| Lateral veins | 15–20 per side | 9–15 per side | 10–16 per side |
| Length of staminate inflorescence | 3.5–5.5 cm | 3–7.5 cm | 3.5–9.5 cm |
| Length of perianth of staminate flowers | 2–2.8 mm | 1.5–2.2 mm | 2.5–2.8 mm |
| Filament column | 0.5–0.6 mm; not constricted at apex | 0.2–0.4 mm; constricted at the apex | 0.9–1 mm; constricted at the apex |
| Anther length | 0.6–0.9 mm | 0.6–0.7 mm | 0.6–1 mm |
| Fruit | 2.7–3.5 × 1.5–2.5 cm (Fig. |
2.1–3.8 × 1.7–2 cm (Fig. |
(2.7–) 3.5–4.5 × (1.9–) 2.3–2.9 cm (Fig. |
| Pericarp thickness | 3.2–3.8 mm | 1–2 mm | (2.7–) 3–4.7 mm |
| Seed | ca. 2.5 × 1.3 cm | 1.6–2.2 × 1.4–1.6 cm | 2.5–2.8 × 1.5–1.7 cm |
Notes
The holotype, deposited at Field Museum (F), represents two sheets with hand written annotation (“Sheet 1 of 2,” “Sheet 2 of 2”), which suggests that they represent a multi-sheet specimen of the same plant (ICN Art. 8.3) (
The specimen B. Hammel et al. 24041 (
The specimens [P. H.] Allen 5763 (type; F, USJ) and [P. H.] Allen 6727 (F, GH), cited as V. guatemalensis in The Rain Forests of Golfo Dulce (Allen, 1956), correspond with this new species.
Virola calophylloidea has recently been considered synonymous with V. calophylla (e.g.
Brazil. Acre: Rio Jurua & Rio Moa, Serra da Moa, 30 Apr 1971 (♂ fl), P. J. M. Maas et al. P12659 (
Specimens examined
Costa Rica. Puntarenas: Golfito, alrededores de la estación Agujas, 300 m elev., 22 May 2000 (st), L. Acosta et al. 1389 (
Virola amistadensis , sp. nov.
Diagnosis
Similar to Virola calophylla in its abaxial leaf surface that is whitish and covered with stellate, sessile trichomes whose centre is reddish-clear to reddish and contrasting in colour with the hyaline branches and the lateral veins that are well-spaced. Virola amistadensis can be distinguished from V. calophylla by its shorter leaf blades [12.3–22.8 (–27) cm vs. (15–) 20–60 cm long] with a usually acute or sometimes attenuate base (vs. usually deeply cordate to truncate), shorter staminate inflorescence (3–7.5 cm vs. 12–30 cm long) and fruits with an obtuse base (vs. usually truncate).
Type
Panama. Bocas del Toro: Vicinity of Fortuna Dam, below pass on Chiriquí Grande road, ca. 800 m elev., 27 Jun 1986 (♀ fl), G. McPherson 9717 (holotype:
Description
Tree 4–13 m tall, no recorded DBH; bark and exudate not described in herbarium specimens. Twigs 0.18–0.42 cm thick, inconspicuous but densely strigulose, glabrescent, trichomes irregularly stellate to dendritic, ferruginous to whitish. Leaves: petiole 1.4–2.3 × 0.18–0.23 cm, slightly canaliculate, pubescent, trichomes stellate; leaf blades 12.3–22.8 (–27) × 4.4–9.5 (–12.5) cm, elliptic to widely elliptic; adaxial surface leaves pale to dark brown (sometimes shining) when dry, glabrous or occasionally with scattered stellate trichomes; abaxial surface usually whitish-greyish when dry, but can be very light to dark brown, densely but inconspicuously pubescent, trichomes stellate, sessile, ferruginous or sometimes with the central part of the trichome reddish, contrasting in colour with the hyaline branches to reddish-clear, with 4–8 branches, the branches ca. 0.02 mm, persistent (the surface with a dense layer of squamiform hyaline structures, especially obvious at high resolution); lateral veins 9–15 per side, 3–5 veins per 5 cm, (1.2–) 1.4–2.5 cm apart, the same colour as the adaxial surface, on adaxial surface flat to slightly elevated or slightly sunken, on abaxial surface raised, free or slightly anastomosing near the margin and without forming a very marked intramarginal vein; tertiary veins barely visible or indistinct on both surfaces; midvein adaxially raised, slightly sunken in a channel, abaxially raised, rounded, tomentose, adpressed pubescent to glabrate; base usually acute or sometimes attenuate, not revolute, flat; margin not revolute; apex acuminate. Staminate inflorescences 3–7.5 cm long, axillary, axes flattened, pubescent, with trichomes stellate, ferruginous; peduncle 1–2 × 0.09–0.16 cm; bracts not seen; terminal fascicles lax, with 2–11 flowers. Staminate flowers with the pedicel 0.7–1 mm long; receptacle sometimes ca. 1 mm wide; perianth 1.5–2.2 mm long, infundibuliform, brown when fresh (possibly due to indumentum), connate for ca. 0.8 mm of length, abaxial surface pubescent with brown trichomes, adaxial surface pubescent, especially on the lobes and margins; lobes 3, ca. 1.2 × 0.7 mm; stamens 3 (–6), the filament column 0.2–0.4 mm long, glabrous, straight, conspicuously thickened throughout its length, constricted at the apex; anthers 0.6–0.7 mm long; apiculus 0.1–0.15 mm long, apiculate, connate or slightly separated at the apex. Pistillate inflorescences 4.3–5 cm, similar to staminate inflorescences; peduncle 1.5–1.8 × 0.18–0.25 cm; bracts not seen; terminal fascicles of 4—10 flowers. Pistillate flowers in terminal fascicles of 4–10 flowers; with the pedicel 1.5–2 mm long; perianth 3.4–3.8 mm long, infundibuliform, brown-yellow when fresh, connate for 2.2–2.5 mm of length, abaxial surface pubescent, with brown trichomes, adaxial surface pubescent, especially on the lobes and sparsely pubescent basally; lobes 3, 1–1.4 × 1–1.2 mm; gynoecium 1.8–2 × 0.8–1.1 mm, densely pubescent, ovate, sessile; stigmatic lobes 0.4–0.5 mm, erect. Infructescence 2.7–6.3 cm long, with 1–5 fruits, peduncle 1–2.5 × 0.25–0.5 cm. Fruits 2.1–3.8 × 1.7–2 cm, ovoid to globose, shortly stipitate, densely tomentose, the trichomes irregularly stellate, ferruginous, persistent, the surface smooth to rugulate when dry, the line of dehiscence slightly carinate, the base obtuse, the apex acute to obtuse, brown to brown-yellow when fresh; pericarp 1–2 mm thick; pedicel 0.4–0.9 cm long; seed 1.6–2.2 × 1.4–1.6 cm, the testa when dry pale brown; aril described as red, pale brown or blackish when dry, membranaceous, dry in texture, thin, laciniate in narrow bands.
Distinctive characters
Virola amistadensis is recognised by its elliptical to widely elliptical leaf blades with lateral veins that are well separated (Fig.
Etymology
The specific epithet, amistadensis, refers to Parque Internacional La Amistad, a UNESCO World Heritage Site, shared between Costa Rica and Panama where the holotype and some of the paratypes of this species were collected.
Distribution
Virola amistadensis is known from Costa Rica (Limón) and Panama (Bocas del Toro and Veraguas; Fig.
Preliminary conservation status
Virola amistadensis is Endangered following IUCN criteria B1a and B2a. This species is known from 4 localities and has an EOO of 2,573 km2 and an AOO of only 28 km2. Only nine specimens were verified in this study. While it occurs in protected areas, its montane habitat is particularly prone to habitat disturbance.
Common names
None recorded.
Phenology
Flowering of V. amistadensis has been recorded in April, June and July and fruiting in January to March, May, June and December.
Field characters
Plants are trees that are 4–13 m tall. Flowers have a yellow-brown perianth and brown fruits.
Discussion
Herbarium specimens of this new species usually have been identified as Virola calophylla (Figs
In Mesoamerica, Virola amistadensis is similar to V. allenii (Figs
Comparison of Virola amistadensis with the morphologically most similar species.
| Characters | V. amistadensis | V. macrocarpa |
|---|---|---|
| Leaf blades | 12.3–22.8 (–27) × 4.4–9.5 (–12.5) cm; on abaxial side, densely but inconspicuously pubescent | 20–40 × 7–11 cm; on abaxial side, inconspicuously puberulent* |
| Lateral veins | 9–15 per side, 3–5 veins per 5 cm, (1.2–) 1.4–2.5 cm apart | 14–16 per side*, 4–5 veins per 5 cm, 0.8–1.5 cm apart |
| Petiole | 0.18–0.23 cm thick | 0.3–0.5 cm thick |
| Fruit size | 2.1–3.8 × 1.7–2 cm (Fig. |
2.7–3.3 × 2–2.3 cm* (Fig. |
| Infructescence peduncle length | 1–2.5 cm | 2.5–3 cm |
| Pedicel length in fruit | 0.4–0.9 cm | 0.3–0.5 cm* |
| Pericarp | 1–2 mm thick, with persistent trichomes | 1.8–3 mm thick, with caducous trichomes |
| Seed size | 1.6–2.2 × 1.4–1.6 cm | 2.2–2.5 × 1.5–1.7 cm* |
| Tree size | 4–13 m | 20–30 m† |
Notes
Specimens from Veraguas Province (Panama), have smaller leaf blades and lateral veins that are more deeply sunken on the adaxial surface than the specimens from Limón and Bocas del Toro provinces.
Specimens examined
Costa Rica. Limón: Parque Internacional La Amistad, subiendo por la fila entre la margen derecha del Río Uren y la Quebrada Crori, Croriña, 650 m elev., 17 Jul 1989 (♂ fl), A. Chacón 194 (
Virola chrysocarpa , sp. nov.
Diagnosis
Species similar to Virola koschnyi due to many characteristics of the leaf, including overall shape, number of lateral veins and stalked trichomes. It differs in leaf blades with pubescent adaxial surfaces that are rough to the touch in herbarium specimens (vs. adaxial surface glabrous to glabrescent and smooth) and abaxial surfaces that are hirsute to hirsutulous (vs. tomentose) with trichomes that have few (3–6 vs. 4–10), but long branches (0.2–0.6 mm vs. 0.1–0.2 mm long), staminate flowers with a longer filament column (1.3–1.5 mm vs. 0.7–0.9 [–1.4)] mm long) and fruits with an acute to apiculate apex (vs. typically obtuse).
Type
Costa Rica. Puntarenas: Golfito, Parque Nacional Corcovado, Estación Sirena, 10 m elev., 06 Feb 1994 (♂ fl), R. Aguilar 3082 (holotype:
Description
Tree
15–45 m × 25–50 cm DBH; bark sometimes described as reddish to reddish-brown. Exudate described as light red but without specifying from which part or red from the trunk. Twigs 0.18–0.28 cm thick, terete, flattened laterally to slightly angulate, hirsute tomentose, trichomes dendritic, yellowish or very pale brown. Leaves: petiole 1–1.6 (–2) × 0.15–0.28 cm, canaliculated, pubescent, the trichomes dendritic; leaf blades (17.5–) 24.2–28.8 × 7.6–10 cm, obovate to oblong; adaxial surface of mature leaves olivaceous, brown to greyish when dry, hirsute to hirsutulous, asperous (in new leaves hirsute, the trichomes dendritic-stellate, pediculate, asperous to the touch); abaxial surface similar in colour to the adaxial surface when dry, densely hirsute to hirsutulous, trichomes dendritic to dendritic-stellate, yellowish to pale brown, pediculate, with 3–6 branches, the branches 0.2–0.6 mm long, persistent; lateral veins 28–32 per side, with 5–7 (–11) veins per 5 cm, (0.5–) 0.7–1.3 cm apart, the same colour as the adaxial surface or sometimes contrasting in colour, on adaxial surface flat to slightly sunken, on abaxial surface conspicuous and raised, straight to slightly arcuate, anastomosing near the margin, forming an intramarginal vein; tertiary veins usually inconspicuous adaxially, conspicuous abaxially; midvein adaxially flat, pubescent, abaxially raised, rounded, pubescent; base usually markedly cordate, not revolute, flat; margin flat, sometimes ciliolate; apex acuminate. Staminate inflorescences 4–8.5 cm long, usually at nodes lacking leaves or, on few occasions, in the axis of leaves, axes slightly flattened, densely pubescent, the trichomes dendritic, yellowish to pale brown; peduncle 1.5–4 × 0.13–0.19 (–0.3) cm; bracts 0.5–0.8 × 0.3–0.5 cm, pubescent on both sides, caducous; terminal fascicles dense, with 15–30 + flowers. Staminate flowers with the pedicel 2.8–3.5 mm long; receptacle 2–4 mm wide; perianth 2–3 mm long, subglobose to rhomboid, yellow when fresh, connate for 0.6–1.5 mm of length, abaxial surface pubescent, with pale brown, yellowish or golden trichomes, adaxial surface with few scattered trichomes, especially on the lobes; lobes 3, 1.5–2.3 × 0.6–1.5 mm; stamens 3, the filament column 1.3–1.5 mm long, thin, not constricted at the apex; anthers 0.6 mm long; apiculus apparently absent, the apex obtuse; pollen 28 µm, with bilateral symmetry, boat shaped to elliptic grain, exine reticulate, exine structure tectate-perforate (based on
Distinctive characters
Virola chrysocarpa is distinguishable for its leaf blades with pubescent adaxial surfaces that are rough to the touch in mature leaves (at least in herbarium specimens) and abaxial surfaces that are hirsute to hirsutulous with trichomes with long branches (0.2–0.6 mm long) (Fig.
