Citation: Christensen KI, Zarrei M, Kuzmina M, Talent N, Lin C, Dickinson TA (2014) Crataegus ×ninae-celottiae and C. ×cogswellii (Rosaceae, Maleae), two spontaneously formed intersectional nothospecies. PhytoKeys 36: 1–26. doi: 10.3897/phytokeys.36.6784
Crataegus monogyna Jacq. is naturalized in North America, where it has hybridized with native diploid hawthorns at least twice. We provide names for the two nothospecies (as well as for the corresponding nothosections and nothoseries), referring to existing documentation in the literature for nothosp. nov. Crataegus ×ninae-celottiae K.I. Chr. & T.A. Dickinson (C. monogyna × C. punctata Jacq.). New data are provided to further document nothosp. nov. Crataegus ×cogswellii K.I. Chr. & T.A. Dickinson (C. monogyna × C. suksdorfii (Sarg.) Kruschke). In both cases, the striking differences in leaf shape between most New World hawthorns and Old World section Crataegus, and the intermediacy of the hybrids, account for the relative ease with which these hybrids can be recognized. Finally, new sequence data from ITS2 and chloroplast DNA barcoding loci confirm the genetic relationships between the two nothospecies and their respective parents.
North America, hawthorn, hybridization, diploid, leaf shape, ITS2, DNA barcodes
Crataegus monogyna Jacq. is a widespread species of Crataegus sect. Crataegus that occurs in much of Europe, northern Africa and western Asia. Within the area of its natural distribution it hybridizes with several other species of sect. Crataegus, e.g., Crataegus laevigata (Poir.) DC., Crataegus rhipidophylla Gand., Crataegus meyeri Pojark., Crataegus pentagyna Waldst. & Kit. ex Willd., Crataegus orientalis M. Bieb., and Crataegus azarolus L., as well as Crataegus nigra Waldst. & Kit. of sect. Sanguineae (
Sampling. Because the occurrence of Crataegus monogyna and its hybrids is sporadic, most of our samples are non-random, and merely attempt to document the co-occurrence of the parental species and (or) their hybrids (Table 1). Only in the case of the hybrid swarm found at the Cogswell-Foster Preserve in Linn Co., Oregon (site OR1), have we used either the throw of a pair of dice or ignorant person sampling (
Sites in Canada and the United States at which collections of native and naturalized diploid (unless indicated otherwise) Crataegus were made as vouchers for morphological, chemical, and molecular (boldface) observations (Fig. 1–3; Tables 2–4). Sampled individuals are listed by their collector and collection number; principal collector is T. A. Dickinson (D) unless indicated otherwise, as follows: JC, J. Coughlan; CAR, Rebecca Dotterer; EH, E. Harris; EL, E. Y. Y. Lo; RML, R. M. Love; MP, M. A. Purich; Z, P. Zika.
| State/Province | ||||
|---|---|---|---|---|
| Site | Location | Taxon | Individuals | |
| British Columbia | ||||
| BC16 | Central Kootenay R.D., Robson, Broadwater Road, Broadwater Road S side | Crataegus monogyna | 2008-26 | |
| BC | Central Kootenay R.D., Winlaw, next to Winlaw general store (10 miles S of Slocan) on bank of small creek (tributary of Slocan River). | Crataegus suksdorfii Probably polyploid | RML9313 | |
| California | ||||
| CA11 | Humboldt Co., Hwy 36, 6.8 air km W of Bridgeville | Crataegus monogyna | JC001 | |
| CAR4 | Trinity Co., T37N R7W S17 | Crataegus suksdorfii Polyploid? | CAR042 | |
| CAR5 | Siskiyou Co., flood plain of the Scott R., N side of Fay Lane, between jct. Hwy 3 and bridge | Crataegus suksdorfii | 2006-16, 2006-18, 2006-19, 2006-22, CAR044 | |
| CAR7 | Siskiyou Co., T26N R11W S17 | Crataegus suksdorfii Polyploid? | CAR048 | |
| CRRR01 | Sonoma Co., Ragle Ranch, W of Sebastopol | Crataegus monogyna | JC003 | |
