Citation: Robinson H, Keeley SC, Skvarla JJ, Chan R (2014) Two new genera, Hoffmannanthus and Jeffreycia, mostly from East Africa (Erlangeinae, Vernonieae, Asteraceae). PhytoKeys 39: 49–64. doi: 10.3897/phytokeys.39.7624
Two genera of Vernonieae subtribe Erlangeinae with Type A pollen, 5-ribbed achenes, and blunt-tipped sweeping hairs on the styles are described as new, Hoffmannanthus with one species and with Vernonia brachycalyx O. Hoffm. as type, and Jeffreycia with five known species, with Vernonia zanzibarensis Less. as type. Vernonia abbotiana O. Hoffm. is neotypified and is an older name for V. brachycalyx.
Africa, Compositae, Erlangeinae, Hoffmannanthus, Jeffreycia, new genera, Vernonieae
The dismantling of the overly broad concept of Vernonia Schreb. in the Old World was begun by
Specimens examined were from the U.S. National Herbarium in Washington, DC. Microscopic structures were examined mostly using material mounted in Hoyer’s Solution (
The genera Hoffmannanthus and Jeffreycia, described here as new, are evidently closely related, but of these only Hoffmannanthus has had its DNA sequenced (
The typical element of the subtribe Erlangeinae with the genera Erlangea Sch. Bip., Bothriocline Oliv. ex Benth., and Cyanthillium Blume consists of herbaceous plants with mostly lophate, triporate pollen, symmetrically T-shaped hairs, and sharply pointed sweeping hairs on the styles. In contrast, the two genera described herein are shrubbier or weakly arborescent with sublophate, tricolporate pollen having a continuous perforated tectum between the colpi, simple or asymmetrical non-T-shaped hairs, and blunt tips on the sweeping hairs.
The two new genera, Hoffmannanthus and Jeffreycia (Fig. 1), share one feature found in many Old World Vernonieae, namely the sweeping hairs which are restricted to the branches of the style and do not extend onto the upper shaft, a feature otherwise a defining characteristic of the tribe Vernonieae and the subfamily Cichorioideae. Jeffreycia may be the most extreme in this character, with the sweeping hairs usually failing to even reach the bases of the style branches.