Vegetative characteristics of Virola chrysocarpa A tree showing deciduous nature of the species B trunk C buttress D branch showing new leaves E leaf blades on abaxial surface F leaf blades on abaxial surface, showing margin detail and venation G leaf apex H new branch and leaf base. Photos by Reinaldo Aguilar.
Etymology
The specific epithet, chrysocarpa, is derived from the Greek chryso (gold) and carpo (fruit). This is in reference to its common name, “fruta dorada” (golden fruit), which is used by locals of the Osa Peninsula, Costa Rica, where this species is frequent.
Distribution
Virola chrysocarpa is known from Costa Rica (Puntarenas and San José) and Panama (Chiriquí) (Fig.
Preliminary conservation status
Possible Near Threatened: This species has a small estimated AOO (60 km2), though a relatively large estimated EOO of 5,334 km2. Its eighteen known specimens represent eleven localities. This limited number of specimens warrants a Possible NT status, though additional collection efforts may demonstrate the lack of conservation threat for this poorly known species.
Common names
Costa Rica: fruta dorada. Panama: bogamani.
Phenology
Herbarium specimens of flowering Virola chrysocarpa have been collected in December to March and fruiting specimens from March to June. Herbarium specimens with pistillate flowers were not observed. In the Osa Peninsula, leaves fall completely during the dry season, which occurs in November to February (
A study of vegetative, flowering and fruiting phenology has been published by
Field characters
Plants are large trees with boles that are straight and do not begin to branch until they reach a great height, with buttresses, up to 1.6–2.5 m tall. Bark is sometimes described as finely fissured. The new leaves are lime green in colour. Twigs, petioles and leaf blades on both surfaces (especially the youngest ones) are covered with golden, brown-reddish to rusty-red trichomes. Flowers have yellow or yellow-cream perianth and anthers. Mature fruits are yellow, orange or ferruginous (possibly due to their indumentum). Seeds are brown or blackish and covered with a red to scarlet aril.
Discussion
Virola chrysocarpa resembles a morphological group of species from South America that includes V. caducifolia W. A. Rodrigues, V. decorticans Ducke, V. guggenheimii W. A. Rodrigues, V. multicostata Ducke, V. multinervia Ducke, V. polyneura W. A. Rodrigues and V. rugulosa (Spruce) Warb. These species are characterised by having leaves that are evidently pubescent, some with dendritic to irregularly dendritic pediculate trichomes on the abaxial surface and leaf blades with numerous, conspicuous and comparatively dense lateral veins; staminate flowers with anthers that are subequal to or shorter than the filament column; and fruits with thick pericarp. Additionally, these species tend to be large, sometimes deciduous trees with cordate leaf bases and staminate flowers with the anthers that are obtuse at the apex. Table
Comparison of Virola chrysocarpa with the most morphologically similar species.
| Character | V. chrysocarpa | V. caducifolia ‡ | V. decorticans ‡ | V. koschnyi | V. guggenheimii ‡ | V. multicostata ‡ | V. multinervia ‡ | V. polyneura ‡ | V. rugulosa ‡ |
|---|---|---|---|---|---|---|---|---|---|
| Petiole length | 1–1.6 (–2) cm | 0.5–0.35 cm | 0.7–2 cm | 0.5–1.5 cm | 0.5–1 (–2) cm | 0.2–0.4 cm | 0.4–1.5 cm | 1–2.5 cm | 0.8–1.1 cm† |
| Leaf size | (17.5–) 24.2–28.8 × 7.6–10 cm | 10–42 × 3.5–12.5 cm | 25–60 × 11–21 cm | 14.1–29.9 × 4.2–8.7 cm | 5–22 (–25.5) × 2–6.5 (–10) cm | 20–28 × 4–10 cm | 25–45 × 8–16 cm | 5.5–11 × 4–8.5 cm | 20–27 × 7–9.5 cm |
| Adaxial pubescence | Pubescent | Glabrous | Pubescent (pilose) | Glabrous | Pubescent (sparsely strigulose) | Glabrous or pubescent on the veins† | Glabrous | Glabrous (pubescent on the midvein) | Glabrous (pubescent on the midvein) |
| Trichomes on abaxial side | Pediculate | Sessile | Pediculate | Pediculate | Pediculate | Pediculate† | Pediculate | Pediculate | Pediculate |
| No. of lateral veins | 28–32 | 48–60 (–69) | 45–60 | (16–) 20–35 | 24–58 | 50–60 | 40–60 | 30–50 | 23–27 |
| Staminate infls. length | 4–8.5 cm | 18 cm | 22 cm | 5–11 cm | 14 cm | ca. 15 cm | 15–20 cm | 5 cm | 25 cm |
| Staminate perianth length | 2–3 mm | 1–1.4 mm | 1.5–1.8 mm | 2–2.5 mm | 1–1.5 mm | ca. 1 mm | 1.2–1.5 mm | None recorded | 1.3–1.5 mm† |
| Filament column length | 1.3–1.5 mm | 0.7–0.9 mm | 0.3–0.4 mm | 0.7–0.9 (–1.4) mm | 0.3–0.4 mm | None recorded | ca. 0.6 mm§ | None recorded | Not described |
| Anther length | 0.6 mm | 0.4 mm | 0.5–0.6 mm | 0.5–0.7 (–1) mm | 0.4–0.5 mm | None recorded | ca. 0.5 mm§ | None recorded | Not described |
| Fruit size | 2.4–2.9 × 1.7–1.8 cm | 2.5–3 × 1.3–2.5 cm | 2.7–3.5 × 1.7–2.2 cm | 1.9–3.1 × 1.5–1.9 cm | 2–2.8 × 1.5–2 cm | 2–3.5 × 1.8–2.5 cm | 2–3 × 1.5–2.5 cm | 2–2.3 × 1.5–1.8 cm | 1.5–2 × 1.5–1.7 cm |
| Pericarp thickness | 1.8–2.5 mm | 2–3 mm | Not described | 1.2–3.1 mm | 2–4 mm | 2–5 mm | 1.5–4 mm | 1–2 mm | 3 mm |
| Leaves phenology | Deciduous | Deciduous | Evergreen | Evergreen | Evergreen | Deciduous | Deciduous | Evergreen | Evergreen |
In Mesoamerica, Virola chrysocarpa resembles and has been confused with, V. koschnyi (e.g.
Notes
The illustration presented in Manual de Plantas de Costa Rica (
Specimens examined
Costa Rica. Puntarenas: Osa, Parque Nacional Corcovado, Estación San Pedrillo, 10–100 m elev., 19 Feb 1994 (♂ fl), R. Aguilar 3125 (
Virola elongata
Virola elongata (Benth.) Warb. Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 68: 178. 1897.
Myristica elongata
Benth. Hooker’s J. Bot. Kew Gard. Misc. 5: 5. 1853. Type. Brazil. “Brasilia prope Borba”, Aug. 1828, [L.] Riedel 116 or s.n. (holotype: K; isotypes: B destroyed, BM, C, G, LE, P, S; fide
Distinctive characters
Virola elongata it is recognized by its relatively small leaf blades [10.8–18.5 × 2.7–3.7 (–5.1) cm] with a sparsely pubescent abaxial surface with stellate to dendritic-stellate trichomes that are usually sessile (Fig.
Distribution
In Mesoamerica, Virola elongata is only known in Panama (Colón, Panamá and San Blas) (Fig.
Common names
None recorded in Mesoamerica.
Phenology
Specimens of Virola elongata with flowers were collected in July, October and September and with fruits in January, February, April, July, August and October. Collections with pistillate flowers were not seen.
Field characters
Plants are trees between (3–) 7–15 m tall and 0.7–12 cm DBH. Bark cuts are sometimes aromatic. Leaf blades are light green or whitish abaxially. Flowers have a yellow or yellow-orange perianth.
Selected specimens seen
Panama. Colón: Camino a la zona maderera de Santa Rita, no elev., 03 Oct 1968 (♂ fl), M. D. Correa & R. L. Dressler 1078 (
Virola fosteri , sp. nov.
Diagnosis
Species resembling Virola multiflora due its small leaf blades and fruits, similar leaf shape and inconspicuous stellate, sessile trichomes on the abaxial leaf surface. Both species also occur on the Caribbean slope of Mesoamerica. They differ in the shape of the leaf base (revolute in V. fosteri vs. not revolute in V. multiflora), the length of the filament column (0.9–1.3 mm vs. 0.7–1 mm long) and anthers (0.6–0.9 mm vs. 0.3–0.6 mm long) and thickness of the pericarp (1.5–2.5 mm vs. 0.7–1 mm thick).