| Idaho | ||||
| ID10 | Benewah Co., T44N R1W S8, Soldier Creek, W side of Hwy 3 just N of RR crossing and St. Mary’s R. | Crataegus suksdorfii Probably polyploid | D1608 | |
| Montana | ||||
| MT1 | Powell Co., Dry Creek, N side, edge of meadow and gallery forest | 4× Crataegus suksdorfii | D1614, D1619 | |
| Ontario | ||||
| NTON23 | City of Toronto, Centennial Park, Etobicoke | Crataegus punctata | MP71 | |
| Crataegus ×ninae-celottiae | MP24, MP73 | |||
| ON21 | Bruce Co., Eastnor Twp., Barrow Bay, E side Hwy 9 at S.R. 15 | Crataegus punctata | Dickinson & Nguyen BB4 | |
| ON31 | Middlesex Co., Ilderton, SE corner Denfield Side Road and Ilderton Road (Hwy 16) | Crataegus punctata | EH52, MP56, MP61, 2003-79 | |
| ON40 | City of Toronto, Ashbridges Bay Park | Crataegus punctata | MP35 | |
| ON45 | Durham R.M., Bowmanville, floodplain of Bowmanville Creek | Crataegus monogyna | MP82, MP83, MP98 | |
| Crataegus ×ninae-celottiae | 2002-13, MP84, MP85, MP86 | |||
| Crataegus punctata | MP81 | |||
| ON46 | Perth Co., E side Thames R. North Branch 2 km S of Motherwell | Crataegus punctata | 2008-72A | |
| Oregon | ||||
| OR1 | Linn Co., Willamette Valley, Cogswell-Foster Preserve | Crataegus monogyna (diploid) | EL74, EL78, EL80, EL83, OR1-5, OR1-8, OR1-9, OR1-10, OR1-11, OR1-12, OR1-16 | |
| Triploid Crataegus monogyna | RML C-2003-25 | |||
| Crataegus ×cogswellii | 99FW7-1, 99FW7-2, 99FW7-3, 99FW7-6, 99FW7-7, 99FW7-8, 99FW7-9, 2009-36, EL68, EL71, EL73, EL76, EL77, EL79, EL81, EL82, EL84, EL85, OR1-2, OR1-3, OR1-4, OR1-6, OR1-7, OR1-13, OR1-14, OR1-15, OR1-17, OR1-18, OR1-19, OR1-20, RML8718 | |||
| Crataegus suksdorfii | EL68, EL69, EL72, EL75, OR1-1, RML8709 | |||
| OR | Lane Co, City of Eugene | Crataegus ×cogswellii | RML C-2003-12, RML C-2003-13, RML9304 | |
| OR4 | Douglas Co., Upper Elk Meadow, 28 miles SSE Cottage Grove | Crataegus suksdorfii Probably polyploid | RML8758, RML8767, RML8768 | |
| OR11 | Columbia Co., Sauvie Island, Willow Park Island, Willow Bar Islands beach, just N of Columbia-Multnomah county line, on bank of Columbia River | Crataegus monogyna | EL108 | |
| Crataegus ×cogswellii | Z18482 | |||
| Crataegus suksdorfii | JC117, JC118, JC119 | |||
| OR18 | Jackson Co., Rogue River, Old Stage Rd. 80 m NE of Rogue River Hwy/99 | Crataegus suksdorfii | JC039 | |
| OR22 | Linn Co., Corvallis, KOA Campground, 440 m from hwy 34 on Oakville Rd. SW. specimen 150 m SE of camp entrance | Crataegus suksdorfii | JC060 | |
| OR35 | Skamania Co., Cascade Locks, 110 m N of Cascade Locks Rd., on N side of Forest Ln. | Crataegus suksdorfii | JC092 | |
| OR37 | Multnomah Co., Columbia River Gorge National Scenic Area, 1.5 km NE of Troutdale | Crataegus suksdorfii | JC098, JC102 | |
| OR38 | Columbia Co., Diblee Pt., Site 350 m N of Dike Rd., 1.8 km WNW of Lewis and Clark Bridge | Crataegus suksdorfii | JC136 | |
| Washington | ||||
| WA | Clark Co. S of mouth of Lewis River, ca. 1.5 air miles NNW of Ridgefield | Crataegus suksdorfii | Z18485 | |
| WA8 | Skamania Co., Gifford Pinchot National Forest, Zig Zag Lake, 9 mi NW of Wind R. | Crataegus suksdorfii Probably polyploid | Brooks s.n. | |
| WA10 | Skamania Co., Gifford Pinchot National Forest, Upper Goose Creek Meadow | Crataegus suksdorfii Probably polyploid | RML8909 | |
Results of Neighbor-joining clustering of sequence data for chloroplast DNA barcode loci. GenBank accession numbers indicate cluster affiliation (Cluster 1 or 2) for Crataegus species and their putative hybrids. Details of the BOLD data can be found at dx.doi.org/10.5883/DS-CRATMONO. See Table 1 for sites and collectors; eight-digit ROM Green Plant Herbarium (TRT) accession numbers identify vouchers.