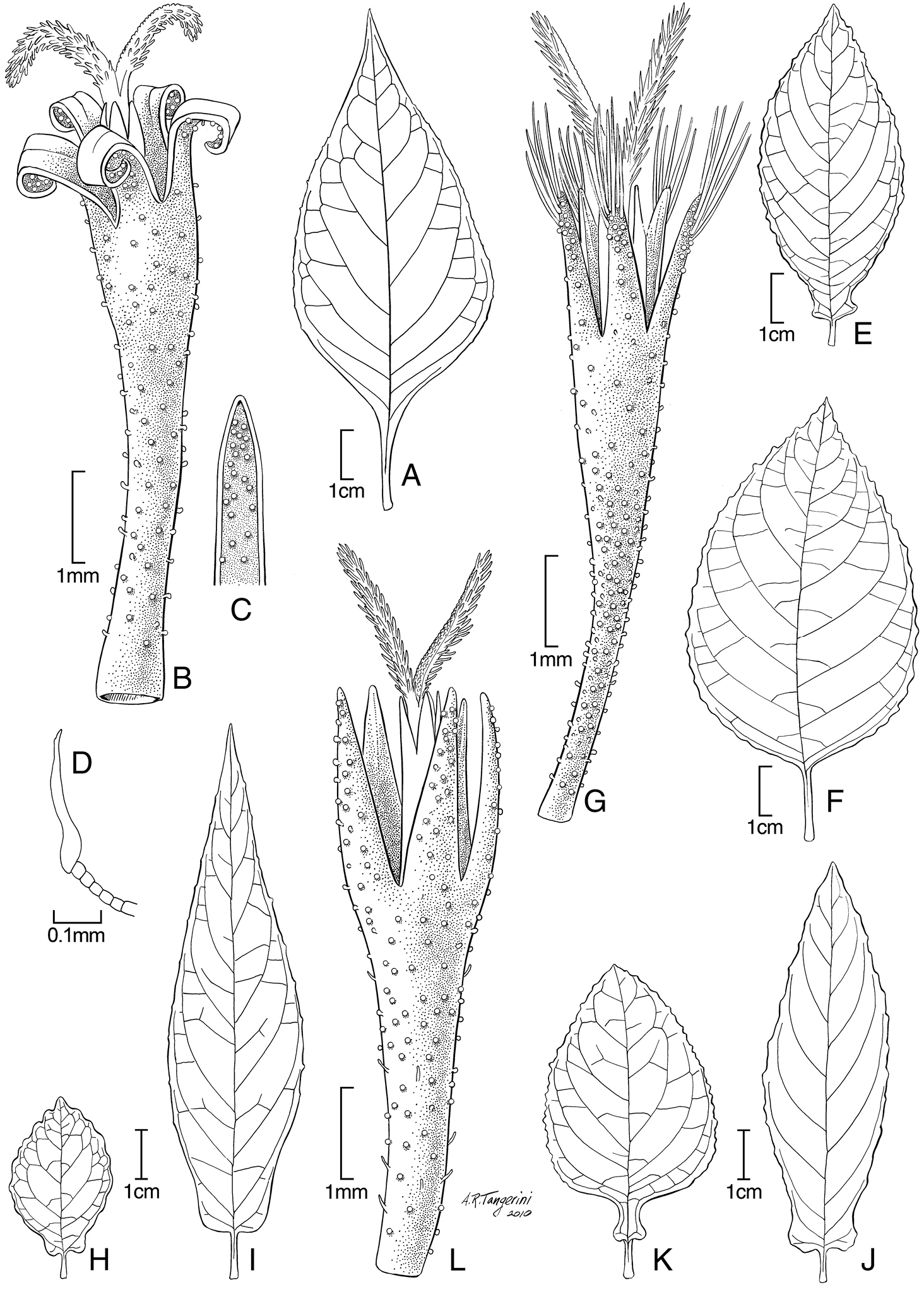
A–D Hoffmannanthus abottianus O. Hoffm. E–G Jeffreycia zanzibarensis (E form with panduriform leaves F, G typical form) H Jeffreycia hildebrandtii I Jeffreycia amaniensis J Jeffreycia usambarensis K, L Jeffreycia zeylanica. A, F, H–K leaf B, G, L corolla C lobe of corolla D stem hair (A, D from Kenya, Gichuon 10, US B, C from Ethiopia, Burger 1816, US E from Rulangaranga et al. 83, US F, G from Tanzania, Faulkner 3866, US H from Tanzania, Stuhmann 7537, US I from Tanzania, Peter O III 7, US J from Tanzania, Peter O IV 15, US K, L from Sri Lanka, Silva 4, US).
These genera are treated here together to allow a more effective direct comparison. The most obvious differences between Hoffmannanthus and Jeffreycia are found in the hairs of the stems, the shape of the leaves, and in details of the corolla lobes. The hairs on the stems of Hoffmannanthus have a rather long, uniseriate multicellular stalk with an elongate, asymmetrically mounted horizontal cap cell at the tip, these hairs being what could be called L-shaped (Fig. 1D). In contrast, the hairs on the stems of Jeffreycia species are simple and unbranched. The leaves of Hoffmannanthus have long petioles below the distinct basal acumination of the blade, and have no auricles on the blades (Fig. 1A). The leaves of all Jeffreycia except the typical variant of the type species, Jeffreycia zanzibarensis (Less.) H. Rob., S. Keeley & Skvarla have short petioles and blades with auricles projecting laterally at the base (Fig. 1E, F, I–K). The corolla lobes in Hoffmannanthus are oblong-triangular, and usually recurved at maturity (Fig. 1B, C). Jeffreycia has corolla lobes that are strictly lanceolate, the sides not parallel in any part, but evenly convergent from the base to the tip (Fig. 1G, L). The lobes are erect though sometimes withered when dry, but never recurved. A less obvious difference is the tendency for the pappus bristles in Hoffmannanthus to be sordid or even rufous and broader in the distal half, while those of Jeffreycia tend to be white and narrowed above.