Type
Panama. Bocas del Toro: Isla Colón, Aprox. a 8 km al NE de los laboratorios del Instituto Smithsonian de Investigaciones Tropicales, Big Creek, 5 m elev., 23 Apr 2009 (♂ fl), C. Galdames, M. Stapf, K. Toribio & Arsenio 6422 (holotype: PMA!* [094201, PMA92162]; isotypes:
Description
Tree
(15–) 20–35 m × 35–60 cm DBH; bark brown or reddish. Exudate described as watery-reddish possibly from the bark, damage to any part of the plant causes the flow of a watery exudate that turns reddish moments later. Twigs 0.12–0.24 cm thick, terete to slightly angulate, puberulent, trichomes stellate, yellowish to pale brown. Leaves: petiole 0.4–0.7 (–1) × 0.07–0.12 cm, canaliculate, densely tomentose to sparsely pubescent, the trichomes stellate; leaf blades 7.8–12 × 1.4–2.7 cm, narrowly elliptical or oblong to oblanceolate; adaxial surface dark brown, light brown or blackish when dry, glabrous, the surface smooth; abaxial surface pale brown to reddish-brown when dry, puberulent, trichomes stellate, sessile, yellowish to pale brown, with 4–8 branches, each branch ± 0.03–0.05 mm long, persistent; lateral veins 16–24 per side, 10–15 veins per 5 cm, 0.2–0.5 (–0.7) cm apart, the same colour as the adaxial surface, on adaxial surface sunken, on abaxial surface flat to slightly elevated, arcuate-ascending, slightly anastomosing near the margin and not forming a marked intramarginal vein; tertiary veins adaxially almost indistinct to slightly sunken, abaxially almost indistinct; midvein adaxially canaliculate, glabrous, abaxially raised, laterally compressed and sometimes resembling a cutting edge, tomentose to sparsely pubescent; base attenuate, revolute; margin revolute (especially near the base) or flat; apex acute to acuminate. Staminate inflorescences 2.5–5.3 cm long, axillary, usually in the axil of terminal leaves, axes flattened to irregularly angled, tomentose, with trichomes stellate, yellowish to pale brown; peduncle 0.9–17 × 0.13–0.25 cm; bracts 2–5 × ca. 2.5 mm, tomentose on both surfaces, the indumentum more clustered on the external side, caducous; terminal fascicles dense, with 5–15+ flowers. Staminate flower with the pedicel 1–2 mm long; receptacle 1.5–2.3 mm wide; perianth 2–2.5 (–3) mm long, subglobose, yellow, orange or yellow-orange when fresh, connate for 0.5–0.8 mm of length, abaxial pubescent, with golden to yellowish trichomes, adaxial surface glabrous somewhat pubescent near the lobes; lobes 3, 1.5–2.6 × (0.6–) 1.3–1.8 mm; stamens 3 (–6), the filament column 0.9–1.3 mm long, glabrous, straight or rarely thickened near the base in some flowers (McPherson 20148), thin, not constricted at the apex; anthers 0.6–0.9 mm long; apiculus small enough to as appear absent, acute to obtuse. Pistillate inflorescences 1.3–3.9 cm long, axillary, with trichomes on the axes similar to those of the staminate inflorescences; peduncle 0.7–2.2 × 0.08–0.14 cm; bracts not seen; terminal fascicles of 4–7 flowers. Pistillate flowers with the pedicel 1.5–2.5 mm long; perianth 2–3 mm long, subglobose, yellow when fresh, connate by 0.6–0.8 mm long, abaxial surface pubescent with golden to yellowish trichomes, adaxial surface sparsely pubescent, the indumentum on the lobes; lobes 3, 1.2–1.5 (–2.5) × 0.7–2.1 mm; gynoecium 1.6–2.4 × 1.1–1.4 mm, densely pubescent, globose to subglobose, stipitate; stigmatic lobes ca. 0.6 mm, erect. Infructescence 2.5–3 cm long, with 1–3 fruits, peduncle 1.5–1.7 × 0.15–0.38 cm. Fruits 1.5–2.3 × 1.2–1.8 cm, ovoid, sessile or very shortly stipitate, tomentose, the trichomes stellate, reddish-brown, the surface rugose when dry, the line of dehiscence canaliculate or smooth, the base obtuse to rounded, the apex obtuse, yellow, orange or golden brown when fresh; pericarp 1.5–2.5 mm thick; pedicel 0.4–0.5 cm long; seed ca. 1.6 × 0.9 cm, the testa pale brown when dry, very slightly grooved; aril usually described as red when fresh, reddish-brown when dry, coriaceous, oily, somewhat thick, laciniate in narrow bands. Germination epigeal, seedling cryptocotylar, epicotyl hairy, moderately dense, stellate and sessile (
Distinctive characters
Virola fosteri is recognised by its small leaf blades (7.8–12 × 1.4–2.7 cm) and fruits (1.5–2.3 × 1.2–1.8 cm) (Figs
Virola fosteri A lower trunk and buttresses B branch with staminate inflorescences C leaf blades showing adaxial (left) and abaxial (right) surface; also demonstrating the revolute base D branch with staminate inflorescences and leaf blades on abaxial surface E close up of staminate inflorescences F fruit G aril covering the seed H seed Photos by Rolando Pérez (A), Carmen Galdames (B, D, E), Steven Paton (C, F, G, H); all photos from https://stricollections.org/portal/index.php.
Etymology
The specific epithet honours one of its collectors, Robin B. Foster (1945–), ecologist and botanist at Field Museum in Chicago (F) who pioneered the cataloguing of the flora of Barro Colorado Island (BCI) in Panama, where V. fosteri occurs. Robin noted on one of his collections (R. B. Foster 2931) that it could represent a new species. In addition, on the same herbarium sheet, he observed one of the taxonomic characters that we here use to distinguish this as a new species: “Leaves are consistently small throughout the tree and on juvenile plants.”
Distribution
Virola fosteri is known from Costa Rica (Limón) and Panama (Bocas del Toro, Colón, Panamá, San Blas and Veraguas) (Fig.
Preliminary conservation status
Virola fosteri is Vulnerable following IUCN criterion B2a. While the EOO for this species is large (25,645 km2), the small AOO (40 km2) with only eight known localities warrants its conservative status.
Common names
Panama: bogamani, fruta dorada.
Phenology
Flowering of Virola fosteri has been recorded in January to April, June and October and production of fruits in January to April.
Field characters
Plants are large trees with tall buttresses. Bark exudes reddish watery exudate when damaged. Their small leaves are white or grey below. Flowers have pale orange or yellow perianth. The mature fruit is yellowish or golden brown with a red aril and brown seed.
Discussion
In addition to the characteristics presented in diagnosis, Virola fosteri tends to have a higher number of and sunken (vs. plane) lateral veins per side than V. multiflora (16–24 vs. 10–18 per side), denser trichomes with longer branches on the abaxial leaf surface (Fig.
It is also comparable to V. micrantha A. C. Sm. from Colombia due to the similar size of the leaf blades, which also have sessile stellate trichomes on the abaxial surface and short staminate inflorescences. Virola micrantha is a name apparently ignored in recent publications (
Virola coelhoi W. A. Rodrigues (Colombia; S. Defler 411,
In Mesoamerica, other species with leaf blades that are covered with stellate and sessile trichomes on the abaxial surface (Fig.
Notes
The species referred to as Virola sp. B in the Manual de Plantas de Costa Rica (
Specimens examined
Costa Rica. Limón: Talamanca. San Miguel, Asacode, sendero a San Miguel, 30–100 m elev., 18 Jan 1997 (♂ fl), J. González et al. 1632 (
Virola guatemalensis
Virola guatemalensis (Hemsl.) Warb. Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 68: 220. 1897.
Myristica guatemalensis Hemsl. Biol. Cent.-Amer., Bot. 3. 66–67. 1882. Type. Guatemala. [no specific data in location], [no date], [seeds], [G. U.] Skinner s.n. (holotype: K!*).
Distinctive characters
Virola guatemalensis is distinguished by many characters of its leaf, including overall size [12.3–17.5 (–24.1) × (2.4–) 3.8–5.5 (–8.9) cm], inconspicuous pubescence of tiny stellate and sessile trichomes on abaxial surfaces (Fig.
Distribution
Virola guatemalensis is known from Mexico (Chiapas, Oaxaca and Veracruz), Guatemala (Sololá) and Honduras (Yoro) (Fig.
Common names
Mexico: cacao, cacao volador (Chiapas), cacaotillo, cedrillo (Veracruz), k’ik’ che’ [Lacandon name]. Guatemala: chucul, palo de sebo, cacao volador, cacao cimarrón. Honduras: sangre.
Phenology
Flowering of Virola guatemalensis has been recorded in April and May and fruit production in January, March, August, October and December.
Field characters
Plants are trees between 12–35 m high and 45–130 cm DBH with a straight trunk, sometimes with moderately sized buttresses. The bark is variously described as smooth or fissured and scaly and is brown to greyish-brown in colour and exudes watery reddish transparent sap when damaged. Flowers have yellow, green-yellowish or brown perianth. The mature fruit is yellow with a red aril.
Discussion
While the name Virola guatemalensis has been applied to herbarium specimens from Costa Rica and Panama in the past, those are here interpreted as a distinct species, V. montana. Based on our interpretation, V. guatemalensis is restricted to Mexico, Guatemala and Honduras. It is distinguished from V. montana by a series of characters, described below. While V. laevigata was typified with material from the Pacific slope in Chiriquí, Panama and is frequently considered a synonym of V. guatemalensis (e.g.
Notes
The specimens, identified in
The specimen J. A. Steyermark 47624 (
Virola guatemalensis, along with another Mesoamerican species, V. koschnyi (see below), produce the largest grains of pollen in Virola (
Selected specimens seen
Mexico. Chiapas: Tila, Chewupaj, 1000 m elev., 10 Dec 1982 (fr), A. Méndez 5223 (
Virola koschnyi
Virola koschnyi
Warb. Repert. Spec. Nov. Regni Veg. 1: 71. 1905. Type. Costarica [Costa Rica]. [Alajuela] San Carlos, Th. Koschny s.n. (lectotype, here designated: F!*, fragment with B photo [649050, F0360191F]); Costa Rica. Heredia. [Sarapiqui] Parque Nacional Braulio Carrillo, bosque primario frente al Puesto La Ceiba, 450 m elev., 23 Dec 1988 (♂ fl), M. Ballestero 72 (epitype, designated here:
Virola merendonis Pittier., Contr. U.S. Natl. Herb. 20: 453. 1922. Type. [Honduras]. [Copán:] Collected in the forests of Cuchillitas, between Arranca Barba Hills and Mohanes, in the Cordillera de Merendon, borders of Guatemala and Honduras, in fruit, [10–] 18 May 1919 [fr], H. Pittier 8530 (holotype: US!*; isotype: NY!*).
Virola costaricensis nomen nudum
Distinctive characters
Virola koschnyi can be recognised by the densely tomentose leaf undersides with pediculate, dendritic trichomes (Fig.
Virola koschnyi A lower trunk and buttresses B trunk C branching D leaf blades on abaxial surface E leaf blades on adaxial surface F twig, petiole and leaf base G leaf venation on adaxial surface (left) and abaxial surface (right) H branch with fruits, showing the interior part of the fruit I seed J mature fruit. All photos by Reinaldo Aguilar from https://sura.ots.ac.cr/florula4/index.php, except G by D. Santamaría-Aguilar.
Distribution
Virola koschnyi is known from Mexico (Chiapas), Guatemala (Izabal and Petén), Belize (Cayo, Toledo and Stann Creek), Honduras (Atlántida, Cortés, Gracias a Dios and Olancho), Nicaragua (Atlántico Norte, Atlántico Sur, Jinotega and Río San Juan), Costa Rica (Alajuela, Guanacaste, Heredia, San José and Limón), and Panamá (Bocas del Toro) (Fig.
We consider this species restricted to Mesoamerica. The collections identified as V. koschnyi from Ecuador in Catalogue of the Vascular Plants of Ecuador (i.e. C. H. Dodson et al. 6465 [RPSC, SEL];
Common names
Guatemala: Cedrillo, drago, sangre. Belize: banak, black banak. Honduras: bának, sebo, sangre de montaña, sangre real. Nicaragua: banak blanco, banak colorado, cebo, fruta dorada, sebo. Costa Rica: achiotillo, fruta dorada.
Phenology
Flowering of Virola koschnyi has been recorded in January, March and May, July, September, October and December. Only three herbarium specimens with pistillate flowers were seen, two from Costa Rica and one from Nicaragua. Fruits are produced throughout the year, though most often collected in March, May and June.
Field characters
Plants are trees (4–) 10–70 m tall and 12–80 cm DBH, with a trunk that is straight with well-developed buttresses. The bark is described as scaly and falling off in small plates or as smooth, red-brown or whitish in colour; clear, red or reddish exudate is released when damaged. Flowers usually have yellow perianth, though it can sometimes be white, brown, or orange. The mature fruit is yellow, orange or brown, with a red or pink-red aril. The testa of the seed is black, brown or white.
Typification
Virola koschnyi was described by Otto Warburg based on a collection made by Theodor Koschny (
The only known original material of Virola koschnyi is a fragmentary specimen accompanied with a photograph at the Field Museum. The photo shows the original specimen was composed of three fertile branches with leaves (two with inflorescences and one with fruit), with a handwritten annotation of “V. costaricensis Warb”, presumably by Warburg, though this name was never validly published. The fragment material consists of pieces of leaves, two inflorescence branches with a few immature flowers and a broken fruit comprising pericarp, testa and seed. Since they are mounted with the photo of the Berlin specimen, these fragments presumably originated from the holotype. Although it is not ideal type material due to its fragmentary nature, the specimen at F appears to be the only extant original material and is sufficient to confirm that it coincides with the protologue and concept of the species used and so is here designated as lectotype. In order to ensure the precise application of the name, given the fragmentary type material, an epitype was selected from the studied specimens.
