Research Article |
|
Corresponding author: Ludovica Santilli ( santilli.ludovica@gmail.com ) Corresponding author: Nicolás Lavandero ( nglavand@uc.cl ) Academic editor: Leandro Giacomin
© 2022 Ludovica Santilli, Fernanda Pérez, Claire de Schrevel, Philippe Dandois, Hector Mondaca, Nicolás Lavandero.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Santilli L, Pérez F, de Schrevel C, Dandois P, Mondaca H, Lavandero N (2022) Nicotiana rupicola sp. nov. and Nicotiana knightiana (sect. Paniculatae, Solanaceae), a new endemic and a new record for the flora of Chile. PhytoKeys 188: 83-103. https://doi.org/10.3897/phytokeys.188.73370
|
Abstract
Nicotiana knightiana is recorded for the first time for the flora of Chile. A new species of Nicotiana, endemic to the coast of the Coquimbo region is described and illustrated. Molecular analysis placed the new species within the N. sect. Paniculatae, as sister to N. cordifolia, an endemic to Juan Fernandez islands. The new species can be considered critically endangered (CR) according to the IUCN categories due to its restricted and fragmented distribution, small population number, and the threat that urbanization and mining activities represent for the conservation of the biodiversity of the area.
Keywords
Coquimbo, endemism, exotic species, Nicotianoidae, systematics, taxonomy
Introduction
Nicotiana L. is one of the largest genera in the Solanaceae with 75 recognised species (
Since
The aim of the present work is to record Nicotiana knightiana Goodsp. for the first time for the flora of Chile and describe a new species of Nicotiana endemic to Chile, determining its phylogenetic position and conservation status.
Methods
Herbarium and fieldwork
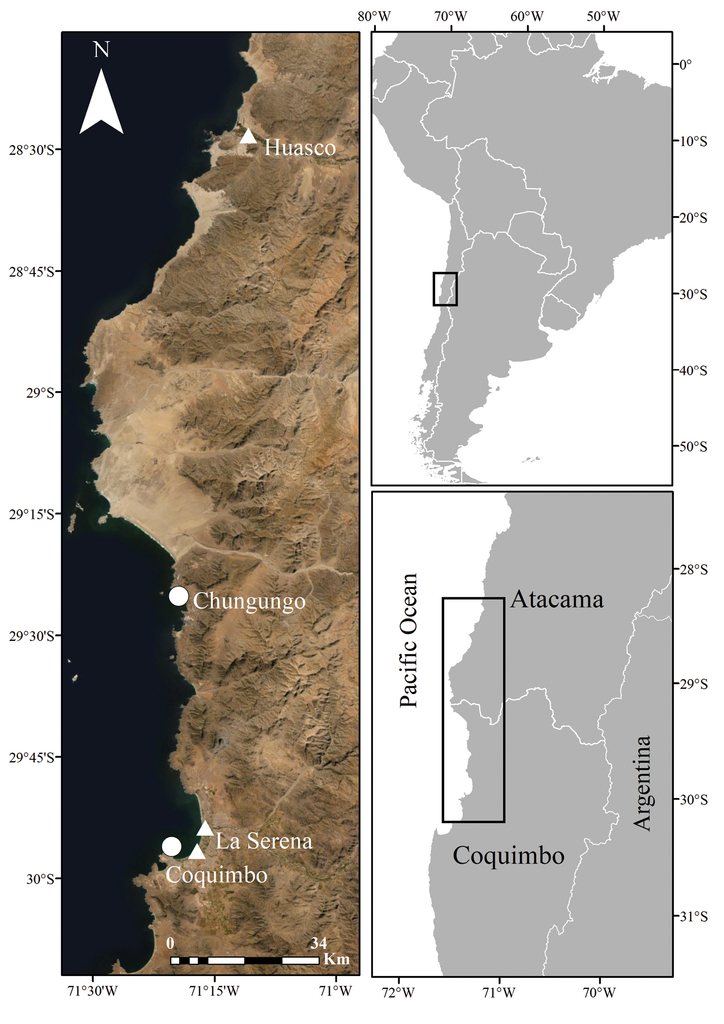
Fieldwork was carried out during November 2020 in Fuerte Lambert and during March 2020 in the proximity of the rivermouth of Elqui river, within the city of La Serena, Coquimbo region (Fig.