The second genus treated here, Jeffreycia, includes three of the species mistakenly placed in Gymnanthemum in the subtribe Gymnantheminae by
A genus that is possibly closely related to Jeffreycia is the recently described Uniyala H. Rob. & Skvarla of India and Sri Lanka (
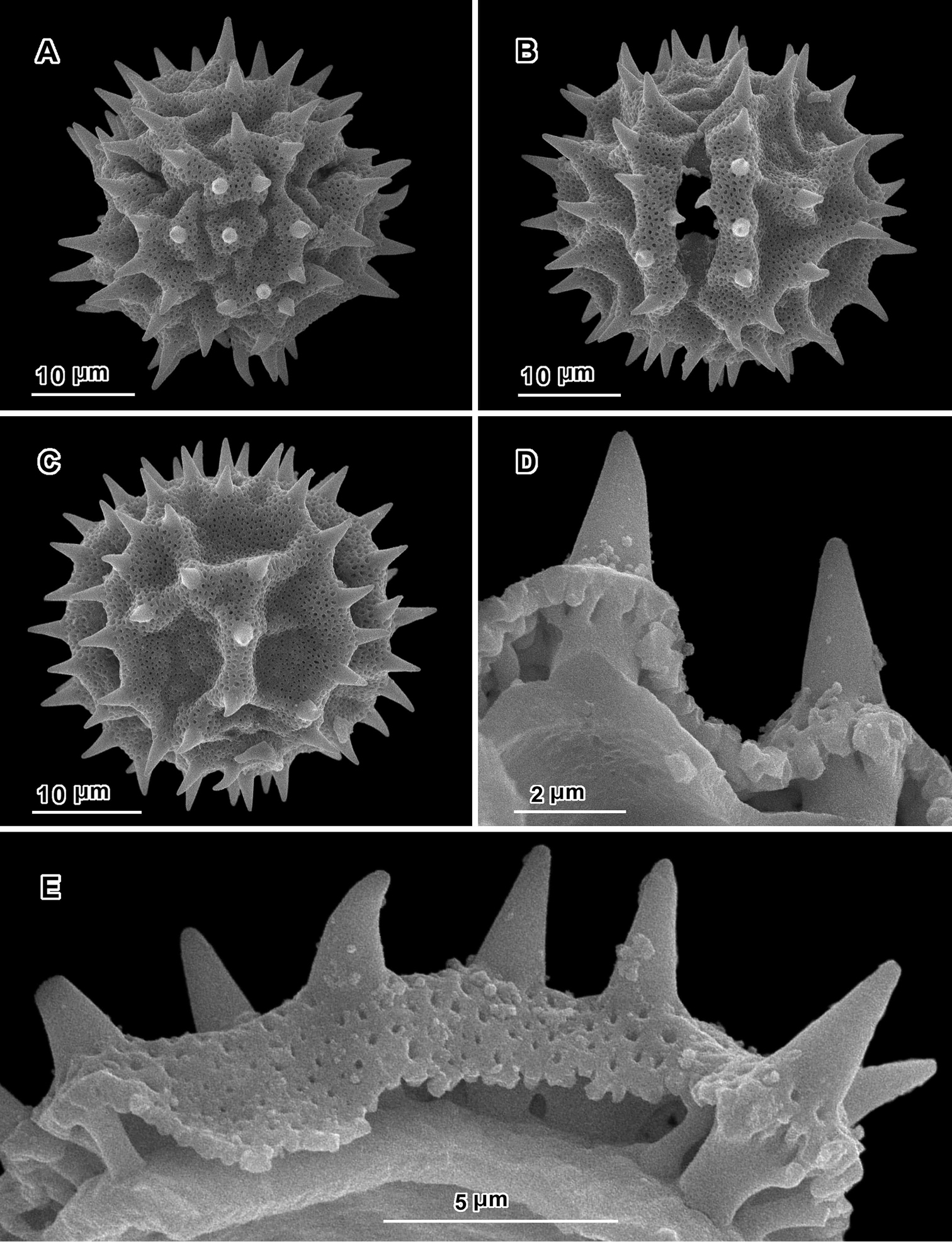
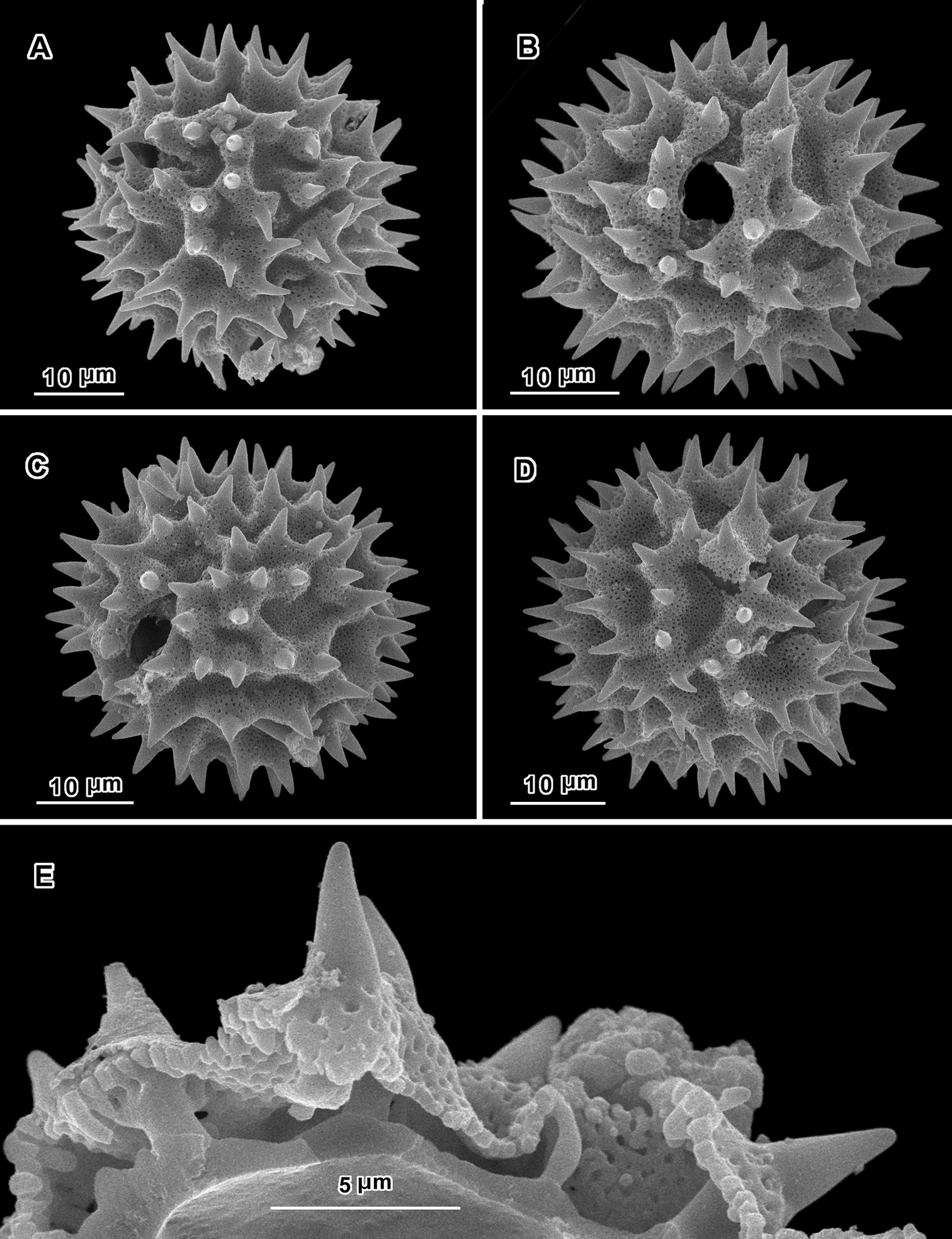
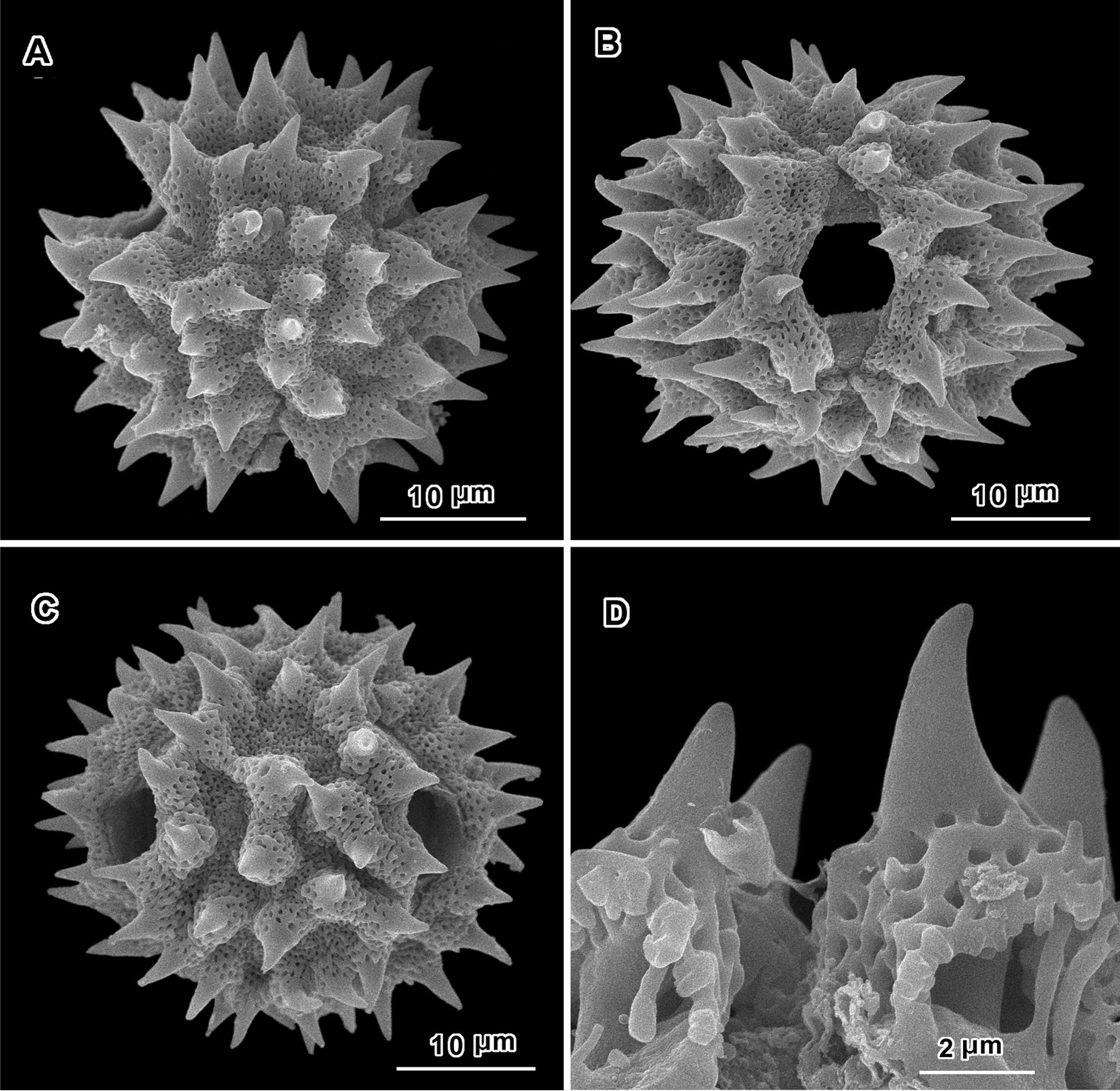
An apparent additional distinction between Uniyala and both Jeffreycia and Hoffmannanthus is the pollen. In all three genera, the