Notes
Most of the specimens from the Pacific slope of Costa Rica and Panama previously identified as V. koschnyi are here interpreted as V. chrysocarpa. The similarities and differences between V. koschnyi and V. chrysocarpa are discussed under the latter species. While most fruiting specimens of V. koschnyi have obtuse to rounded apices, G. Herrera 1092 (
Selected specimens seen
Mexico. Chiapas: Ocosingo, En ejido Chaju, 150 m elev., 17 Mar 1993 (fl), E. Martínez & C. H. Ramos 26336 (
Virola laevigata
Virola laevigata Standl. Publ. Field Mus. Nat. Hist., Bot. Ser. 4(8): 209. 1929. Type. Panama. Province of Chiriquí, Progreso, [July–Aug.] 1927 [♂ fl], G. P. Cooper & G. M. Slater 308 (holotype: F!*; isotypes: NY!*, WIS!*, US!*).
Distinctive characters
Virola laevigata is distinguished by its glabrous or nearly glabrous vegetative parts (i.e. twigs, mature leaf blades on both surfaces [Fig.
Distribution
Virola laevigata is known from Costa Rica (Puntarenas and San José) and Panama (Chiriquí) (Fig.
Common names
Costa Rica: fruta dorada. Panama: bogamani.
Phenology
Flowering of Virola laevigata has been recorded in January, May, July and November. Fruits are produced from December to February. Pistillate flowers were not present on herbarium sheets studied.
Virola laevigata A branch with staminate inflorescences B leaf blades on abaxial surface, inset showing the glabrous midvein C twig, petiole and leaf on adaxial surface D close up of staminate inflorescences E leaf base F leaf apex, inset showing leaf punctations G branch with immature fruits H close-up of immature fruit I open fruit, showing the aril. Photos by Reinaldo Aguilar.
Field characters
Plants are trees 9–40 m tall and 35–60 cm DBH with a straight trunk and small (ca. 20 cm tall), triangular buttresses. The bark is described as finally grooved, smooth, flaking in vertical strips or scaly and is grey, blackish or reddish in colour, with exudate that is reddish or colourless and oxidising to reddish-cream. The leaves are bright green on both sides and have numerous pellucid dots that are most visible against the light. Flowers have yellow, yellow-brown or yellowish perianth, sometimes with a slight aroma in staminate flowers (N. Zamora & T. D. Pennington 1583, but the specimen label states pistillate flower). The mature fruit is yellow with a red aril (when immature, it is white). In the Osa Peninsula, where this species is frequent, it prefers riparian habitats.
Discussion
Virola laevigata has traditionally been considered a synonym of V. guatemalensis (e.g.
In addition to Virola guatemalensis, herbarium specimens of V. laevigata have been determined as V. surinamensis (interpreted here as V. nobilis). However, V. laevigata is distinguished by its glabrous or almost glabrous abaxial leaf surface (Fig.
Notes
The seedlings of V. laevigata are described by
Selected specimens seen
Costa Rica. Puntarenas: Golfito, 1 km antes de llegar a La Palma, 8 m elev., 16 Jan 1993 (fr), R. Aguilar 1585 (
Virola megacarpa
Virola megacarpa
A. H. Gentry. Ann. Missouri Bot. Gard. 62(2): 474. 1975. Type. Panama. Colón: Santa Rita Ridge, 23 Mar 1972 [fr], [A. H.] Gentry & [J. D.] Dwyer 4804 (holotype:
Distinctive characters
Virola megacarpa can be recognised by its large and oblong leaf blades (20.3–37 × 7–13 cm) with numerous [(32–) 40–50 per side], dense lateral veins and a densely pubescent abaxial surface with dark brown to ferruginous dendritic trichomes (Fig.
Distribution
Virola megacarpa is only known from Panama (Colón, Panamá, San Blas and Veraguas) (Fig.
This species is attributed to Colombia in
A. Virola megacarpa A branch with fruits, both leaf blade surfaces shown B fruit. Virola nobilis from Barro Colorado C lower trunk and buttress D branch with fruits, leaf blades on both surfaces E pistillate flowers F staminate flower and bud G fruits. Photos by Carmen Galdames (A, B), Rolando Pérez (C, D, G), Steven Paton (E, F); all photos from https://stricollections.org/portal/index.php.
Common names
None recorded.
Phenology
The only observed herbarium specimen with flowers (these staminate) was collected in August. Fruits were collected in February and March and August to November.
Field characters
Plants are trees 12–30 m tall and 21.5–53 cm DBH. Damaged bark releases exudate that is red or that oxidises reddish-brown. Flowers have pale yellow perianth. Fruits are densely pubescent with brown trichomes and a red aril.
Discussion
Vegetatively, Virola megacarpa can be confused with V. koschnyi and some specimens have been identified as the latter (e.g. G. de Nevers & H. Herrera 7917,
Specimens examined
Panama. Colón: East of Portobelo, 50–100 m elev., 12 Oct 1992 (fr), G. McPherson & M. Richardson 15873 (
Virola montana , sp. nov.
Diagnosis
Species most similar to Virola guatemalensis, from which it differs by the mostly caducous (vs. persistent) trichomes on the abaxial leaf surface that have 3–10 branches and are 0.2–0.6 mm long (vs. 3–6 branches 0.05–0.1 mm long), staminate flowers with a shorter filament column (0.6–0.9 mm vs. 1–1.2 mm long) and fruits with a thicker pericarp (3.2–5 mm vs. 0.4–1 [–2.5] mm thick).
Type
Costa Rica. Cartago: Jiménez, Taus de Pejibaye, 900 m elev., 06 Apr 1994 (♂ fl), E. Lépiz & J. F. Morales 284 (holotype:
Description
Tree 6–35 m × (4–) 10–50 cm DBH; bark not described. Exudate described on one occasion as orange in bark, branches and fruits. Twigs 0.13–0.4 cm thick, angulate to lightly compressed, densely tomentose to puberulent, trichomes dendritic, yellowish, brownish to ferruginous. Leaves: petiole 0.5–1.2 × 0.16–0.3 cm, canaliculate, densely to sparsely pubescent, the trichomes dendritic to irregularly stellate; leaf blades (11.2–) 15–30.5 × (3.9–) 4.5–7.4 cm, oblong-elliptic; adaxial surface on mature leaf blades dark brown when dry, reddish-brown or greyish-blackish, glabrous or with trichomes very sparse and scattered, the surface smooth; abaxial surface pale brown, dark brown or whitish-greyish when dry, sparsely pubescent, but usually densely to sparsely pubescent along the lateral veins and midvein (in new leaves, blades with a dense layer of trichomes that fall readily when touched, covering the entire surface), trichomes dendritic or rarely irregularly stellate, sessile, yellowish to pale brown, with 3–10 branches 0.2–0.6 mm long, caducous; lateral veins (15–) 18–30 per side, (4–) 6–9 veins per 5 cm, 0.5–0.9 (–1.5) cm apart, the same colour as the adaxial surface or sometimes lighter, flat or very slightly sunken on adaxial surface, raised on abaxial surface, straight to slightly arcuate (especially towards the distal part), slightly anastomosing near the margin and without forming a very marked intramarginal vein; tertiary veins usually visible on both surfaces; midvein adaxially flat to slightly canaliculate, glabrous or with scattered trichomes, sometimes densely pubescent at the base, abaxially raised, rounded, densely tomentose (with trichomes that fall very easily to the touch) to glabrescent; base acute to rounded, not revolute, flat; margin flat; apex acute to acuminate. Staminate inflorescences 3–9 cm long, axillary either at the junction with a leaf or at leafless nodes, axes slightly flattened to irregularly angled, densely tomentose, with trichomes dendritic, brown to yellowish-brown; peduncle 1.7–3.5 × 0.05–0.12 cm; bracts 3–7 × 1.9–4 cm, pubescent on both sides, caducous; terminal fascicles dense, with 7–20 + flowers. Staminate flowers with the pedicel 1.7–3.4 mm long; receptacle 1.5–3 mm wide; ; perianth (1.6–) 2–2.7 mm, subglobose, yellow, greenish-white or brown, possibly by the indumentum), connate by (0.2–) 0.5–0.8 mm long, abaxial surface pubescent, with brown to ferruginous trichomes, adaxial surface glabrous at the base, sparsely pubescent on the lobes; lobes 3, 1.5–2 × 1–1.4 (–1.8) mm; stamens 3 (–6), the filament column 0.6–0.9 mm long, straight or sometimes slightly thickened at the base and somewhat narrow at the apex, thin, not constricted at the apex; anthers 0.5–0.8 mm long; apiculus ca. 0.07–0.1 mm, inconspicuously apiculate. Pistillate inflorescences 3.5 cm long, at leafless nodes, with trichomes on the axes similar to those of the staminate inflorescences; peduncle 1.8–2.5 × 1–2 cm; bracts not seen; terminal fascicles of 5–6 flowers. Pistillate flowers with the pedicel 3.5–4 mm long; perianth 3.5–4.6 mm long, globose to subglobose, pale brown when fresh (possibly by the indument), connate by 1–1.5 mm long, abaxial surface pubescent, with brown trichomes, adaxial surface sparsely pubescent; lobes 3, 2.5–3.5 × 1.5–2.2 mm; gynoecium 2–2.7 × 1.5–1.6 mm, densely pubescent, subglobose, stipitate; stigmatic lobes ca. 0.3 mm, erect. Infructescence 3–6.2 cm long, with 1 (–2) fruits, peduncle 2–3.3 × 0.19–0.3 cm. Fruits (2.8–) 3–3.6 × 2–2.5 cm, ovoid-ellipsoid, sessile, densely tomentose to tomentulose, the trichomes dendritic to irregularly stellate, pale brown to ferruginous, the surface commonly rugulose or smooth when dry, the line of dehiscence carinate, the base obtuse, the apex acute, orange, golden or yellowish-brown when fresh; pericarp 3.2–5 mm thick; pedicel 0.7–1 cm long; seed 2.2–2.5 × ca. 1.5 cm, the testa dark brown, almost smooth; aril usually described as red when fresh, yellowish- or reddish-brown when dry, coriaceous, oily in texture, thick, laciniate in narrow bands distally.
Distinctive characters
Virola montana is recognised by twigs, new leaf undersurfaces, petioles and inflorescences covered in indument of dendritic to irregularly stellate, caducous trichomes, with long branches; on the underside of the leaf, this indument is mainly found on the midvein and the lateral veins (Fig.
Etymology
The specific epithet refers to the montane habitat where the species has been collected.
Distribution
Virola montana is known from Costa Rica and Panama, where it has been collected on the Caribbean slope in the provinces of Alajuela, Cartago, Guanacaste, Heredia and Limón in Costa Rica and Bocas del Toro in Panama; it has only been collected on the Pacific slope in Puntarenas (Costa Rica) (Fig.
Preliminary conservation status
Virola montana is of Least Concern following IUCN guidelines. It has both a large EOO (14,606 km2) and AOO (104 km2) and is known from nineteen localities.
Common names
Costa Rica: fruta dorada.
Phenology
Flowering of Virola montana has been recorded in January, March to May, November and December; only two herbarium specimens with pistillate flowers were seen. Fruits have been collected in March and June to December.
Field characters
Leaf blades are lustrous, dark green above and whitish or silver below. Flowers have yellow, cream, greenish-white or brown perianth. Mature fruits are yellow, pale brown, brown yellow or orange. The seed is brown with a red aril.
Discussion
As far as we know, the first specimen of this species was collected 115 years ago by Henri F. Pittier (1857–1950) in the mountains of El Rosario de Orosi, Cartago, Costa Rica (H. Pittier 16628, NY-2 sheets!*). Paul C. Standley treated this specimen as V. koschnyi in “Flora of Costa Rica” (
| Character | V. montana | V. guatemalensis |
|---|---|---|
| Leaf blades size | (11.2–) 15–30.5 × (3.9–) 4.5–7.4 cm | 12.3–17.5 (–24.1) × (2.4–) 3.8–5.5 (–8.9) cm |
| Indument and trichomes on abaxial surface | Sparsely pubescent, but usually densely to sparsely pubescent primarily along the lateral veins and midvein; trichomes with 3–10 branches, the branches 0.2–0.6 mm long, caducous (Fig. |
Puberulent, trichomes over the entire surface; trichomes with 3–6 branches, the branches 0.05–0.1 mm long, persistent (Fig. |
| Lateral veins | (15–) 18–30 per side, on abaxial side raised (Figs |
13–21 per side, on abaxial side slightly raised or flat (Figs |
| Filament column | 0.6–0.9 mm long | 1–1.2 mm long |
| Pericarp thickness and presence of a carina | 3.2–5 mm thick, carinate | 0.4–1 (–2.5) mm thick, smooth or slightly carinate |
In addition to Virola guatemalensis, herbarium specimens of this new species have been determined as three other species: V. koschnyi, V. sebifera and V. surinamensis (here treated as V. nobilis). Vegetatively, V. montana can be distinguished from these species by its mature leaves that are abaxially sparsely pubescent (vs. covering the entire surface of the leaf blades abaxially). The first two species are distinguished by pediculate trichomes on abaxial surface of leaf blades (vs. sessile in V. montana, these primarily present in young leaves). Finally, V. nobilis has trichomes with short branches [0.05–0.1 mm (Fig.
