Short Communication |
|
Corresponding author: Jovani B. S. Pereira ( jovanibio@gmail.com ) Academic editor: Angelo Troia
© 2019 Jovani B. S. Pereira, Ana Maria Giulietti, Vali J. Pott, Maurício T. C. Watanabe.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Pereira JBS, Giulietti AM, Pott VJ, Watanabe MTC (2019) Rediscovering two Isoetes species in the Brazilian Amazon and Cerrado after 167 years. PhytoKeys 135: 105-117. https://doi.org/10.3897/phytokeys.135.46624
|
Abstract
Isoetes amazonica and I. gardneriana were the first two species of the genus to be collected from Brazil. Isoetes amazonica was gathered by Richard Spruce in the Amazon basin near Santarém in the state of Pará in 1850. Isoetes gardneriana was collected by George Gardner in the current Dianópolis in Tocantins State in 1843. Despite being known for a long time by botanists, these species have not been recollected since then, which raised questions about their taxonomic recognition, current distribution ranges and conservation status. Fieldwork efforts led to the rediscovery of I. amazonica and I. gardneriana after 167 years. These collections enrich our understanding of their habitats and morphologies. We provide here re-descriptions for these species. Based on IUCN criteria, Isoetes amazonica and I. gardneriana should be assigned as data deficient (DD) and endangered (EN), respectively. The rediscovery of these species raises hopes that other areas in Amazon and Cerrado biomes harbour I. amazonica and I. gardneriana, respectively. This study will serve as a basis towards the conservation of these species.
Keywords
Aquatic plants, conservation status, endemic species, fieldworks, Isoetaceae, taxonomy
Introduction
Brazil presents the greatest diversity of plants in the world (
The lycophyte genus Isoetes L. is globally distributed with an estimated 250 species (
Isoetes amazonica A. Braun and I. gardneriana Kunze ex A. Braun were the first two Isoetes species to be collected and described from Brazil. Isoetes amazonica was first collected by Richard Spruce in September 1850 from inundated shores of the Tapajós river near Santarém municipality in the state of Pará (
Motivated by these issues, we embarked on an attempt to rediscover these species in both the type localities and other similar environments in Amazon basin and Brazilian Cerrado.
Material and methods
For Isoetes amazonica, fieldwork was carried out along both banks of the Tapajós river, near the district Alter do Chão, municipality of Santarém, in the state of Pará, Brazil, in September 2016 and July 2017. For Isoetes gardneriana, fieldwork efforts were carried out along the margins of the Preto river in Formosa do Rio Preto (Bahia) in January 2018. Additional efforts to find this species took place in other Brazilian Cerrado areas: Ondas river, Barreiras (Bahia) ‒ ca. 200 km away from the type location ‒ in January 2018; Parque Nacional Serra da Mesa (Maranhão) ‒ 500 km away from the type location ‒ in November 2017; Parque Nacional da Serra do Cipó ‒ 900 km away from the type location ‒ in June 2018; Fazenda Modelo, Campo Experimental da Embrapa, Terenos (Mato Grosso do Sul) ‒ 1200 km away from the type location ‒ in November 2017.
Besides field trips, specimens from the following herbaria were consulted to check for previous records of these species (acronyms following
We checked the total monthly precipitation and average monthly maximum and minimum temperatures of the environments of these species’ localities to understand the influence of both flooding and drought in their habitats and life forms. For I. amazonica, the climatic data were collected from the meteorological station located in Belterra in the state of Pará and made available by “Instituto Nacional de Meteorologia” (
Habitat, life form, colour, size and ornamentation of the mega- and microspores, the proportion of the sporangium wall covered by the velum and the sporangial wall colouration were used in the identification of the species. The megaspores and microspores were analysed using scanning electron microscopy (SEM). Images of the spores were made by transferring the spores to aluminium stubs coated with a carbon adhesive. The stubs were then coated with gold-palladium-alloy in a sputter-coater for 180 s and then digitally imaged using a Zeiss SIGMA VP.