pollen is approximately the same size, ca. 30 µm in diam. when dry, up to 50 µm in diam. in fluid, type A tricolporate or sublophate with a continuous perforated tectum between the colpi. However, in Uniyala the pollen has incipient muri more defined with fewer, larger incipient lacunae (
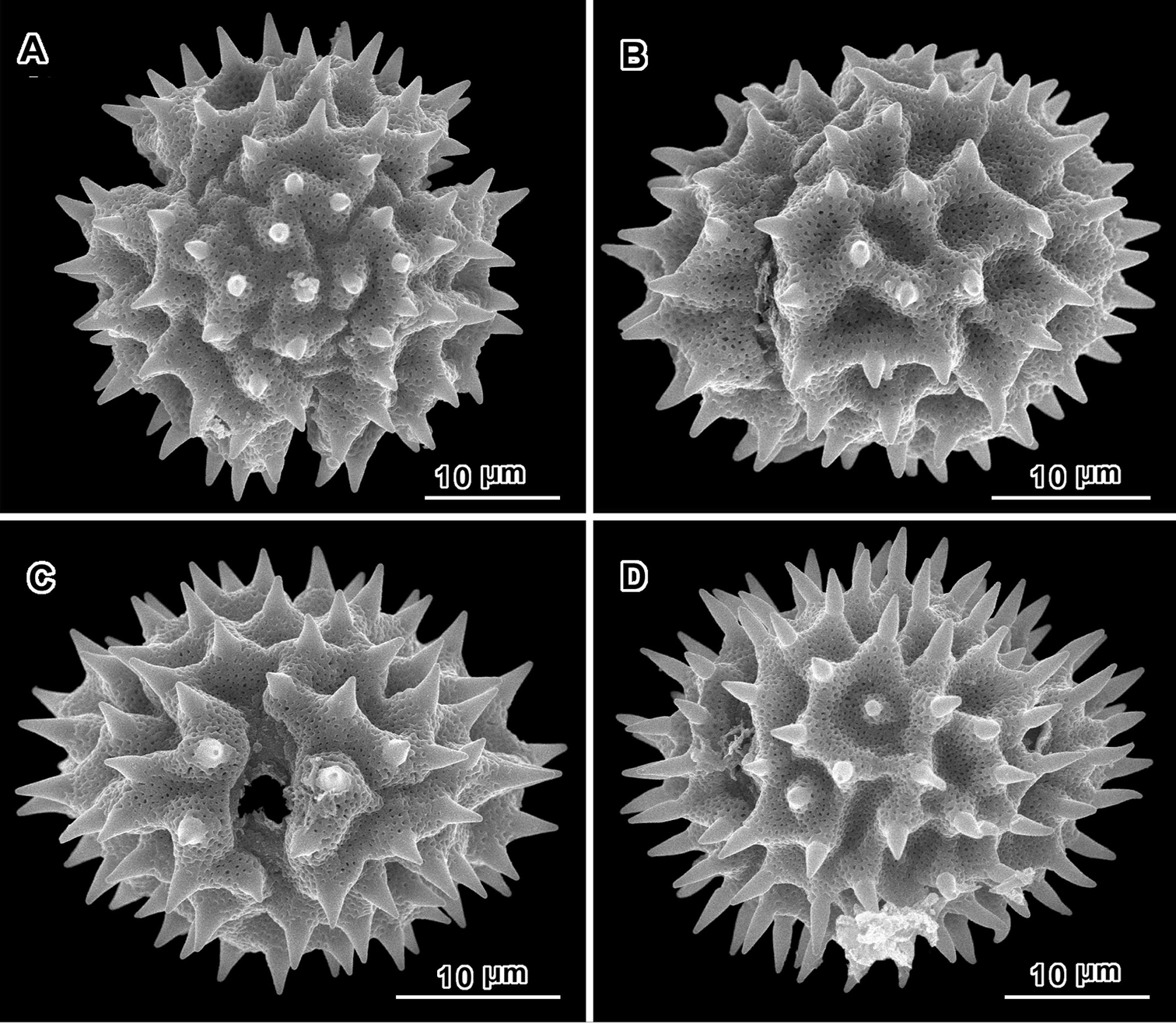
Scanning electron micrographs of Hoffmannanthus abbotianus pollen (Kenya, Gichuon 10, US). A polar view B equatorial view C lateral view D, E views of fractured grains.
Scanning electron micrographs of Jeffreycia zanzibarensis pollen (Tanzania, Faulkner 3866, US). A polar view B equatorial view C oblique lateral view D lateral view E view of fractured grain.
Scanning electron micrographs of Jeffreycia zeylanica pollen (Ceylon, K. Wirawan 695, US). A polar view B equatorial view C lateral view D fractured grain.
Scanning electron micrographs of Jeffreycia pollen: A, B J. usambarensis (Ost-Africa, A. Peter 0 IV 15, US) C, D J. hildebrandtii (Kenya, J. Lauranos 12468, US). A polar view B, D lateral view C equatorial view.
The generic segregates of Vernonia in tropical Africa are only partially resolved. A treatment of all but two species in Southern Africa is nearly complete, but it does not include any close relatives of the two genera described here. Nevertheless, the presently recognized tropical African genera that have previously been placed in Vernonia can be partially distingushed by the following key. Its utility is limited by the number of segregates of tropical African Vernonieae that remain untreated.