Based on the number of herbarium specimens collected, Virola montana is the most common montane species of Virola in southern Mesoamerica and it is usually the only species where it occurs. However, three fruiting specimens of Virola sp., represented by R. Aguilar et al. 4327 (
Virola montana A juvenile tree showing branching pattern B leaf blades on adaxial side C leaf blades on abaxial side D venation. Virola multiflora. E Branch with staminate inflorescences, inset showing twig and flowers F twig, leaf blade on abaxial surface and inflorescences, inset showing immature fruit G lateral view of a perianth H close-up of staminate flowers. Photos by J. Esteban Jiménez (A–D); B. Hammel (E–H), Indiana Coronado (F, inset).
Specimens examined
Costa Rica. Alajuela: Reserva Biológica Monteverde, Río Peñas Blancas, parcela de Badilla, 800 m elev., 23 Oct 1988 (fr), E. Bello 470 (
Virola multiflora
Virola multiflora (Standl.) A. C. Sm. Brittonia 2: 499. 1938.
Dialyanthera multiflora
Standl. Publ. Field Mus. Nat. Hist., Bot. Ser. 8: 12. 1930. Type. [Belize] British Honduras. In jungle, Stann Creek Railway, alt. 30 m, 16 Jul 1929 [♂ fl], W. A. Schipp 279 (holotype: F!*; isotypes: A!*, BM!*, G-2 sheets!*, GH!*, K!*,
Virola brachycarpa Standl. Publ. Field Mus. Nat. Hist., Bot. Ser. 11: 131. 1932. Type. [Belize] British Honduras. Stann Creek Valley, 13 Jan 1932 [fr], J. A. Burns 20 (holotype: F!*; isotypes: BKL!*, G!*, US!*, WIS!*).
Distinctive characters
Virola multiflora is recognised by its usually small and narrow leaf blades [5.5–15.5 × 1.5–3.6 (–4.8) cm] with an inconspicuously pubescent abaxial surface with stellate and sessile trichomes (Fig.
Distribution
Virola multiflora is known from Belize (Cayo, Stann Creek and Toledo), Honduras (Gracias a Dios), Nicaragua (Atlántico Norte, Atlántico Sur, Jinotega, Matagalpa and Río San Juan) and Costa Rica (Alajuela, Cartago, Heredia and Limón) (Fig.
While V. multiflora is not documented from Guatemala in herbaria,
Common names
Belize: banak, bastard banak. Honduras: asang banak, bának, banak almuk, báhanak luhpia, sangre, sebo álmut, sebo negro. Nicaragua: conchillo, samo. Costa Rica: fruta dorada.
Phenology
Flowering of Virola multiflora has been recorded in March to August, with a noted peak in July; just one herbarium specimen with pistillate flowers was seen and it is from Nicaragua. Fruits were collected in December through April.
Field characters
Plants are trees between 6–30 m tall and 17–35 cm DBH. Bark exudes latex red. The leaf blades are sometimes whitish abaxially. The flowers usually have yellow, golden or orange perianth. The mature fruit is yellow or orange with a red aril.
Notes
We were not able to locate any Panamanian specimens of V. multiflora: all fertile Panamanian specimens identified as V. multiflora that the first author has studied are actually V. fosteri. However, V. multiflora is to be expected in Panama because it occurs in physiognomically similar forests in Costa Rica near the border. The similarities and differences between V. multiflora and V. fosteri (which is formally described above) are discussed under the latter species.
Selected specimens seen
Belize. Cayo: Hummingbird Highway south of Belmopan, 200–300 ft [60–90 m] elev., 26 Jun 1973 (♂ fl), A. Gentry 8615 (
Virola nobilis
Virola nobilis
A. C. Sm. Brittonia 2: 490. 1938. Type. Panama. Canal Zone, Barro Colorado Island, 07 Jan. 1932 [imm fr], [R. H.] Wetmore, [E. C.] Abbe & [O. E.] Shattuck 155 (holotype: GH!*; isotypes: A!*, F!*,
Distinctive characters
Virola nobilis is recognised by its narrow, oblong leaf blades (9–17.2 [–27.5] × 2.5–5 [–4.7–7.1] cm) with numerous lateral veins (20–30 [25–32] per side) corresponding to 8–11 (5–7 [–9]) veins per 5 cm, as well the stellate, tertiary veins that are slightly sunken above (Fig.
Distribution
Virola nobilis is known from the Pacific slope of Costa Rica (Puntarenas, San José) and the Caribbean slope of Panama (Bocas del Toro, Colón, Panamá, San Blas). It is recorded from 0–400 (–1300) m elevation (Fig.
Common names
Costa Rica: Fruta dorada. Panama: bogamani, coton cuinur gia, sabdurgia (Kuna name).
Phenology
Flowers have been collected in January to April, July, August, November and December and fruits in almost all months except October.
Field characters
Plants are large trees between (5–) 15–40 m tall and 20–70 cm DBH. The trunk is straight, usually with conspicuous buttresses and does not begin to branch until it reaches a great height. The bark is reddish and releases red exudate when damaged. The leaves are whitish with inconspicuous trichomes on the abaxial surface. Flowers have yellow perianth. The mature fruit is yellow to orange with a red aril.
Discussion
This species has usually been treated, identified and included as a synonym of Virola surinamensis (e.g.
Virola otobifolia A branch with leaf blades showing both surfaces and inflorescences B leaf blade, venation and base on abaxial surface C infructescence D fruits E detail of fruit, showing an aril of an immature fruit. Virola surinamensis. F Branch with inflorescences G, H leaf blade on adaxial (G) and abaxial surface (H). I Infructescence J detail of fruits, including a longitudinal section of an immature fruit. Photos by Rolando Pérez (A, B from https://stricollections.org/portal/index.php); Jerry Harrison (C), Alwyn H. Gentry (D, E from http://www.tropicos.org) and John P. Janovec (F–J from http://atrium.andesamazon.org/).
Notes
Virola nobilis exhibits complex variation that requires more fieldwork, ideally in combination with molecular phylogenetic analysis, to clarify species boundaries. For example, specimens from the Pacific slope in Costa Rica (see specimens cited below and Figs
Comparisons of Virola surinamensis with collections of V. nobilis A V. surinamensis (S. Mori & R. Cardoso 17467, NY; inset showing fruits from W. E. Broadway 5264a,
Virola cf. nobilis from the Osa Peninsula, Costa Rica A trunk B branch with fruits C leaf blades on adaxial surface D twig, petiole and leaf base on adaxial surface E exudate on a twig F, G leaf blades on abaxial surface and trichomes (G). H Leaf margin I leaf apex J staminate inflorescences K detail of staminate inflorescences L detail of staminate flower M branch with fruits N fruits O seed. Photos by Reinaldo Aguilar.
Other material that potentially belongs to V. nobilis includes a few collections from Panama, including both infertile and fruting specimens from El Llano Cartí and others from Cerro Jefe (e.g. G. de Nevers & H. Herrera 4333, imm fr,
Selected specimens
Costa Rica. Puntarenas: Golfito, camino a Piro, finca de Adrian, 50 m elev., 16 Apr 2005 (fr), R. Aguilar & X. Cornejo 9739 (
Virola otobifolia , sp. nov.
Diagnosis
Species most similar to Virola macrocarpa in having leaf blades that are discolorous abaxially with stellate, sessile trichomes with a reddish central portion and similar number of lateral veins (10–16) that are not densely spaced. However, it differs in its leaves with dense, but inconspicuously pubescent leaf undersides (vs. inconspicuously puberulent) with 2–4 veins per 5 cm that are 1.7–3 cm apart (vs. 4–5 veins per 5 cm, 0.8–1.5 cm apart) and fruits that are (2.7–) 3.5–4.5 × (1.9–) 2.3–2.9 cm, densely tomentose with persistent trichomes, a rugose surface when dry and a line of dehiscence that is conspicuously carinate (vs. 2.7–3.3 × 2–2.3 cm, tomentellous, with caducous, dust-like trichomes, a smooth surface when dry, the line of dehiscence slightly carinate), as well as the production of thicker pericarp [(2.7–) 3–4.7 mm vs. 1.8–3 mm thick].
Type
Panama. [Panamá: Chepo], El Llano Cartí road 6.9 km N of Panamerican Highway, 350–500 m elev., 23 Jan 1977 (fr), J. P. Folsom 1440 (holotype:
Description
Tree 6–30 m × 24.9–37.3 cm DBH; bark not described. Exudate from the trunk sometimes described as red, watery-red, or black-reddish. Twigs 0.23–0.34 cm, terete to slightly flattened, glabrescent to puberulent, trichomes stellate to irregularly stellate, ferruginous to greyish. Leaves: petiole (1–) 1.3–2 × 0.19–0.44 cm, flat to very slightly canaliculate, tomentose, the trichomes stellate; leaf blades (14–) 18.2–42.5 × (4.1–) 7.3–14.2 cm, oblong to elliptic; adaxial surface of mature leaf blades brown (sometimes shining) when dry, glabrous or sometimes with scattered stellate trichomes (especially along the veins), the surface smooth; abaxial surface when drying whitish-greyish or sometimes very light brown, dense but inconspicuously pubescent, trichomes stellate, sessile, the central part of the trichome usually reddish, contrasting in colour with the hyaline branches to reddish-clear, with 4–11 branches, the branches ± 0.01–0.05 mm, persistent; lateral veins 10–16 per side, 2–4 veins per 5 cm, 1.7–3 cm apart, the same colour as the adaxial surface, on adaxial surface flat to slightly sunken, on abaxial surface slightly elevated, arcuate, slightly anastomosing near the margin and without forming a very marked intramarginal vein; tertiary veins barely visible on both surfaces; midvein adaxially elevated, but submerged in a channel, abaxially raised, triangular to rounded, tomentose; base acute to obtuse, sometimes slightly cordate, not revolute, flat; margin flat to slightly revolute; apex acuminate. Staminate inflorescences 3.5–9.5 cm long, axillary either at a leaf or at a leafless node, axes flattened or irregularly flattened, tomentose, with trichomes irregularly stellate to irregularly dendritic, brown to ferruginous; peduncle 1.4–2.2 × 0.22–0.34 cm long; bracts not seen; terminal fascicles lax, with 5–13 flowers. Staminate flower with the pedicel 1.5–2 mm long; receptacle 0.7–1.5 mm wide; ; perianth 2.5–2.8 mm long, infundibuliform, yellow or brown when fresh, connate by 0.9–1.5 mm long, abaxial surface pubescent with brown trichomes, adaxial surface glabrous or with few trichomes close to the base; lobes 3 (4), 1.2–1.5 × 0.9–1.1 mm; stamens 3 (–6), the filament column 0.9–1 mm long, straight and very thickened throughout its length, fleshy, constricted at the apex; anthers 0.6–1 mm long; apiculus ca. 0.1 mm long, acute to apiculate, connate or slightly separate. Pistillate inflorescences 3.2–4 cm long (immature), axillary, axes flattened or irregularly flattened, tomentose, with trichomes stellate, brown to ferruginous; peduncle 0.6–1.2 × ca. 3.2 cm long; bracts not seen. Pistillate flowers not seen. Infructescence 3.2–5 cm long, with (1–) 2–4 fruits, peduncle 1–3.5 × 0.35–0.53 cm. Fruits (2.7–) 3.5–4.5 × (1.9–) 2.3–2.9 cm, ellipsoid, shortly stipitate, densely tomentose, the trichomes stellate, ferruginous, persistent, the surface rugose when dry, the line of dehiscence conspicuously carinate, the base obtuse to subtruncate, the apex acute to acuminate, brown (possibly by pubescence), although presumably green when fresh; pericarp (2.7–) 3–4.7 mm thick; pedicel 0.5–0.8 (–1) cm long; seed 2.5–2.8 × 1.5–1.7 cm, the testa dark brown when dry, markedly grooved; aril described as red when fresh, brown to blackish when dry, membranaceous, with a dry texture, thin, laciniate in narrow bands distally.