Taxon sampling for phylogenetic analysis
DNA sequences for cpDNA intergenic spacers trnF-trnL, trnS-trnG, and genes ndhF and matK were obtained from GenBank (www.ncbi.nlm.nih.gov/Genbank) for all species of Nicotiana used in
DNA extraction, amplification, sequencing, and phylogenetic analyses
Total genomic DNA was extracted from silica-dried material collected in the field using the Qiagen DNeasy Plant Mini Kit (QIAGEN, Santiago, Chile), following the manufacturer’s instructions. Genomic DNA was used to amplify by PCR the following chloroplast regions: trnL-trnF using the primers c and f (
The assembled sequences were aligned using the MAFFT v7.450 algorithm (
Conservation assessment
The assessment of the conservation status of the new species was made using the International Union for Conservation of Nature criteria (
Results
We could not find any described species of Nicotiana for Chile that matched the morphology of the plants from Elqui river and Fuerte Lambert. A specimen matching the morphology of the species from Elqui river was found at SGO, collected in 2021 in the city of Huasco Bajo, Atacama, approx. 160 km north of La Serena. Plants from Elqui river are 2 m-long, somewhat ineffectively rooted, with branching short perennial shrubs, a pale yellow-green corolla, a dark green limb and eglandular indumentum (Fig.
Molecular phylogenetic analyses
The concatenated DNA matrix contained 4427 nucleotide characters (1554 matK, 1074 ndhF, 932 trnL-trnF and 867 trnS-trnG), representing 60 ingroup and 2 outgroup accessions. Both BI and ML analyses yielded congruent topologies. The topology of the phylogenetic tree constructed in this study is congruent with the clades found by
Phylogeny of Nicotiana resulting from Maximum Likelihood analysis of the plastid regions matK, rps16, trnS-trnG and trnL-trnF. Numbers above and below the branches represent the Posterior probabilities from the BI analysis and bootstrap values from the ML analysis, respectively. The species whose sequences were obtained in the present study are highlighted in bold, while section Paniculatae including N. rustica is highlighted in grey.
Section Paniculatae, including N. rustica, forms a well-supported clade (PP = 1.0, BS = 87). Relationships among the clades largely reflect
Taxonomic treatment
Nicotiana knightiana
Type
Peru. Dept. Arequipa, Prov. Islay, near Mollendo, 40 m, 16 November 1935 (fl, fr), Y. Mexia 04161 (holotype: UC [UC448735 photo!]; isotypes: MO, NA, NY).
Description
Robust annual or short-lived shrub up to 3 m with many new stems at different stages of development arising from a lignified horizontal stem poorly anchored to the soil. Stems herbaceous, green, tomentose. Leaves ovate, undulate, base rounded to subcordate, apex obtuse to acute; bigger leaves 13 × 10 cm, indumentum similar to the stem but much denser on the abaxial side which confers a whitish colour, hairs simple, eglandular, pluricellular, brochidodromous venation, petioles a third or half as long as the leaves. Inflorescence a broad thyrse or lax panicle, 40 cm. Pedicels 0.5–1 cm in mature fruits, covered in glandular hairs. Calyx up to 7 mm, cylindric, tomentose, teethup to 2 mm, triangular. Corolla 20–23 mm excluding the limb (tubular part), tube proper 4 mm, throat 16 mm, pale yellow-green, covered in short, eglandular, hairs, limb bottom 3 mm wide, dark green, same indumentum as tube proper, notched into 5 lobes shorter than 1 mm. Stamens extending below the limb, 19 mm except one slightly shorter, filaments adnate to the tube proper, then free, pubescent in the proximal 6 mm, then glabrous and slightly curved, with stamens bending toward the stigma. Capsule 6–8 mm, ovoid. Seeds mainly subrotund, 0.5–0.7 mm, brown, surface reticulate. Embryo straight.
Distribution and habitat
Nicotiana knightiana grows naturally in the coast of southern Perú in roadsides, pastures and rocky ravines bottoms. It was found in Chile, within the city of La Serena, Coquimbo region, in the proximity of the rivermouth of Rio Elqui, and in the proximity of Avenida Los Pescadores. It was also found growing in the city of Huasco Bajo, Atacama region (Fig.
Phenology
Nicotiana knightiana is found flowering and fruiting between November and May.