| 1 | Leaves triplinervate; corollas sometimes yellow | Distephanus Cass. |
| – | Leaves with pinnate venation; corollas only purplish, bluish or white | 2 |
| 2 | Strictly herbaceous | 3 |
| – | Weakly to strongly woody, prostrate shrubs to small trees | 7 |
| 3 | Perennial herbs with root crown with densely pilose apex; often flowering before leaves appear | Vernonella Sond. |
| – | Annual or perennial herbs with rootstock not densely pilose at apex; flowers usually appearing after leaves | 4 |
| 4 | Mostly small herbs with pollen either sublophate, triporate or pantoporate | (most Erlangeinae and Centrapalinae) Cabobanthus H. Rob., Centrapalus Cass., Cyanthillium Blume, Lettowia H. Rob. & Skvarla, Oocephala (S.B. Jones) H. Rob., Orbivestus H. Rob., Parapolydora H. Rob., Polydora Fenzl, Vernoniastrum H. Rob. |
| – | Pollen lophate with three fully developed colpi | 5 |
| 5 | Weak herbs with involucral bracts bearing smooth broad shields abaxially, not marginally toothed or apically appendaged | Anathura H. Rob. & Skvarla |
| – | Coarse herbs to subshrubs; involucral bracts marginally toothed or apically appendaged | (Linziinae) 6 |
| 6 | Corollas with lobes not longer than the throat; pappus segments flattened; muri of pollen echinate | Baccharoides Moench |
| – | Corollas with lobes longer than throat; pappus of capillary segments; muri of pollen psilate | Linzia Sch.Bip. ex Walp. |
| 7 | Involucral bracts broad, smooth abaxially with broad median shield; inner bracts often deciduous | Gymnanthemum |
| – | Involucral bracts oblong-lanceolate to linear-lanceolate, without broad smooth shield abaxially; inner bracts persistent | 8 |
| 8 | Pollen lophate, with little or no perforated tectum | Ambassa Steetz |
| – | Pollen sublophate, with continuous perforated tectum in intercolpi | 9 |
| 9 | Leaf blades tapering into petiole at base; corolla lobes oblong-lanceolate, recurved; hairs of stems with asymmetric cap cells | Hoffmannanthus |
| – | Leaf blades usually with basal auricles; corollas with erect lanceolate lobes; hairs of stems simple | Jeffreycia |
Vernonia brachycalyx O. Hoffm.
Scrambling shrubs; stems slender with solid pith, somewhat deflected at nodes in upper part of vegetative plant and in inflorescence; hairs of stems L-shaped, with long, multicellular, uniseriate stalk and elongate, horizontal cap cell mounted near one end. Leaves alternate, petioles slender and 7–15 mm long below basal acumination of blade; blades ovate, 6–7 times longer than petiole, 5–10 cm long, 1.5–5.0 cm wide, base broadly obtuse to short-acute, narrowly acuminate at petiole, margins remotely denticulate to nearly entire, apex scarcely to gradually acuminate, surfaces pilosulous and with glandular dots, hairs sparser above, dense on larger veins; secondary veins pinnate, with ca. 6 weak secondary veins on each side of midrib, spreading at ca. 40-45° angles. Inflorescences broadly corymbiform, with branches elongate, mostly with small or insignificant bracteoles at bases; peduncles 2–30 mm long. Heads campanulate; involucre much shorter than florets at maturity; involucral bracts in 2–3 series, persistent, oblong-lanceolate, with acute to short-acuminate tips, puberulous outside, pale at base, midvein broadly greenish, percurrent at tip, lateral margins thinly membranous; receptacle scarcely convex, epaleate, epilose. Florets ca. 15 in a head, homogamous, bisexual; corollas violet to purple, narrowly funnelform, with long basal tube, throat short, lobes narrowly oblong-lanceolate, with glandular dots outside; anthers with triangular apical appendages; base of style slightly enlarged, style shaft glabrous, sweeping hairs on style branches elongate with rounded or blunt tips. Achenes 5-angled, with some glandular dots and short setulae, surface with sparse idioblasts and inner layer with small subquadrate or rounded raphids; pappus pale to sordid or rufous, 2 series, inner pappus of many capillary bristles that are slightly broader in distal half, outer pappus of short narrow scales. Pollen grains 40 µm in diam., Type A, sublophate. 2n = 20 (
The name Hoffmannanthus is considered appropriate, since both of the older species names featured here were published by
The genus contains the single species.
urn:lsid:ipni.org:names:77140772-1
The species occurs from Ethiopia, Congo and Uganda in the north to Angola, Malawi and Zambia to the south.
The type specimen of Vernonia abbotiana O. Hoffm. was destroyed in Berlin during the Second World War, and the species was treated by
Neotype of Vernonia abbotiana O. Hoffm. and lectotype of Vernonia brachycalyx O. Hoffm.
Vernonia zanzibarensis Less.