Distinctive characters
Virola otobifolia is recognised by its large leaf blades with well-spaced lateral veins (Fig.
Etymology
The specific epithet refers to the similarity of the leaf blades with Otoba, another member of Myristicaceae. This epithet was, in part, inspired by the fact that some of the first collections of this new species were initially confused with this genus (as Dialyanthera Warb.; e.g. A. Gentry & S. Mori 14199,
Distribution
Virola otobifolia is only known in Panama (Colón, Panamá and San Blas), where it is found on the Caribbean slope (Fig.
Preliminary conservation status
Virola obtobifolia is Endangered following IUCN criteria B1a and B2a. Justifying this status, it has both a small EOO (3,269 km2) and AOO (36 km2) and is known from only five localities.
Common names
Panama: velario, miguelario; cuinur burwi, putmas (Kuna).
Phenology
Virola otobifolia has been recorded with flowers and fruits in February to April and one collection with fruits was made in October.
Field characters
Exudate is slow to appear and is watery and red-black. Leaf blades are whitish abaxially. Flowers have yellow perianth. Fruits are green, but appearing brown (possibly due to pubescence).
Discussion
Specimens of Virola otobifolia have been confused with and identified as V. macrocarpa (e.g.
| Characters | V. otobifolia | V. macrocarpa |
|---|---|---|
| Leaf blades | (14–) 18.2–42.5 × (4.1–) 7.3–14.2 cm; dense, but inconspicuously pubescent on abaxial side | 20–40 × 7–11 cm; inconspicuously puberulent on abaxial side* |
| Lateral veins | 10–16 per side, 2–4 veins per 5 cm, 1.7–3 cm apart | 14–16 per side*, 4–5 veins per 5 cm, 0.8–1.5 cm apart |
| Petiole | (1–) 1.3–2 × 0.19–0.44 cm | 1.5–2.3* × 0.18–0.23 cm |
| Fruit | (2.7–) 3.5–4.5 × (1.9–) 2.3–2.9 cm; densely tomentose, with persistent trichomes; the surface rugose when dry, the line of dehiscence conspicuously carinate. | 2.7–3.3 × 2–2.3 cm*; tomentellous, with cauducous trichomes that are dust-like when they fall; the surface smooth when dry, the line of dehiscence slightly carinate. |
| Pericarp | (2.7–) 3–4.7 mm thick | 1.8–3 mm thick |
| Habitat | Lowland or premontane rain forest, Central Panama (Panamá, San Blas) at 50–850 m elevation | Montane forests at high elevations Andes of Colombia (Boyacá) at 1100 m elevation* |
Two similar species from Mesoamerica that resemble Virola otobifolia are: V. allenii (Figs
Notes
The holotype, deposited at Missouri Botanical Garden (
Specimens examined
Panama. Colón: Teck Cominco Petaquilla, 200 m elev., 21 Feb 2008 (fl bud), G. McPherson 20135 (
Virola sebifera
Virola sebifera
Aubl. Hist. Pl. Guiane. 2: 904. 1775. Type. French Guiana. Cayenne, 1775, [J.] Aublet s.n., (holotype: BM!*; isotypes: NY photograph of BM holotype). Fide
Myristica panamensis Hemsl. Biol. Cent.-Amer., Bot. 3: 67. 1882. Virola panamensis (Hemsl.) Warb., Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 68: 185. 1897. Syntypes: Panama. shady forest near Cruces, n.d. (fls.), Seemann 545 (BM!*, K!*); [Canal Area], Lion Hill station, [28 Mar 1862, leaves, excl. fr], S. Hayes 618 (K!*).
Virola warburgii Pittier. Contr. U.S. Natl. Herb. 18: 143. 1916. Type. Panama. Panamá, collected in forests along the Chagres River above Alhajuela, 12 May 1911 [fls], H. Pittier 3505 (holotype: US; isotype: BM).
Distinctive characters
Virola sebifera is recognised by its dense pubescence of pediculate and ferruginous trichomes (Fig.
Distribution
Virola sebifera is the most collected and widespread species in Mesoamerica. It is known from Honduras (Gracias a Dios), Nicaragua (Atlántico Norte, Atlántico Sur, Jinotega, Matagalpa and Río San Juan), Costa Rica (Alajuela, Cartago, Guanacaste, Heredia, Limón, Puntarenas and San José) and Panamá (Bocas del Toro, Chiriquí, Coclé, Colón, Darién, Herrera, Panamá, San Blas and Veraguas) (Fig.
Common names
Honduras: walus, banak. Nicaragua: banak, cebo, cebo macho, cebo sirsio, sangre drago. Costa Rica: coton, fruta dorada, miguelario. Panama: bogamani, copidijo, fruta dorada, gorgoran, malagueta de montaña, mancha, sangre, tabegua, velario colorado.
Phenology
Herbarium specimens of flowering and fruiting individuals have been collected throughout the year. In the Osa Peninsula and Golfo Dulce, Costa Rica, flowering occurs in December to May.
Field characters
Plants are shrubs or trees between 8–25 (–40) m tall and 7–40 cm DBH. Trunks are straight, sometimes with small buttresses and with long and horizontal branches. Bark is described as dark, minutely fissured vertically or flaking off in small pieces and aromatic. Exudate from different parts of the plant is red or reddish. The leaf blades are bright green above and whitish to greyish below in living material. Flowers have brown, cream, orange, yellow-brown or yellowish perianth and are sometimes fragrant, with a lemon or sweet aroma. The mature fruit is green and covered by a dense layer of trichomes, with a white seed with a red aril.
Notes
The leaves of this species are very variable in shape and size. Sometimes, a single individual may have leaves of different sizes. Individuals with small leaf blades from Panama (e.g. G. McPherson 15341,
Selected specimens seen
Honduras. Gracias a Dios: Monte Alto de Anastasio, 90 m elev., 17 Mar 1994 (♂ fl), P. R. House 2019 (
Conclusions
In this synopsis of Mesoamerican Virola, 14 species are recognised. Twelve of these are restricted to Mesoamerica, while the other two species extend into South America. Six of the treated species are new; these are all from Costa Rica and Panama, countries with the highest species diversity of Virola, with 10 and 11 species, respectively. We clarified the application of some names, the resurrection of others and the distribution of some species previously considered to occur in Mesoamerica (e.g. South American V. macrocarpa) or South America (e.g. the Mesoamerican V. multiflora). This has resulted in a much modified taxonomy for Virola, summarised here: Mesoamerican collections previously identified as V. calophylla and/or V. macrocarpa are here interpreted as three new species: V. alleni, V. amistadensis and V. otobifolia, while Mesoamerican specimens previously determined as V. surinamensis are treated as V. nobilis. As a result of this, V. calophylla and V. macrocarpa are now restricted to South America and V. surinamensis to South America and the Antilles. Additionally, two new species are identified within what was previously V. guatemalensis: montane collections from the southern range now belong to V. montana and we have resurrected V. laevigata, previously considered a synonym. Under our concept, Virola guatemalensis has a restricted range, found only in northern Mesoamerica. Finally, the majority of V. koschnyi specimens from the Pacific coast of Costa Rica and Panama correspond to V. chrysocarpa, while most of Panama’s collections, identified as V. multiflora, are now considered V. fosteri.
These contributions came to light via detailed comparisons across the complete range of these species thanks to hundreds of collections made by numerous botanists and herbaria who care, maintain and provide access to the specimens, including making them available online.
Acknowledgements
We would like to express our gratitude to the curators of the institutions mentioned in the text (
References
- Acevedo-Rodríguez P, Strong MT (2012) Catalogue of seed plants of the West Indies. Smithsonian Contributions to Botany 98: 1–1192. https://doi.org/10.5479/si.0081024X.98.1
- Achá S, Liesner RL (2014) Myristicaceae. In: Jørgensen PM, Nee MH, Beck SG (Eds) Catálogo de las plantas vasculares de Bolivia. Monographs in Systematic Botany from the Missouri Botanical Garden 127(1), 868–870.
- Aguilar R, Cornejo X, Santamaría-Aguilar D, Tulig M, Bainbridge C, Mori SA (2017 onward) Vascular Plants of the Osa Peninsula, Costa Rica. http://sweetgum.nybg.org/osa/
- Allen PH (1956) The Rain Forests of Golfo Dulce. University of Florida Press Gainesville, 1–417.
- APG IV (2016) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181(1): 1–20. https://doi.org/10.1111/boj.12385
- Aublet JBCF (1775) Histoire des plantes de la Guiane Françoise: Rangées suivant la méthode sexuelle, avec plusieurs mémoires sur différens objects intéressans, relatifs à la culture & au commerce de la Guiane Françoise, & une notice des plantes de l'Isle-de-France. Tome Second. Pierre-François DIDOT, London & Paris 2: 622–976. https://doi.org/10.5962/bhl.title.48831
- Aymard GA, Berry PE, Rodrigues WA (2007) Myristicaceae. In: Funk VA, Hollowell TH, Berry PE, Kelloff CL, Alexander S (Eds) Checklist of the plants of the Guiana Shield (Venezuela: Amazonas, Bolivar, Delta Amacuro; Guyana, Suriname, French Guiana). Contributions from the United States National Herbarium 55: 428–430.
- Balick MJ, Nee MH, Atha DE (2000) Checklist of the vascular plants of Belize. Memoirs of the New York Botanical Garden, 246 pp.
- Bennett BC, Alarcón R (1994) Osteophloeum platyspermum and Virola duckei (Myristicaceae): Newly reported as hallucinogens from Amazonian Ecuador. Economic Botany 48(2): 152–158. https://doi.org/10.1007/BF02908205
- Bentham G (1853) Notes on the American species of Myristica. Hooker’s Journal of Botany and Kew Garden Miscellany 5: 1–9. https://www.biodiversitylibrary.org/item/6323#page/7/mode/1up
- Cardona NF, David HH, Hoyos SEG (2010) Flora de la Miel, Central Hidroeléctrica Miel I, Oriente de Caldas, Guía ilustrada. ISAGEN – Universidad de Antioquia, Herbario Universidad de Antioquia (HUA), Medellín, 228 pp.
- Cardoso D, Särkinen T, Alexander S, Amorim AM, Bittrich V, Celis M, Daly DC, Fiaschi P, Funk VA, Giacomin LL, Goldenberg R, Heiden G, Iganci J, Kelloff CL, Knapp S, Cavalcante de Lima H, Machado AFP, dos Santos RM, Mello-Silva R, Michelangeli FA, Mitchell J, Moonlight P, de Moraes PLR, Mori SA, Nunes TS, Pennington TD, Pirani JR, Prance GT, de Queiroz LP, Rapini A, Riina R, Rincon CAV, Roque N, Shimizu G, Sobral M, Stehmann JR, Stevens WD, Taylor CM, Trovó M, van den Berg C, van der Werff H, Viana PL, Zartman CE, Forzza RC (2017) Amazon plant diversity revealed by a taxonomically verified species list. Proceedings of the National Academy of Sciences of the United States of America 114(40): 10695–10700. https://doi.org/10.1073/pnas.1706756114
- Cogollo Á (2011) Myristicaceae. In: Idárraga-Piedrahita A, Ortiz RDC, Callejas Posada R, Merello M (Eds) Flora de Antioquia: Catálogo de las Plantas Vasculares, Listado de las plantas vasculares del departamento de Antioquia. Universidad de Antioquia, Medellín 2: 641–644.