Specimens examined
Perú. Arequipa: Prov. Islay, Quebrada Canyon, 5–6 km north of Mollendo, 300 m, 29 September 1938 (fl, fr) C.R. Worth & J.L. Morrison 15742 (US); 12 km southeast of Islay, 250–300 m, 28 September 1938 (fl, fr),C.R. Worth & J.L. Morrison 15724 (US). Chile. Atacama: Huasco bajo, 28 m, 16 October 2021 (fl), J.H. Macaya 1782 (SGO). Coquimbo: Prov. del Elqui, La Serena, ribera sur del Río Elqui a ca. 200 m de la desembocadura, 2 m, 23 March 2021 (fl, fr), L. Santilli 210323 (SGO); La Serena, ribera sur del Río Elqui a ca. 200 m de la desembocadura, 1 m, 24 Nov 2018 (fl, fr), A. Ryan (iNaturalist); La Serena, avenida Los Pescadores con Canto del Agua, 3 m, 26 May 2021 (fl), B.L. Bedard (iNaturalist).
Nicotiana rupicola , sp. nov.
Type
Chile. Región de Coquimbo: Prov. Elqui, Comuna de Coquimbo, Fuerte Lambert, 29°56'2.52"S, 71°20'16.46"W, 29 m, 12 November 2020 (fl, fr), N. Lavandero 1011 (holotype: SGO!; isotypes: EIF!, CONC!).
Diagnosis
Nicotiana rupicola is most similar to N. solanifolia, from which it differs by its congested panicle (vs. lax panicle), its short and glabrous corolla up to 18 mm (vs. corolla of 35–50 mm, pubescent), non-retroflexed corolla limb (vs. retroflexed corolla limb), mature capsule included or slightly exserted from calyx, 6–10 mm (vs. more than half the length excluded from calyx at maturity, 12–18 mm).
Description
Perennial shrub up to 2 m with many stems arising from a lignified horizontal stem. Stems lignified, light brown, glabrous. Leaves orbicular to ovate, flat to slightly ondulate, margins slightly revolute, base rounded to cordate, apex retuse to obtuse; bigger leaves 10 × 8.5 cm, reducing their size towards the apex, densely covered in two types of hairs in both sides: simple, straight, pluricellular, up to 1 mm long, and glandular (capitate), straight, pluricellular, 50–600 µm long, brochidodromous venation, petioles a quarter to half as long as the leaves, 0.5–2.5(–5.0) cm long. Inflorescence a compact panicle, up to 35 cm; pedicels up to 0.5 cm, same indumentum as the leaves. Calyx up to 10 mm, cylindric, same indumentum as the leaves, teeth up to 3.5 mm, triangular. Corolla 17–18 mm excluding the limb (tubular part), tube proper up 5.5–6 mm, throat up to 12 mm, yellow, glabrous, limb 4 mm wide, yellow, glabrous or with scattered hairs, notched into 5 lobes. Stamens extending below the limb, similar length; filaments adnate for the first 5 mm to the tube proper, then free, pubescent at the base, then glabrous and slightly curved, with the distal portion bending toward the stigma. Capsule 6–10 mm long, ovoid. Seeds mainly angular, laterally compressed, 0.4–0.6 mm long, dark brown, surface reticulate. Embryo unknown. Chromosome number unknown. (Fig.
Distribution and habitat
Nicotiana rupicola is endemic to Chile where it is currently known from two locations, Fuerte Lambert and Chungungo, both in the region of Coquimbo (Fig.
Phenology
Nicotiana rupicola was found flowering and fruiting in November.
Etymology
The specific epithet derives from the Latin rupes (rock), and cola (dweller), alluding to rocky habitat of this species.
Additional specimens examined (paratypes)
Chile. Región de Coquimbo. Prov. Elqui, Comuna La Higuera, costa al Norte de Chungungo, 7 November 2006 (fl), N. García 3085 (EIF).
Conservation status
Nicotiana rupicola can be considered as Critically Endangered (CR) under the IUCN categories and criteria B1ab(iii); D. The criterion B1 was selected because its extent of occurrence is < 100 km2 (8 km2). The criterion “a” was selected because the distribution is highly fragmented. The criterion “b(iii)” was selected because there is a projected decline in the area, extent and quality of habitat. This area is constantly threatened by the expansion of urbanization that is affecting central-north coastal Chile. One of the locations is currently found at less than 300 m from the residential area of Coquimbo and the habitat is being altered by numerous and increasing amounts of formal and informal paths and human activity (camping, garbage, etc.). Moreover, mining activities within the extent of occurrence, especially Minera Dominga, which pretends to settle between the two known localities, will more likely affect possible unknown populations and the quality of the habitat. The criterion D was selected because we observed less than 50 individuals around the two known localities.