Small to moderate-sized; branching, often scrambling shrubs; stems woody, with narrow solid pith; hairs simple, without cap-cells, sometimes forming loose tomentum. Leaves alternate; petioles distinct, short to elongate; blades ovate to elliptic or panduriform usually with basal auricles, abruptly delimited from petiole at the base, 2.5 to ca. 11 cm long, ca. 1.5–7.5 cm wide, margins crenate or serrate, apices acute to scarcely acuminate, rarely obtuse, upper surface sparsely pilosulous to hispidulous, lower surface sparsely pilosulous to tomentellous, with many glandular dots; secondary veins 4–6 on each side, with unusual somewhat meandering course, spreading at 45–60° angles. Inflorescences terminal, with branches alternate and usually ascending at 30° angles or less, usually with minute bracteoles, sometimes primary bracteoles larger and foliiform; heads crowded at ends of longer branches, with distinct short peduncles; involucral bracts persistent, subimbricate in ca. 4–5 series or with differentiated long, linear-lanceolate basal bracts, bracts, except at base, smooth outside, without median keel; receptacle scarcely convex, epaleate, epilose, with proturberant scars; florets 5–40 in a head; corollas purplish, 5–11 mm long, with some glandular dots outside, few or no hairs below tips, basal tube slender, half as long as the corolla, throat half as long as the limb, ca. as long as the lobes, lobes strictly narrowly lanceolate, with sides straight from base to apex, erect, not recurving, sometimes with stiff hairs at tip; anther thecae without glands, calcarate at base, with narrow tails; endothecial cells without obvious nodes; apical appendages narrowly lanceolate; style with basal node; sweeping hairs with blunt tips, restricted to branches, often lacking for some distance above bases of branches. Achenes 2–4 mm long, with 4 or 5 poorly differentiated angles, with or without glands or setulae, with scattered idioblasts on surface sometimes in vertical series, inner cells of achene wall with distinct firm cell walls, containing small subquadrate raphids; carpopodium stopper-shaped or somewhat turbinate and asymmetrical, with many series of subquadrate, thick-walled cells; pappus white, with inner series capillary, often deciduous, 4.5–7.0 mm long, gradually narrowed to tips, somewhat flattened on outer surface; outer series of short persistent scales, minute to 0.5 mm long. Pollen ca. 40 µm in diam. in fluid, sublophate, tricolporate, with perforated tectum continuous between colpi.
The new genus, Jeffreycia, honors the author of the study of the Vernonieae of East Tropical Africa (
Five species are currently placed in the genus.
In addition to the species listed below,
Regarding the shape of the leaf base, while it is similar to cordate,
urn:lsid:ipni.org:names:77140773-1
Tanzania.
urn:lsid:ipni.org:names:77140774-1
Kenya, Somalia, Tanzania.
urn:lsid:ipni.org:names:77140775-1
Tanzania.
urn:lsid:ipni.org:names:77140776-1
Kenya, Tanzania.
urn:lsid:ipni.org:names:77140777-1
Sri Lanka.
| 1 | Heads with 5–10 florets; with only rather short involucral bracts at base; corollas 5–6 mm long; leaf blades with crenate margins | 2 |
| – | Heads with 20–40 florets; with elongate filiform bracts at base; corollas 7–11 mm long; leaf blades with serrate margins | 3 |
| 2 | Undersurfaces of leaves and branches of inflorescence with short hispidulous pubescence; heads with ca. 10 florets | Jeffreycia hildebrandtii |
| – | Undersurfaces of leaves and branches of inflorescence with long hairs forming tomentum; heads with ca. 5 florets | Jeffreycia zeylanica |
| 3 | Corollas with cluster of long stiff hairs at tips of lobes; leaf blades usually ovate with margins closely serrate; with small bracteoles in the inflorescences | Jeffreycia zanzibarensis |
| – | Corollas lacking cluster of long hairs at tips of lobes; leaf blades oblong or elliptical; with remotely serrate margins; inflorescences with large foliiform primary bracteoles | 4 |
| 4 | Peduncles with appressed stiff hairs; leaf blades shortly pubescent below; inner involucral bracts to ca. 8 mm long | Jeffreycia amaniensis |
| – | Peduncles with mostly spreading, crisped hairs; leaf blades crispate pubescent below, somewhat obscurely pubescent on lamina surface; inner involucral bracts ca. 6 mm long | Jeffreycia usambarensis |
We wish to thank Alice Tangerini, Staff illustrator of the Department of Botany, National Museum of Natural History for the line drawings of the leaves and corollas of Hoffmannanthus and Jeffreycia. We also thank Ingrid Pol-yin Lin of the Department of Botany for the scan of the isolectotype of Vernonia brachycalyx O. Hoffm. designated here as the neotype of Vernonia abbotiana O. Hoffm. Thanks also to the editor, Alexander Sennikov, for many careful observations and corrections.