Since megaspore ornamentations are essential for the correct species identification, the absence of detailed images of spores during the determination process may have potentially led to the name I. gardneriana being misused for several collections of I. panamensis Maxon & C.V.Morton sensu lato. We consulted these materials to check whether the identification was correct or not in these cases. Amongst these materials were collections from: Paraguay in 1878 (Balansa 3294, P [P00170381, P00573953, P04459456]); municipality of Barreiras in Bahia, Brazil, in 1971 (Irwin 31615, P [P01591973]); an area next to type location of I. gardneriana in the municipality of Formosa do Rio Preto in Bahia, Brazil, in 2015 (Labiak 5783, UPCB with duplicates in NY [NY2697584]). In this step, megaspores of these materials were removed, images were taken using SEM and then compared with the type of I. gardneriana. We used both qualitative and quantitative characters to identify the species. The terminology used for the description of the spores follows that of
Results
Rediscovering I. amazonica after 167 years and re-description of the species
Isoetes amazonica
Description
Stems globose, 0.35‒0.7 cm wide, 3-lobate. Leaves 0.45‒1 mm wide at mid length, 4‒17 cm long, 9‒23 per individual, filiform, straight, ascending, apex acute; alae 0.8‒4.5 cm long, extending from the base 1/10 ‒ 1/4 of total leaf length, hyaline, membranaceous, attenuate. Subula present, olive green, trigonal. Labium present, 0.5‒0.7 × 0.9‒1.1 mm long, cordate. Ligule 2.5‒3 × 1‒1.2 mm, hyaline, triangular. Velum 0.1‒0.2 mm along the lateral edges of the sporangium, rudimentary. Sclerified phyllopodia absent. Sporangium at the base of the leaf, 2‒2.5 × 1.8‒2.5mm, elliptic, hyaline, light brown, brown dots present or absent. Megaspores white, 420‒512 (‒590) µm in equatorial diameter (average = 490 µm), trilete; laesurae as wide as high or higher than wide, 40‒53 × 35‒47 µm; proximal surface verrucate, projections 24‒41.4 × 24‒46 µm; equatorial ridges arched, slightly sinuous; distal surface verrucate, macroscuptural projections 25‒45 × 25‒46 µm. Microspores 28‒32 µm long (average = 30 µm), proximal surface echinate, distal surface sparsely echinate.
Type
Brazil. Province of Pará: inundated places near Santarém, Sept 1850, Spruce 1081, (holotype: B! [B200107121]; isotype: K! [K000574506], P! [P00573942; P00573943]).
Remarks
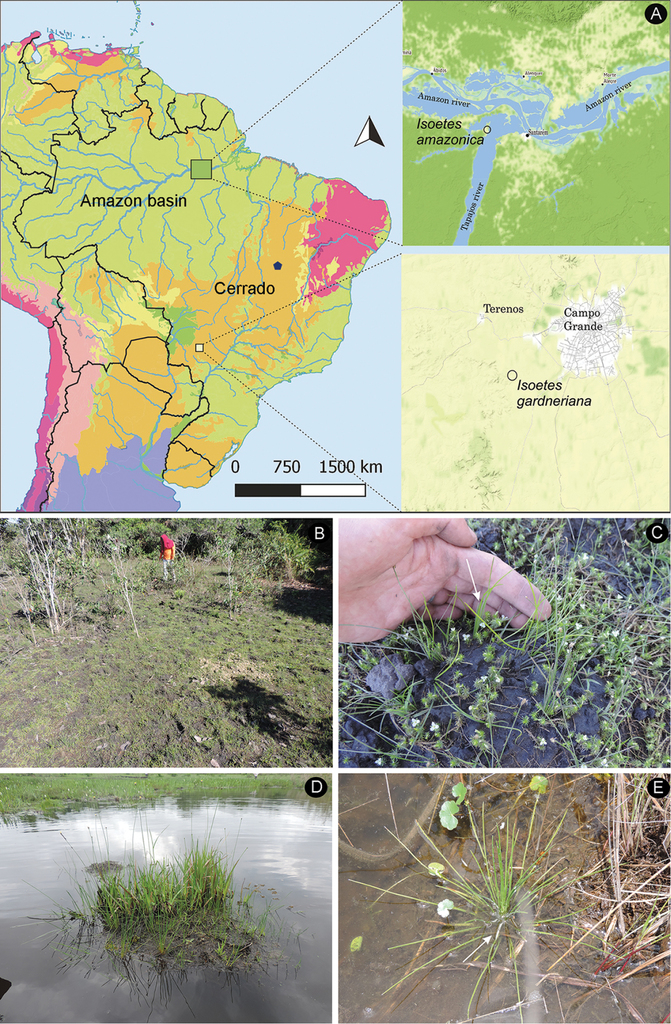
Isoetes amazonica was rediscovered at its type location in July 2017 after 167 years (Pereira 1015, MG). This species was found in a single area at approximately 2.5 km from the left bank of the Tapajós river at the geographical coordinates 2°24'15.15"S, 55°3'1.89"W (Figure
Geographic distribution, habit and habitat of Isoetes amazonica and I. gardneriana A location where Isoetes amazonica and I. gardneriana were rediscovered in Brazil (type location of I. gardneriana in blue pentagon) B–C Isoetes amazonica (Pereira 1015, MG): B habitat C habit D–E Isoetes gardneriana (Pereira 1028, MG): D habitat E habit.