Key to the species of Nicotiana sect. Paniculatae found in Chile
| 1 | Inflorescence a congested panicle, corolla tube glabrous except for sparse hairs on the limb | N. rupicola |
| – | Inflorescence a lax panicle, corolla tube entirely covered by trichomes | 2 |
| 2 | Corolla tube 3–5 cm, indumentum of corolla made of glandular trichomes | N. solanifolia |
| – | Corolla tube 2–2.3 cm, indumentum of corolla made of eglandular trichomes | 3 |
| 3 | Corolla tube 2 cm, limb dark green, continental Chile | N. knightiana |
| – | Corolla tube 2–3 cm, limb yellow or purple, Juan Fernandez Islands | 4 |
| 4 | Corolla tube purple, Alejandro Selkirk Island | N. cordifolia subsp. cordifolia |
| – | Corolla tube yellow, Santa Clara Island | N. cordifolia subsp. sanctaclarae |
Discussion
The characters that proved to be useful to differentiate species of Nicotiana sect. Paniculatae are the type of inflorescence, the types of trichomes and distribution of the indumentum, as well as the size and colour of flowers. N. knightiana resembles most N. paniculata from which it differs on account of its shorter flowers with dark green limbs (vs. yellow) (Fig.
It is worth mentioning that N. solanifolia has been erroneously reported for the Coquimbo region based on a collection held at K (Cuming 860), which is the type of Nicotiana breviloba Jeffr., considered by
Molecular analyses showed that plants from Elqui river are likely correctly identified as N. knightiana and that our initial conjectures about the phylogenetic position of N. rupicola as part of the sect. Paniculatae were confirmed (Fig.
An important question regards whether N. knightiana has to be considered native or introduced to Chile. Either the species was never noticed or collected during the last two centuries of botanical expeditions, and presents naturally disjunct populations, being almost 1500 km apart from the closest population found in Peru, or it was recently introduced in Chile by anthropogenic means. The earliest evidence of its presence dates back to 2018 (iNaturalist) and it seems to have been established and possibly expanded to the surrounding area in sites with similar ecological conditions to the river mouth of Elqui river, such as Huasco Bajo. The production of abundant and small seeds, together with the ability of some species to grow in a broad range of open and disturbed habitats, is considered as a common adaptation that ensures high probability of dispersal and establishment. Such is the case for Nicotiana paniculata, N. glauca, and N. plumbaginifolia Viv. that are considered invasive species (
Nicotiana rupicola presents a restricted distribution limited to a small portion of the coastal area of northern Chile, where it grows on two locations on easily accessible coastal rocky cliffs at less than 300 m from urbanization. The population from Fuerte Lambert is situated in an area where urban expansion has caused major damage to the vegetation. The coastal area between Tongoy and Coquimbo is catalogued as a site of interest for the conservation of woody and succulent species due to its high diversity and rates of endemism (
Additional specimens examined
Nicotiana solanifolia. Chile. Antofagasta: [Antofagasta Province]. Lomas de Taltal, near road from Taltal to the panamericana, 430 m, 25 October 2002 (fl), M. Ackermann 500 (SGO); bei Hueso Parado, nahe von Taltal, 400 m, 9 July 1972 (fl, fr), O. Zöllner 5942 (L); Quebrada de Taltal, 410 m, 17 September 1992 (fl, fr), S. Teillier, P. Rundel & P. García 2850 (F); Hueso Parado, s.d., s.c. (SGO); Ravine ca. 16 km north from Paposo, 207 m, 21 November 2008 (fl), R. Baines, M. Gardner, P. Hechenleitner, C. Morter & D. Rae 38. (E); Mirador above the Thermoelectric plant