- Cogollo Á, Velásquez-Rúa C, García N (2007) Las miristicáceas. In: García N (Ed.) Libro Rojo de Plantas de Colombia. Las magnoliáceas, las miristicáceas y las podocarpáceas. Serie Libros Rojos de Especies Amenazadas de Colombia. Bogotá, Colombia. Instituto Alexander von Humboldt – CORANTIOQUIA – Jardín Botánico Joaquín Antonio Uribe de Medellín - Instituto de Ciencias Naturales de la Universidad Nacional de Colombia – Ministerio de Ambiente, Vivienda y Desarrollo Territorial 5: 155–192.
- Condit R, Pérez R, Daguerre N (2011) Trees of Panama and Costa Rica. Princeton University Press, New Jersey, 494 pp. https://doi.org/10.1515/9781400836178
- Cornejo X, Mori SA, Aguilar R, Stevens H, Douwes F (2012) Phytogeography of the trees of the Osa Peninsula, Costa Rica. Brittonia 64(1): 76–101. https://doi.org/10.1007/s12228-011-9194-0
- Correa MD, Galdames C, Stapf M (2004) Catálogo de las Plantas Vasculares de Panamá. Smithsonian Tropical Research Institute, Panamá, 599 pp.
- Croat TB (1978) Flora of Barro Colorado Island. Stanford University Press, Stanford, California, 960 pp.
- Dauby G, Stévart T, Droissart V, Cosiaux A, Deblauwe V, Simo-Droissart M, Sosef MSM, Lowry PP II, Schatz GE, Gereau RE, Couvreur TLP (2017) ConR: An R package to assist large-scale multispecies preliminary conservation assessments using distribution data. Ecology and Evolution 7(24): 11292–11303. https://doi.org/10.1002/ece3.3704
- de Candolle A (1856) Ordo CLXIII. Myristicaceae. In: de Candolle A (Ed. ) Prodromus Systematis Naturalis Regni Vegetabilis 14: 189–208.
- de Wilde WJJO (1991) The genera of Myristicaceae as distinguished by their inflorescences, and the description of a new genus, Bicuiba. Beiträge zur Biologie der Pflanzen 66: 95–125.
- Ducke A (1936) Notes on the Myristicaceae of Amazonian Brazil with descriptions of new species. Journal of the Washington Academy of Sciences 26(5): 213–222. https://www.jstor.org/stable/24530511
- Ducke A (1945) New forest trees and climbers of the Brazilian Amazon. Boletim Técnico do Instituto Agronômico de Norte 4: 8–12.
- Duke JA (1962) Myristicaceae. Flora of Panama, Part IV. Fascicle 5. Annals of the Missouri Botanical Garden 49(3–4): 214–225. https://doi.org/10.2307/2394708
- Duke JA (1975) Ethnobotanical observations on the Cuna Indians. Economic Botany 29(3): 278–293. https://doi.org/10.1007/BF02873178
- Flores EM (2010) Virola koschnyi Warb. In: Vozzo JA (Ed.) Manual de Semillas de Árboles Tropicales. Departamento de Agricultura de los Estados Unidos, Servicio Forestal (USDA), Washington, 887 pp.
- Flores-Vindas E, Obando-Vargas G (2014) Árboles del Trópico Húmedo, Importancia socioeconómica. Second edition. Editorial Tecnológico de Costa Rica, 11–996.
- Frankie FW, Baker HG, Opler PA (1974) Comparative Phenological Studies of Trees in Tropical Wet and Dry Forests in the Lowlands of Costa Rica. Journal of Ecology 62(3): 881–919. https://doi.org/10.2307/2258961
- Garwood NC (2009) Seedlings of Barro Colorado Island and the Neotropics. Cornell University Press, Ithaca, 645 pp.
- Gentry AH (1975) Additional Panamanian Myristicaceae. Annals of the Missouri Botanical Garden 62(2): 474–479. https://doi.org/10.2307/2395207
- Gentry AH (1993) A field guide to the families and genera of woody plants of northwest South America (Colombia, Ecuador, Peru), with supplementary notes on herbaceous taxa. Conservation International, Washington, D.C., 895 pp.
- Gentry AH (2001) Myristicaceae. In: Stevens WD, Ulloa Ulloa C, Pool A, Montiel OM (Eds) Flora de Nicaragua. Monographs in Systematic Botany from the Missouri Botanical Garden 85(1), 1542–1545.
- Gómez-Laurito J, Ortiz R (2004) Lista con Anotaciones de las Angiospermas de la Reserva Biológica Alberto Brenes (Microcuencas de los ríos San Lorenzo y San Lorencito), Costa Rica. Lankesteriana 4(2): 113–142. https://doi.org/10.15517/lank.v4i2.21614
- Gradstein SR (2016) Virola. In: Bernal R, Gradstein SR, Celis M (Eds) Catálogo de plantas y líquenes de Colombia. Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá. http://catalogoplantasdecolombia.unal.edu.co
- Grayum MH, Hammel BE, Troyo S, Zamora N (2004) Historia/History. In: Hammel BE, Grayum MH, Herrera C, Zamora N (Eds) Manual de Plantas de Costa Rica. Vol. I. Introducción. Monographs in Systematic Botany from the Missouri Botanical Garden 97: 1–50.
- Haber W (2014) Plantas Vasculares de Monteverde. Apéndice 1. In: Nadkarni NM, Wheelwright NT (Eds) Monteverde: ecología y conservación de un bosque nuboso tropical (versión actualizada y ampliada en español). Oxford University Press, 744–805. http://digitalcommons.bowdoin.edu/scholars-bookshelf/3/
- Hallé F, Oldeman RAA, Tomlinson PB (1978) Tropical Trees and Forests, An Architectural Analysis. Springer-Verlag Berlin Heidelberg, New York, 441 pp. https://doi.org/10.1007/978-3-642-81190-6_1
- Hemsley WB (1882) Botany. In: Godman FD, Salvin O (Eds) Biologia Centrali-Americana; or Contributions to the Knowledge of the Fauna and Flora of Mexico and Central America. R. H. Poret and Dulau & Co. , London 3: 1–711.
- Howe HF (1981) Dispersal of a Neotropical Nutmeg (Virola sebifera) by birds. The Auk: Ornithological Advances 98(1): 88–98. https://doi-org.eres.qnl.qa/10.1093/auk/98.1.88
- Howe HF (1982) Fruit production and animal activity in two tropical trees. In: Leigh EG Jr, Stanley Rand A, Windsor DM (Eds) The Ecology of a tropical forest: seasonal rhythms and long-term changes. Smithsonian Institution Press, Washington, 189–200.
- Howe HF (1993) Aspects of variation in a neotropical seed dispersal system. In: Fleming TH, Estrada A (Eds) Frugivory and seed dispersal: ecological and evolutionary aspects. Vegetation 107/108, 149–162. https://doi.org/10.1007/978-94-011-1749-4_10
- Howe HF, Vande Kerckhove GA (1980) Nutmeg dispersal by tropical birds. Science 210(4472): 925–927. https://doi.org/10.1126/science.210.4472.925
- Janovec PJ (2000) A systematic study of Compsoneura (A. DC.). Warb., a Neotropical Member of the Nutmeg family (Myristicaceae). PhD Thesis, Texas A & M University.
- Jaramillo TS, Muriel P, Rodrigues WA, Balslev H (2000) Myristicaceae novelties from Ecuador. Nordic Journal of Botany 20(4): 443–447. https://doi.org/10.1111/j.1756-1051.2000.tb01586.x
- Jaramillo TS, Muriel P, Balslev H (2004) Myristicaceae. In: Harling GW, Andersson L (Eds) Flora of Ecuador. University of Göteborg, Göteborg 72: 1–101.
- Jiménez Q (2007) Myristicaceae. In: Hammel BE, Grayum MH, Herrera C, Zamora N (Eds) Manual de Plantas de Costa Rica. Vol. VI. Monographs in Systematic Botany from the Missouri Botanical Garden 111: 684–691.
- Jiménez Madrigal Q, Grayum MH (2002) Vegetación del Parque Nacional Carara, Costa Rica. Brenesia 57–58: 25–66.
- Jørgensen PM, León-Yánez S (1999) Catalogue of the Vascular Plants of Ecuador. Monographs in Systematic Botany from the Missouri Botanical Garden 75: 1–1181.
- Knapp S (2013) A revision of the Dulcamaroid Clade of Solanum L. (Solanaceae). PhytoKeys 22(0): 1–428. https://doi.org/10.3897/phytokeys.22.4041
- Kühn U, Kubitzki K (1993) Myristicaceae. In: Kubitzki K, Rohwer JG, Bittrich V (Eds) The Families and Genera of Vascular Plants. Flowering Plants. Dicotyledons. Magnoliid, Hamamelid, and Caryophyllid families. Springer Verlag, Berlin 2: 457–467. https://doi.org/10.1007/978-3-662-02899-5_53
- Lambright DD (1981) The comparative anatomy and morphology of Virola (Myristicaceae). PhD Thesis, Miami University, Oxford, Ohio.
- Ley López JM, Chacón Madrigal E (2017) Las Plántulas de Árboles y Palmas de la Península de Osa. San José, Costa Rica, 3–183.
- Little EL (1970) New Tree Species from Esmeraldas, Ecuador. Phytologia 19(4): 251–269. https://doi.org/10.5962/bhl.part.14772
- Lobo J, Aguilar R, Chacón E, Fuchs E (2008) Phenology of tree species of the Osa Peninsula and Golfo Dulce region, Costa Rica. In: Weissenhofer A, Huber W, Mayer V, Pamper S, Weber A, Aubrecht G (Eds) Natural and Cultural History of the Golfo Dulce Region, Costa Rica. Stapfia 88: 547–555.
- Mari Mut JA (2018) Notes on the etymology of Aublet’s generic names, 1–17. https://archive.org/details/aubletgen [Last updated: April 30, 2018]
- Mitchell JD (2002) Myristicaceae (Nutmeg Family). In: Mori SA, Cremers G, Gracie CA, de Granville JJ, Heald SV, Hoff M, Mitchell JD (Eds) Guide to the vascular plants of Central French Guiana. Memoirs of The New York Botanical Garden 76(2), 525–532.
- Monro AK, Santamaría-Aguilar D, González F, Chacón O, Solano D, Rodríguez A, Zamora N, Fedele E, Correa MDA (2017) A first checklist to the vascular plants of La Amistad International Park (PILA), Costa Rica-Panama. Phytotaxa 322(1): 1–283. https://doi.org/10.11646/phytotaxa.322.1.1
- Moreira JI, Riba-Fernández P, Lobo J (2017) Toucans (Ramphastos ambiguus) facilitate resilience against seed dispersal limitation to a large‐seeded tree (Virola surinamensis) in a human‐modified landscape. Biotropica 49(4): 502–510. https://doi.org/10.1111/btp.12427
- Moya Roque R, Rodríguez González A, Olivares Gutiérrez C (2014) Árboles maderables de la Península de Osa, madera y corteza. Editorial Tecnológico de Costa Rica, Cartago, 344 pp.
- Nelson CH (2008) Catálogo de las Plantas Vasculares de Honduras. Secretaría de Recursos Naturales y Ambiente, Tegucigalpa, 1576 pp.
- Pennington TD, Sarukhánn J (2016) Árboles Tropicales de México, Manual para la Identificación de Campo de las Principales Especies. Universidad Nacional Autónoma de México y Fondo de Cultura Económica. Ciudad de México 3: 9–511.
- Pérez R, Condit R (2018) Tree Atlas of Panama. http://ctfs.si.edu/webatlas/maintreeatlas.php
- Pesce C (2009) Oleaginosas da Amazônia. Second edition. Belém: Museu Paraense Emílio Goeldi; Brasília: Ministério do Desenvolvimento Agrário 23–334.
- Pitman NCA, Mogollón H, Dávila N, Ríos M, García‐Villacorta R, Guevara J, Baker TR, Monteagudo A, Phillips OL, Vásquez‐Martínez R, Ahuite M, Aulestia M, Cardenas D, Cerón CE, Loizeau PE, Neill DA, Núñez P, Palacios WA, Spichiger R, Valderrama E (2008) Tree community change across 700 km of Lowland Amazonian Forest from the Andean Foothills to Brazil. Biotropica 40(5): 525–535. https://doi.org/10.1111/j.1744-7429.2008.00424.x
- Pittier H (1916) New or noteworthy plants from Colombia and Central America (V). Contributions from the United States National Herbarium 18(4): 143–171.