New record of I. gardneriana at about 1200 km away from its type location and re-description of the species
Isoetes gardneriana
Description
Stems globose, 2.5‒4 cm wide, 3 or 4-lobate. Leaves 1.0‒1.8 mm wide at mid length, 32‒45 cm long, 30‒90 per individual, linear, straight, ascending, apex acute; alae 7‒15 cm long, extending from the base 1/5‒2/5 of total leaf length, hyaline or light brown, chartaceous, attenuate. Subula present, olive green, trigonal. Labium present, 2.5‒3.5 × 4‒6 mm, cordate. Ligule not observed. Velum > 0.4 mm along the lateral edges of the sporangium, rudimentary. Sclerified phyllopodia absent. Sporangium at the base of the leaf, 8‒18 × 4.3‒7 mm, oblong, hyaline, brown dots absent. Megaspores grey, 490‒650 µm in equatorial diameter (average = 540 µm), trilete; laesures higher than wide, 40‒50 × 11‒16 µm; proximal surface tuberculate, macrosculptural projections 20‒39 × 13‒24 µm; equatorial ridges arched, straight; distal surface tuberculate, projections 24‒44 × 17‒34 µm. Microspores 33‒40 µm long (average = 37 µm), proximal and distal surface smooth or sparsely microechinate.
Type
Brazil. Province of Goyaz: Missiones Duro, Sept 1841, Gardner 3563, (holotype: B! [B200107577]; isotype: BM [BM000097912,] E! [E00429095], K! [K000574505]).
Remarks
Despite our intensive fieldwork efforts in the Brazilian Cerrado, I. gardneriana was only rediscovered in Terenos in the state of Mato Grosso do Sul at the geographical coordinates 20°33'32"S, 54°47'23"W. This area is located at about 1200 km away from its type location (Fig.
Isoetes gardneriana was found in a pond along with Rhynchospora corymbosa (L.) Britton, Pontederia cordata L. and Xyris spp. (Fig.
Morphologically, the individuals have ascending leaves, rudimentary vela, elliptic sporangia and 3-lobate corms (or more rarely 4). The megaspores are brown, sparsely verrucate, 490‒650 µm diameter (vs. 548‒615 µm), with knife-like laesurae (Fig.
On the other hand, none of the analysed herbarium collections appeared (Balansa 3294, Irwin 31615 and Labiak 5783) to be I. gardneriana. The megaspores of these collections are both qualitatively and quantitatively distinct from the type of I. gardneriana. The Balansa and Labiak collections have baculate-tuberculate megaspores and Irwin’s collection revealed baculate-clavate megaspores (Fig.
Boxplots showing quantitatively the variation in the size of the macro-ornamentation projections of the megaspores of I. gardneriana and I. panamensis sensu lato. In the proximal surface, the projects of the macro-ornamentation are narrower and shorter in I. gardneriana than in I. panamensis s.l. In the distal surface, the macro-ornamentation projections are slightly narrower and considerably shorter in I. gardneriana than in I. panamensis s.l..
Discussion
Although fieldwork investigation is fundamental to improve our understanding about how human impacts on biological systems can be recognised, mitigated or averted, fieldwork has considerably decreased in the past decades with negative implications for global biodiversity conservation (
Both proper habitat and taxonomic identification of species are the first steps towards conserving biodiversity. Amongst the aquatic macrophytes, Isoetes is one of the most threatened groups (
Even though I. amazonica was collected only during the dry season, we can make inferences about its habitat conditions and life forms during the year, using climatic data (see
Despite the importance of habitat data for species characterisation, they provide a limited amount of information for species distinction if two or more similar species occupy the same habitat and/or show morphological convergence due to habitat adaptation (e.g.