below Quebrada Paposo, 680 m, 1 December 2004 (fl, fr), :M. Dillon & M. Finger 8670 (SGO); Paposo, Peralito, 15 November 1959 (fl), A. Torres s.n. (SGO); Paposo, borde quebrada, 24 October 2009, A. Moreira & F. Luebert 1205 (SGO); Camino Paposo-Caleta Blanco Encalada, Queb. Miguel Diaz, 160 m, 15 November 1996, R. Rodriguez 3131 (SGO); El Rincón, al N de Paposo, 17 September 1941 (fl, fr), C. Muñoz & G. Johnson 2877 (SGO); Quebrada el Despoblado, 25–26 August 1992 (fl), J.C. Torres s.n. (SGO); Taltal-Paposo, September 1909 (fl, fr), K. Reiche s.n. (SGO); Paposo, entrada a la Qda. Los Peralitos, 30 September 2005 (fl, fr), M. Muñoz 4607 (SGO); 10 km al sur de Caleta Blanco Encalado, 200–800 m, 11 December 1940 (fl), W. Biese 3209 (SGO). Taltal, Quebrada de Taltal, 410 m, 17 September 1992 (fl, fr), S. Teiller, P. Rundel, P. García 2837 (SGO); 6 Km east of Taltal, 300–600 m, 14 October 1938 (fl, fr), C.R. Worth & J.L. Morrison 16122 (US); Cerro Perales, ca. 5 km E of Taltal, 550–960 m, 27 September 1988 (fl, fr), M.O. Dillon, D. Dillon & V. Pobleto 5536 (F); Quebrada Rinconada, ca. 5 Km N of Caleta Paposo, 250 m, 25 October 1988 (fl), M.O. Dillon, D. Dillon & B. Tay 5741 (F);
Atacama: [Chañaral Province]. Parque Nacional Pan de Azúcar, Quebrada de Coquimbo, 10 November 1987, Paez s.n. (SGO); Chañaral, 24 October 1985 (fl), G. Nieuwenhuizen 132–27 (SGO); Camino Chañaral a Flamenco, 3.5 km al interior camino izquierdo desde Portofino, quebrada y cono de deyección, 14 October 1992 (fl, fr), M. Muñoz 3095 (SGO). [Copiapó Province]. Caldera, Quebrada León, 20 m, October 1924 (fl), E. Werdermann. 437 (E, SI, F); Quebrada de los leones, Caldera, 1888 (fl), W. Geisse s.n. (Type of Nicotiana cardiophylla Phil.) (SGO); Caldera, September 1900, K. Reiche s.n. (SGO). [Huasco Province]. Camino Carrizal Bajo – Huasco, 30 m, 13 October 1991 (fl, fr), S. Teillier, L. Villaroel & R. Torres 2579 (SGO); sector Aguada Tongoy, road to Los Bronces near Corral El Sauce – road junction, 276 m, 6 December 2004 (fl, fr), P. Baxter, M. Gardner, P Hechenleitner, P.I. Thomas & C. Zamorano 1877 (E, SGO); Carrizal Bajo, September 1885 (fr), F. Philippi s.n. (SGO); Camino Carrizal Bajo, km 50, 2 November 1991 (fl, fr), M. Muñoz, S. Teillier & I. Meza 2944 (SGO); Camino de vuelta Carrizal a Canto de Agua en Qda. Exposición sur, 23 September 1977 (fl, fr), M. Muñoz 1119 (SGO); Carrizal, September 1885, F. Philippi s.n. (SGO).
Nicotiana cordifolia. Chile. Valparaíso: [Valparaíso Province]. Archipiélago de Juan Fernandez, Isla Santa Clara, Bahía Matriz, 12 December 1998 (fl, fr), P. Danton s.n. (SGO); Isla Masafuera, s.d. (fl), R. A. Philippi 730 (F), Isla Masafuera, October 1854 (fl, fr), P. Germain s.n. (SGO); Isla Masatierra, San Juan Bautista, Conaf Garden, 56 m, 13 December 2003 (fl, fr), M. Gardner, P. Hechenleitner & M. Tobar (E)
Acknowledgements
The authors thank staff at SGO and EIF for receiving the specimens and allowing revision of specimens under their care, Sebastian Teillier for providing literature of Nicotiana, Gabriel Marianjel Donoso for his help in the field, Gisela Stotz for logistic support during fieldwork, Elisa Guzman for sharing images of the flora of the Elqui river, Matías Tobar for providing images of Nicotiana cordifolia for comparison, Nicolás García for sharing data and information about the new species, Jorge Macaya for providing a new locality for Nicotiana knightiana in Huasco Bajo and Gioconda Peralta for the support provided with molecular work (CONICYT-FONDEQUIP EQM150077). We would also like to thank Andrés Moreira-Muñoz and João Renato Stehmann for their helpful comments on the submitted manuscript. Herbarium and lab work were funded by the ANID/FONDECYT grant 1211765.