- Pittier H (1922) New or noteworthy plants from Colombia and Central America (VIII). Contributions from the United States National Herbarium 20(12): 453–487.
- Pittier H (1978) Plantas usuales de Costa Rica. Editorial Costa Rica, 1–329.
- Plotkin MJ, Schultes RE (1990) Virola: A Promising Genus for Enthnopharmacological Investigation. Journal of Psychoactive Drugs 22(3): 357–361. https://doi.org/10.1080/02791072.1990.10472561
- Poinar Jr G, Steeves R (2013) Virola dominicana sp. nov. (Myristicaceae) from Dominican amber. Botany 91(8): 530–534. https://doi.org/10.1139/cjb-2013-0019
- Prance GT (1970) Notes on the use of plant Hallucinogens in Amazonian Brazil. Economic Botany 24(1): 62–68. https://doi.org/10.1007/BF02860638
- Quesada Quesada FJ, Jiménez Madrigal Q, Zamora Villalobos N, Aguilar Fernández F, González Ramírez J (1997) Árboles de la Península de Osa. INBio, Santo Domingo de Heredia, 1–411.
- Riba Hernández JP (2017) Conservación, estructura poblacional y ecología reproductiva de la fruta dorada (Virola surinamensis: Myristicaceae) un árbol dioico maderable en la Península de Osa, Costa Rica. PhD Thesis, Universidad de Costa Rica, Costa Rica.
- Rodrigues WA (1972) A ucuuba de várzea e suas aplicações. Acta Amazonica 2(2): 29–47. https://doi.org/10.1590/1809-43921972022029
- Rodrigues WA (1977) Novas espécies de Virola Aubl. (Myristicaceae) da Amazônia. Acta Amazonica 7(4): 459–471. https://doi.org/10.1590/1809-43921977074459
- Rodrigues WA (1980) Revisão taxonômica das espécies de Virola Aublet (Myristicaceae) do Brasil. Acta Amazonica 10(1): 1–127. https://doi.org/10.1590/S0044-59672006000100001
- Rodrigues WA (1989) A New Venezuelan Virola (Myristicaceae). Annals of the Missouri Botanical Garden 76(4): 1163–1164. https://doi.org/10.2307/2399703
- Rodrigues WA (2008) Myristicaceae. In: Hokche O, Berry PE, Huber O (Eds) Nuevo Catálogo de la Flora Vascular de Venezuela. Fundación Instituto Botánico de Venezuela, Caracas, 512–515.
- Rodrigues WA (2010) Flora da Bahia: Myristicaceae. Sitientibus. Série Ciências Biológicas 10(1): 138–146.
- Rodrigues W (2015) Myristicaceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB10197
- Rodrigues WA, Aymard CGA, Berry PE (2001) Myristicaceae. In: Steyermark JA, Berry PE, Holst BK (Eds) Flora of the Venezuelan Guayana. Missouri Botanical Garden Press 6: 734–747.
- Sabatier D (1997) Description et biologie d'une nouvelle espèce de Virola (Myristicaceae) de Guyane. Adansonia sér. 3 19(2): 273–278.
- Sánchez Hernández S, Mendoza Briseño MA, García Hernández RV (2017) Diversificación de la sombra tradicional de cafetales en Veracruz mediante especies maderables. Revista Mexicana de Ciencias Forestales 8(40): 7–18. https://doi.org/10.29298/rmcf.v8i40.32
- Schultes RE (1954) A new narcotic snuff from the Northwest Amazon. Botanical Museum Leaflets 16(9): 241–260.
- Schultes RE (1969) De plantas toxicariis e mundo novo tropicale commentationes V: Virola as an orally administered hallucinogen. Botanical Museum Leaflets 22(6): 229–240.
- Schultes RE (1979) Evolution of the identification of the myristicaceous hallucinogens of South America. Journal of Ethnopharmacology 1(3): 211–239. https://doi.org/10.1016/S0378-8741(79)80013-6
- Schultes RE, Holmstedt B (1968) De plantis toxicariis e Mundo Novo tropicale commentationes II. The vegetal ingredients of the myristicaceous snuffs of the northwest Amazon. Rhodora 70: 113–160.
- Shorthouse DP (2010) SimpleMappr, an online tool to produce publication-quality point maps. http://www.simplemappr.net [accessed several times 2018]
- Smith AC (1953) Studies of South America plants, XIII. Journal of the Washington Academy of Sciences 43(7): 203–212.
- Smith AC (1956) Studies of South American plants, XV. American Journal of Botany 43(8): 573–577. https://doi.org/10.1002/j.1537-2197.1956.tb10536.x
- Smith AC, Wodehouse RP (1938) The American species of Myristicaceae. Brittonia 2(5): 393–510. https://doi.org/10.2307/2804799
- Soares Maia JG, Rodrigues WA (1974) Virola theiodora como alucinógena e tóxica. Acta Amazonica 4(1): 21–23. https://doi.org/10.1590/1809-43921974041021
- IUCN Standards and Petitions Subcommittee (2014) Guidelines for using the IUCN Red List categories and criteria. Version 11. Standards and Petitions Subcommittee. http://www.iucnredlist.org/documents/RedListGuidelines.pdf
- Standley PC (1928) Flora of the Panama canal zone. Contributions from the United States National Herbarium 27: 1–416.
- Standley PC (1929) Studies of American plants I. Publications of the Field Museum of Natural History, Botanical Series 4(8): 197–299.
- Standley PC (1930) Flora of Yucatan. Publications of the Field Museum of Natural History, Botanical Series 3(3): 157–492.
- Standley PC (1931) Flora of the Lancetilla Valley, Honduras. Publications of the Field Museum of Natural History, Botanical Series 10: 1–418. https://doi.org/10.5962/bhl.title.2352
- Standley PC (1932) New plants from British Honduras. Publications of the Field Museum of Natural History, Botanical Series 11(4): 127–142. https://doi.org/10.5962/bhl.title.2271
- Standley PC (1937) Flora of Costa Rica. Part II. Publications of the Field Museum of Natural History, Botanical Series 18(2): 399–780.
- Standley PC, Steyermark JA (1946) Myristicaceae. In: Standley PC, Steyermark JA (Eds) Flora of Guatemala – Part IV. Fieldiana: Botany 24(4), 294–299.
- Steeves RAD (2011) An Intrageneric and Intraspecific Study of Morphological and Genetic Variation in the Neotropical Compsoneura and Virola (Myristicaceae). PhD. Thesis, The University of Guelph, 295 pp.
- ter Steege H, Pitman NCA, Sabatier D, Baraloto C, Salomão RP, Guevara JE, Phillips OL, Castilho CV, Magnusson WE, Molino J-F, Monteagudo A, Núñez Vargas P, Montero JC, Feldpausch TR, Coronado ENH, Killeen TJ, Mostacedo B, Vasquez R, Assis RL, Terborgh J, Wittmann F, Andrade A, Laurance WF, Laurance SGW, Marimon BS, Marimon B-H, Guimarães Vieira IC, Amaral IL, Brienen R, Castellanos H, Cárdenas López D, Duivenvoorden JF, Mogollón HF, Matos FDA, Davila N, Garcia-Villacorta R, Stevenson Diaz PR, Costa F, Emilio T, Levis C, Schietti J, Souza P, Alonso A, Dallmeier F, Montoya AJD, Fernandez Piedade MT, Araujo-Murakami A, Arroyo L, Gribel R, Fine PVA, Peres CA, Toledo M, Aymard CGA, Baker TR, Ceron C, Engel J, Henkel TW, Maas P, Petronelli P, Stropp J, Zartman CE, Daly D, Neill D, Silveira M, Paredes MR, Chave J, Lima Filho DA, Jorgensen PM, Fuentes A, Schongart J, Cornejo Valverde F, Di Fiore A, Jimenez EM, Penuela Mora MC, Phillips JF, Rivas G, van Andel TR, von Hildebrand P, Hoffman B, Zent EL, Malhi Y, Prieto A, Rudas A, Ruschell AR, Silva N, Vos V, Zent S, Oliveira AA, Schutz AC, Gonzales T, Trindade Nascimento M, Ramirez-Angulo H, Sierra R, Tirado M, Umana Medina MN, van der Heijden G, Vela CIA, Vilanova Torre E, Vriesendorp C, Wang O, Young KR, Baider C, Balslev H, Ferreira C, Mesones I, Torres-Lezama A, Urrego Giraldo LE, Zagt R, Alexiades MN, Hernandez L, Huamantupa-Chuquimaco I, Milliken W, Palacios Cuenca W, Pauletto D, Valderrama Sandoval E, Valenzuela Gamarra L, Dexter KG, Feeley K, Lopez-Gonzalez G, Silman MR (2013) Hyperdominance in the Amazonian tree flora. Science 342(6156): 1243092. https://doi.org/10.1126/science.1243092
- ter Steege H, Vaessen RW, Cardenas D, Sabatier D, Antonelli A, Mota de Oliveira S, Pitman NCA, Jørgensen PM, Salomão RP (2016) The discovery of the Amazonian tree flora with an updated checklist of all known tree taxa. Scientific Reports 6(1): 29549. https://doi.org/10.1038/srep29549
- ter Steege H, Mota de Oliveira S, Pitman NCA, Sabatier D, Antonelli A, Guevara Andino JE, Aymard GA, Salomão RP (2019) Towards a dynamic list of Amazonian tree species. Scientific Reports 9(1): 3501. https://doi.org/10.1038/s41598-019-40101-y
- Thiers B (2019) Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih/
- Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten. https://doi.org/10.12705/Code.2018
- Ulloa Ulloa C, Acevedo-Rodríguez P, Beck S, Belgrano MJ, Bernal R, Berry PE, Brako L, Celis M, Davidse G, Forzza RC, Gradstein SR, Hokche O, León B, León-Yánez S, Magill RE, Neill DA, Nee M, Raven PH, Stimmel H, Strong MT, Villaseñor JL, Zarucchi JL, Zuloaga FO, Jørgensen PM (2017) An integrated assessment of the vascular plant species of the Americas. Science 358(6370): 1614–1617. https://doi.org/10.1126/science.aao0398
- Vásquez MR, Rojas GRDP, Monteagudo MAL, Valenzuela GL, Huamantupa Ch I (2018) Catálogo de los árboles del Perú. Q'euña. Revista de la Sociedad Botánica del Cusco 9: 1–607.
- Vásquez Martínez R (1997) Flórula de las Reservas Biológicas de Iquitos, Perú: Allpahuayo-Mishana, Explornapo Camp, Explorama Lodge. Monographs in Systematic Botany from the Missouri Botanical Garden 63: 1–1046.
- Vásquez Martínez R (2010) Myristicaceae. In: Vásquez Martínez R, Rojas Gonzáles R, van der Werff H (Eds) Flora del Río Cenepa, Amazonas, Perú. Angiospermae (Gentianaceae–Zingiberaceae). Monographs in Systematic Botany from the Missouri Botanical Garden 2: 1061–1072.
- Villaseñor JL (2016) Checklist of the native vascular plants of Mexico/ Catálogo de las plantas vasculares nativas de México. Revista Mexicana de Biodiversidad 87(3): 559–902. https://doi.org/10.1016/j.rmb.2016.06.017
- Walker JW, Walker AG (1979) Comparative Pollen Morphology of the American Myristicaceous Genera Compsoneura and Virola. Annals of the Missouri Botanical Garden 66(4): 731–755. https://doi.org/10.2307/2398916
- Warburg O (1897) Monographie der Myristicaceen. Nova Acta Academiae Caesareae Leopoldino-Carolinae Germanicae Naturae Curiosorum 68: 1–680.
- Warburg O (1905) Myristicaceae costaricense (XXVI). Repertorium Specierum Novarum Regni Vegetabilis 1(5–6): 71–72. https://doi.org/10.1002/fedr.19050010503
- Williams LO (1964) Tropical American plants, VI. Fieldiana. Botany 31(2): 17–48. https://doi.org/10.5962/bhl.title.2420
Supplementary material
List of exsiccatae
Data type: List of specimens examined.