| Taxon / site / BOLD / tree / TRT | Cluster 1 – sections Coccineae and Douglasia | Cluster 2 – section Crataegus | ||
|---|---|---|---|---|
| rbcL-a | trnH-psbA | rbcL-a | trnH-psbA | |
| Crataegus punctata | ||||
| NTON23 TRT103 MP71 TRT00002237 | KC251377 | KC251652 | ||
| ON31 TRT096 MP61 TRT00002228 | KC251375 | KC251650 | ||
| ON31 TRT105 MP56 TRT00002223 | KC251372 | KC251647 | ||
| ON40 TRT101 MP35 TRT00002203 | KC251374 | KC251649 | ||
| ON45 TRT104 MP81 TRT000047 | KC251378 | KC251653 | ||
| ON46 TRT210 2008-72A TRT00000908 | KC251373 | KC251648 | ||
| Crataegus ×ninae-celottiae | ||||
| NTON23 TRT106 MP24 TRT00002199 | KC251376 | KC251651 | ||
| NTON23 TRT203 MP73 TRT00002239 | KC251350 | KC251624 | ||
| ON45 TRT201 MP85 TRT00002250 | KC251348 | KC251622 | ||
| ON45 TRT202 MP86 TRT00002251 | KC251351 | KC251625 | ||
| ON45 TRT204 MP84 TRT00002249 | KC251349 | KC251623 | ||
| Crataegus monogyna | ||||
| BC16 TRT209 2008-26 TRT00002452 | KC251343 | KC251617 | ||
| CA11 TRT274 JC001 TRT00020101 | KC251338 | KC251612 | ||
| CRRR01 TRT275 JC003 TRT00020102 | KC251341 | KC251615 | ||
| ON31 TRT109 2003-79 TRT00000395 | KC251340 | KC251614 | ||
| ON45 TRT108 MP82 | KC251342 | KC251616 | ||
| ON45 TRT190 MP83 TRT00002248 | KC251339 | KC251613 | ||
| ON45 TRT211 MP98 TRT00029476 | KC251336 | KC251610 | ||
| OR1 TRT005 EL80 TRT00000413 | KC251347 | KC251621 | ||
| OR1 TRT006 EL83 TRT00000415 | KC251346 | KC251620 | ||
| OR1 TRT007 EL74 TRT00000416 | KC251344 | KC251618 | ||
| OR TRT030 RML C-2003-25 TRT00000420 | KC251337 | KC251611 | ||
| OR11 TRT143 EL108 TRT00000417 | KC251345 | KC251619 | ||
| Crataegus ×cogswellii | ||||
| OR1 TRT206 EL71 TRT00002650 | KC251627 | |||
| OR1 TRT207 EL85 TRT00002654 | KC251626 | |||
| OR1 TRT208 EL79 TRT00002657 | KC251352 | |||
| Crataegus suksdorfii | ||||
| CAR5 TRT129 2006-19 TRT00001569 | KC251419 | KC251692 | ||
| CAR5 TRT133 2006-22 TRT00001563 | KC251418 | KC251691 | ||
| CAR5 TRT140 2006-16 TRT00001567 | KC251417 | KC251690 | ||
| CAR5 TRT141 2006-18 TRT00001568 | KC251416 | KC251689 | ||
| OR1 TRT205 EL68 TRT00001724 | KC251424 | KC251699 | ||
| WA TRT146 Z18485 TRT00001805 | KC251415 | KC251688 | ||
Voucher specimens for cloned ITS2 data, listing site number (Table 1), collection number, ROM Green Plant Herbarium (TRT) accession numbers, and the GenBank accession numbers for individual clones.
| Taxa | Voucher | GenBank accession number |
|---|---|---|
| Crataegus suksdorfii | OR18 Coughlan, Zarrei, and Shaw JC039 (TRT00020137) | KC173887, KC173888, KC173889, KC173890, KC173891, KC173892, KC173893 |
| OR22 Coughlan, Zarrei, and Shaw JC60 (TRT00020146) | KC173587, KC173588, KC173589, KC173590, KC173591, KC173592 | |
| OR35 Coughlan, Zarrei, and Shaw JC092 (TRT00020153) | KC173957, KC173958, KC173959, KC173960, KC173961, KC173962, KC173963, KC173964 | |
| OR37 Coughlan, Zarrei, and Shaw JC98 (TRT00020159) | KC173595, KC173596, KC173597, KC173598, KC173599, KC173600, KC173601, KC173602, KC173603, KC173604 | |
| OR37 Coughlan, Zarrei, and Shaw JC102 (TRT00020163) | KC174113, KC174114, KC174115, KC174116, KC174117 | |
| OR11 Coughlan, Zarrei, and Shaw JC117 (TRT00020172) | KC174118, KC174119 | |
| OR11 Coughlan, Zarrei, and Shaw JC118 (TRT00020232) | KC174178, KC174179, KC174180, KC174181, KC174182, KC174183 | |
| OR11 Coughlan, Zarrei, and Shaw JC119 (TRT00020234) | KC174144, KC174145, KC174146, KC174147, KC174148, KC174149, KC174150 | |
| OR38 Coughlan, Zarrei, and Shaw JC136 (TRT00020242) | KC173605, KC173606, KC173607, KC173608, KC173609 | |
| CAR5 Dickinson and Lo 2006-16 (TRT00001567) | KC173531, KC173532, KC173533, KC173534, KC173535, KC173536, KC173537, KC173538 | |