References
- Alharthi AS, Abd-ElGawad AM, Assaeeda AM (2021) Influence of the invasive shrub Nicotiana glauca Graham on the plant seed bank in various locations in Taif region, western of Saudi Arabia. Saudi Journal of Biological Sciences 28(1): 360–370. https://doi.org/10.1016/j.sjbs.2020.10.014
- Aoki S, Ito M (2000) Molecular phylogeny of Nicotiana (Solanaceae) based on the nucleotide sequence of the matK gene. Plant Biology 2(3): 316–324. https://doi.org/10.1055/s-2000-3710
- Bachman S, Moat J, Hill AW, de la Torre J, Scott B (2011) Supporting Red List threat assessments with GeoCAT: geospatial conservation assessment tool. In: Smith V, Penev L (Eds) e-Infrastructures for data publishing in biodiversity science. ZooKeys 150: 117–126. https://doi.org/10.3897/zookeys.150.2109
- Ballester B, Carrasco C, Del Desierto C (2016) Nicotianas litorales del desierto de Atacama: Historia de registro y consumo de tabaco cimarrón (Nicotiana solanifolia Warp.). Taltalia (9): 69–87.
- Bremer B, Bremer K, Heidari N, Erixon P, Anderberg AA, Olmstead RG, Källersjö M, Barkhordarian E (2002) Phylogenetics of asterids based on three coding and three non-coding chloroplast DNA markers and the utility of noncoding DNA at higher taxonomic levels. Molecular Phylogenetics and Evolution 24(2): 274–301. https://doi.org/10.1016/S1055-7903(02)00240-3
- Chase MW, Knapp S, Cox AV, Clarkson JJ, Butsko Y, Joseph J, Parokonny AS (2003) Molecular systematics, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae). Annals of Botany 92(1): 107–127. https://doi.org/10.1093/aob/mcg087
- Clarkson JJ, Knapp S, Garcia VF, Olmstead RG, Leitch AR, Chase MW (2004) Phylogenetic relationships in Nicotiana (Solanaceae) inferred from multiple plastid DNA regions. Molecular Phylogenetics and Evolution 33(1): 75–90. https://doi.org/10.1016/j.ympev.2004.05.002
- Clarkson JJ, Kelly LJ, Leitch AR, Knapp S, Chase MW (2010) Nuclear glutamine synthetase evolution in Nicotiana: Phylogenetics and the origins of allotetraploid and homoploid (diploid) hybrids. Molecular Phylogenetics and Evolution 55(1): 99–112. https://doi.org/10.1016/j.ympev.2009.10.003
- Clarkson JJ, Dodsworth S, Chase MW (2017) Time-calibrated phylogenetic trees establish a lag between polyploidisation and diversification in Nicotiana (Solanaceae). Plant Systematics and Evolution 303(8): 1001–1012. https://doi.org/10.1007/s00606-017-1416-9
- Edler D, Klein J, Antonelli A, Silvestro D (2020) raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods in Ecology and Evolution 12(2): 1–5. https://doi.org/10.1111/2041-210X.13512
- Felsenstein J (1981) Evolutionary trees from DNA sequences: A maximum likelihood approach. Journal of Molecular Evolution 17(6): 368–376. https://doi.org/10.1007/BF01734359
- Gairola S, El-Keblawy A, Mahmoud T (2016) A note on the current distribution of Nicotiana plumbaginifolia (Solanaceae) in the United Arab Emirates. National Academy Science Letters 39(6): 461–464. https://doi.org/10.1007/s40009-016-0490-9
- Gallo AG, de la Torre WW, Rodríguez VM (2008) Especies vegetales consideradas invasoras de hábitats, en la Historia Natural de Canarias. Lazaroa 29: е49.
- Goodspeed TH (1954) The genus Nicotiana. Chronica Botanica Company, Waltham, Mass, USA, V. 16.
- Hamilton MB (1999) Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Molecular Ecology 8(3): 521–523.
- Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics (Oxford, England) 17(8): 754–755. https://doi.org/10.1093/bioinformatics/17.8.754
- Hunziker AT (2001) Genera Solanacearum. The genera of Solanaceae illustrated, arranged according to a new system. Gantner ARG, Verlag K & Ruggell G, 500 pp.
- IUCN (2017) Guidelines for using the IUCN red list categories and criteria, version 13. Prepared by the Standards and Petitions Subcommittee of the IUCN Species Survival Commission. http://www.iucnredlist.org/documents/RedListGuidelines.pdf
- Johnson LA, Soltis DE (1995) Phylogenetic inference in Saxifragaceae sensu stricto and Gilia (Polemoniaceae) using matK sequences. Annals of the Missouri Botanical Garden 82(2): 149–175. https://doi.org/10.2307/2399875
- Johnston IM (1936) A study of the Nolanaceae. Proceedings of the American Academy of Arts and Sciences 71(1): 1–87. https://doi.org/10.2307/20023213
- Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30(4): 772–780. https://doi.org/10.1093/molbev/mst010
- Katoh K, Misawa K, Kuma KI, Miyata T (2002) MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30(14): 3059–3066. https://doi.org/10.1093/nar/gkf436
- Mehmood F, Ubaid Z, Shahzadi I, Ahmed I, Waheed MT, Poczai P, Mirza B (2020) Plastid genomics of Nicotiana (Solanaceae): Insights into molecular evolution, positive selection and the origin of the maternal genome of Aztec tobacco (Nicotiana rustica). PeerJ 8: e9552. https://doi.org/10.7717/peerj.9552
- Nylander JAA (2004) MrModeltest v2. Program distributed by the author.
- Olmstead RG, Sweere JA (1994) Combining data in phylogenetic systematics: An empirical approach using three molecular data sets in the Solanaceae. Systematic Biology 43(4): 467–481. https://doi.org/10.1093/sysbio/43.4.467
- Philippi RA (1856) Bemerkungen über die Flora der Insel Juan Fernández. Botanische Zeitung, Berlin 14: 641–650.
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67(5): 901–904. https://doi.org/10.1093/sysbio/syy032
- Reiche K (1903) Estudio crítico sobre la Flora de Chile. Solanaceae. Anales de la Universidad de Chile 5: 378–390.
- Rodriguez R, Marticorena C, Alarcón D, Baeza C, Cavieres L, Finot VL, Fuentes N, Kiessling A, Mihoc M, Pauchard A, Ruiz E, Sanchez P, Marticorena A (2018) Catálogo de las plantas vasculares de Chile. Gayana. Botánica 75(1): 1–430. https://doi.org/10.4067/S0717-66432018000100001
- Rodríguez-Caballero G, Roldán A, Caravaca F (2020) Invasive Nicotiana glauca shifts the soil microbial community composition and functioning of harsh and disturbed semiarid Mediterranean environments. Biological Invasions 22(10): 2923–2940. https://doi.org/10.1007/s10530-020-02299-1
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MRBAYES 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Systematic Biology 61(3): 539–542. https://doi.org/10.1093/sysbio/sys029
- Shaw J, Lickey EB, Beck JT, Farmer SB, Liu W, Miller J, Siripun KC, Winder CT, Schilling EE, Small RL (2005) The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. American Journal of Botany 92(1): 142–166. https://doi.org/10.3732/ajb.92.1.142
- Silvestro D, Michalak I (2012) raxmlGUI: A graphical front-end for RAxML. Organisms, Diversity & Evolution 12(4): 335–337. https://doi.org/10.1007/s13127-011-0056-0
- Squeo FA (2000) Libro rojo de la flora nativa y de los sitios prioritarios para su conservación: Región de Coquimbo. Ediciones de la Universidad de La Serena, La Serena, Chile.
- Stamatakis A (2014) Raxml version 8: A tool for phylogenetic analysis andpost-analysis of large phylogenies. Bioinformatics (Oxford, England) 30(9): 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
- Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17(5): 1105–1109. https://doi.org/10.1007/BF00037152
- Walpers WG (1844) Synopsis solanacearum, scrophularinarum, orobanchearum et labiatarum in Repertorium botanices systematicae. The New York Botanical Garden 3: е12.
Supplementary material
GenBank accession numbers
Data type: Xlsx file.
Explanation note: GenBank accession numbers for the trnL-trnF, trnS-trn-G, matK and ndhF sequences used in this study. GenBank accessions in bold are new to this study.