Additionally, an Isoetes population from Itaparica lake in Xique-Xique (Bahia State) in north-eastern Brazil was tentatively identified as I. amazonica (Harley 19109, K). However, despite its resemblance to I. amazonica by size of megaspores and number and size of leaves, the presence of brown sporangium (vs. hyaline) and its occurrence in Caatinga (vs. Amazon) leads us to believe that this population is either a variant of I. luetzelburgii U. Weber or an undescribed species.
The geographical distribution of the species is crucial in assessing their conservation status (
Isoetes amazonica is currently known from a single locality next to a cattle farm and, thus, it is prone to the effects of human activities within a short time. However, given its potential occurrence in other areas in the Amazon basin and the lack of current knowledge about its distribution range, I. amazonica should be assessed as data deficient (DD), according to IUCN criteria (
In conclusion, the rediscovering of these species raises hopes that other areas in Amazon and Cerrado biomes still harbour Isoetes amazonica and I. gardneriana, respectively. We hope that these rediscoveries spark research towards a deeper understanding of the life history of Isoetes and provide information for any future efforts to protect Isoetes amazonica and I. gardneriana from extinction.
Acknowledgements
We thank Daniela Zappi, Diego Pinangé, Paulo Labiak, Raymond Harley and Thaís Almeida for assistance in fieldworks. We are also grateful to Jim Hickey, Alexandre Salino and Angelo Troia for their valuable comments on the manuscript. ICMbio provided licence permits to JBSP (35897). CNPq provided a Senior Grant to AMG. This study was supported through a fellowship from Capes/ITV (88887.130616/2016) to JBSP.
References
- Carvalho FM, Marco P, Ferreira LG (2009) The Cerrado into-pieces: Habitat fragmentation as a function of landscape use in the savannas of central Brazil. Biological Conservation 142(7): 1392–1403. https://doi.org/10.1016/j.biocon.2009.01.031
- CEMTEC/MS (2019) Centro de Monitoramento do Tempo e Clima no estado do Mato Grosso do Sul, Brasil. http://www.cemtec.ms.gov.br/boletins-meteorologicos [accessed 10.06.2019]
- Forzza RC, Baumgratz JFA, Bicudo CEM, Canhos DAL, Carvalho Jr AA, Coelho MAN, Costa AF, Costa DP, Hopkins MG, Leitman PM, Lohmann LG, Lughadha EN, Maia LC, Martinelli G, Menezes M, Morim MP, Peixoto AL, Pirani JR, Prado J, Queiroz LP, Souza S, Souza VC, Stehmann JR, Sylvestre LS, Walter BMT, Zappi DC (2012) New Brazilian floristic list highlights conservation challenges. Bioscience 62(1): 39–45. https://doi.org/10.1525/bio.2012.62.1.8
- Gardner G (1849) Travels in the Interior of Brazil, Principally Through the Northern Provinces, and the Gold and Diamond Districts, During the Years 1836–1841. Reeve Benham & Reeve, London, 428 pp.
- Hickey RJ, Macluf CC, Link-Pérez M (2009) Isoetes maxima, a new species from Brazil. American Fern Journal 99(3): 194–199. https://doi.org/10.1640/0002-8444-99.3.194
- INMET (2019) Instituto Nacional de Meteorologia, Brasil. http://www.inmet.gov.br/portal/index.php?r=bdmep/bdmep [accessed 4.08.2019]
- IUCN (2016) The IUCN red list categories and criteria, version 3.1. IUCN Red List Unit, Gland, Switzerland and Cambridge, UK. http://www.iucnredlist.org/technical-documents/categories-and-criteria [accessed 4.02.2019]
- Jiménez-Mejías P, Benítez-Benítez C, Fernández-Mazuecos M, Martín-Bravo S (2017) Cut from the same cloth: The convergent evolution of dwarf morphotypes of the Carex flava group (Cyperaceae) in Circum-Mediterranean mountains. PLoS One 12(12): e0189769. https://doi.org/10.1371/journal.pone.0189769
- Kuhn FAM (1884) Isoetaceae. In: Martius CFP (Eds) Flora Brasiliensis v. 1, part 2., 645–648. http://florabrasiliensis.cria.org.br/search?taxon_id=1403 [accessed 4.02.2019]
- Laurance WF, Vasconcelos HL, Lovejoy TE (2000) Forest loss and fragmentation in the Amazon: Implications for wildlife conservation. Oryx 34(1): 39–45. https://doi.org/10.1046/j.1365-3008.2000.00094.x
- Marengo JA, Espinoza JC (2016) Extreme seasonal droughts and floods in Amazonia: Causes, trends and impacts. International Journal of Climatology 36(3): 1033–1050. https://doi.org/10.1002/joc.4420
- Middelboe AL, Markager S (1997) Depth limits and minimum light requirements of freshwater macrophytes. Freshwater Biology 37(3): 553–568. https://doi.org/10.1046/j.1365-2427.1997.00183.x
- Moreira SM, Pott A, Pott VJ, Damasceno GA (2011) Structure of pond vegetation in a vereda in the Brazilian Cerrado. Rodriguésia 62(4): 721–729. https://doi.org/10.1590/S2175-78602011000400002
- Murphy K, Efremov A, Davidson TA, Molina-Navarro E, Fidanza K, Crivelari TCB, Chambers P, Tapia JG, Varandas SM, Springuel I, Kennedy M, Mormul RP, Dibble E, Hofstra D, Lukács BA, Gebler D, Baastrup-Spohr L, Urrutia-Estrada J (2019) World distribution, diversity and endemism of aquatic macrophytes. Aquatic Botany 158: 103–127. https://doi.org/10.1016/j.aquabot.2019.06.006
- Pereira JBS, Salino A, Arruda A, Stützel T (2016) Two new species of Isoetes (Isoetaceae) from northern Brazil. Phytotaxa 272(2): 141–148. https://doi.org/10.11646/phytotaxa.272.2.5
- Pereira JBS, Stützel T, Schulz C (2017) Isoetes nana, a new species from the coastal mountains of southeastern Brazil. PhytoKeys 89: 91–105. https://doi.org/10.3897/phytokeys.89.20171
- Prado J, Sylvestre LS, Labiak PH, Windish PG, Salino A, Barros ICL, Hirai RY, Almeida TE, Santiago ACP, Kieling-Rubio MA, Pereira AFN, Ollgaard B, Ramos CGV, Mickel JT, Dittrich VAO, Mynssen CM, Schwartsburd PB, Condack JPS, Pereira JBS, Matos FB (2015) Diversity of ferns and lycophytes in Brazil. Rodriguésia 66(4): 1073–1083. https://doi.org/10.1590/2175-7860201566410
- Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A (2007) Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology 143(1–2): 1–81. https://doi.org/10.1016/j.revpalbo.2006.06.008
- R Core Team (2013) A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Wien. http://www.R-project.org/ [accessed 10.08.2018]
- Ríos-Saldaña CA, Delibes-Mateos M, Ferreira CC (2018) Are fieldwork studies being relegated to second place in conservation science? Global Ecology and Conservation 14: e00389. https://doi.org/10.1016/j.gecco.2018.e00389
- Sousa-Baena MS, Garcia LC, Peterson AT, Brotons L (2014) Completeness of digital accessible knowledge of the plants of Brazil and priorities for survey and inventory. Diversity & Distributions 20(4): 369–381. https://doi.org/10.1111/ddi.12136
- Strassburg BBN, Brooks T, Feltran-Barbieri R, Iribarrem A, Crouzeilles R, Loyola R, Latawiec AE, Oliveira Filho FJB, Scaramuzza CAM, Scarano FR, Soares-Filho B, Balmford A (2017) Moment of truth for the Cerrado hotspot. Nature Ecology & Evolution 1: 0099. https://doi.org/10.1038/s41559-017-0099
- Taylor WC, Hickey RJ (1992) Habitat, evolution, and speciation in Isoetes. Annals of the Missouri Botanical Garden 79(3): 613–622. https://doi.org/10.2307/2399755
- Thiers BM (2018) The World’s Herbaria 2017: A summary Report Based on Data from Index Herbariorum (2nd ed.). The New York Botanical Garden Press, 19 pp. https://doi.org/10.3897/biss.2.26440
- Troia A, Pereira JBS, Kim C, Taylor WC (2016) The genus Isoetes (Isoetaceae): A provisional checklist of the accepted and unresolved taxa. Phytotaxa 277(2): 101–145. https://doi.org/10.11646/phytotaxa.277.2.1