| CAR5 Lo and Dickinson 2006-22 (TRT00001563) | KC173522, KC173523, KC173524, KC173525, KC173526, KC173527, KC173528, KC173529, KC173530 | |
| OR1 Lo, Dickinson, and Nguyen EL-68 (TRT00001724) | KC173577, KC173578, KC173579, KC173580, KC173581, KC173582, KC173583, KC173584, KC173585, KC173586 | |
| WA Zika 18485 (=18430, 18417; TRT00001805) | KC173513, KC173514, KC173515, KC173516, KC173517, KC173518, KC173519, KC173520, KC173521 | |
| Crataegus ×cogswellii | OR1 Lo, Dickinson, and Nguyen EL-71 (TRT00002650) | KC173663, KC173664, KC173665, KC173666, KC173667, KC173668 |
| OR1 Lo, Dickinson, and Nguyen EL-79 (TRT00002657) | KC173682, KC173683, KC173684, KC173685, KC173686, KC173687 | |
| OR1 Lo, Dickinson, and Nguyen EL-85 (TRT00002654) | KC173669, KC173670, KC173671, KC173672, KC173673, KC173674, KC173675, KC173676, KC173677, KC173678, KC173679, KC173680, KC173681 | |
| Crataegus monogyna | OR1 Lo, Dickinson, and Nguyen EL-74 (TRT00000416) | KC173650, KC173651, KC173652, KC173653, KC173654 |
| BC16 Dickinson, Lee, and Talent 2008-26 (TRT00002452) | KC173655, KC173656, KC173657, KC173658, KC173659, KC173660, KC173661, KC173662 | |
| ON45 Purich MP98 (TRT00029476) | KC173643, KC173644, KC173645, KC173646, KC173647, KC173648, KC173649 | |
| Crataegus ×ninae-celottiae | ON45 Purich and Talent MP84 (TRT00002249) | KC174184, KC174185, KC174186, KC174187, KC174188, KC174189 |
| ON45 Purich and Talent MP85 (TRT00002250) | KC174190, KC174191, KC174192, KC174193, KC174194, KC174195 | |
| ON45 Purich and Talent MP86 (TRT00002251) | KC173688, KC173689, KC173690, KC173691, KC173692, KC173693 | |
| Crataegus punctata | ON21 Dickinson and Nguyen BB4 (TRT) | KC174266, KC174267, KC174268, KC174269, KC174270, KC174271 |
| ON31 Purich s.n (TRT) | KC174272, KC174273, KC174274, KC174275 | |
| NTON23 Purich, Talent, Nguyen, and Lo MP73 (TRT00002239) | KC173694, KC173695, KC173696, KC173697, KC173698, KC173699, KC173700, KC173701 |
Note that we distinguish the taxon referred to here as Crataegus suksdorfii from the other western North American black-fruited hawthorn with 20 stamens per flower, Crataegus gaylussacia A. Heller. This is because these two taxa are allopatric (
In order to increase our sample for molecular studies we have supplemented field collections of leaf tissue and herbarium vouchers with tissue removed from existing specimens in the ROM Green Plant Herbarium. Historical records of the distribution of Crataegus monogyna were collected from five herbaria across Canada (TRT, MTMG, MT, QFA and UBC). Online databases of Canadian and U.S. herbaria used included ACAD, the Invader Database System of the University of Montana (which contains information for five northwestern states: Idaho, Montana, Oregon, Washington, Wyoming), OSC, and WTU. Distribution maps were prepared from specimen locality data using SimpleMappr (
Morphology. For this study we concentrated on capturing and analyzing leaf shape data, as described elsewhere (
Leaf outline data were collected from two overlapping samples: (1) short shoot leaf spectra (
For each leaf outline we also obtained the area (\(A\)) and perimeter (\(P\)), so as to calculate the inverse of the dissection index described by
Molecular methods. Four DNA barcodes (rbcL, matK, trnH-psbA, and ITS2;
We also analyzed data from another project (Zarrei et al. http://2012.botanyconference.org/engine/search/index.php?func=detail&aid=536 and unpubl. data) in which ITS2 was cloned for a sample of individuals that included 14 Crataegus suksdorfii, four Crataegus monogyna, three Crataegus punctata and two each of the two hybrids (Table 3). Methods for extracting total genomic DNA, marker amplification, cloning, DNA sequencing, and collapsing original sequences to unique sequences (ribotypes) are described elsewhere (Zarrei et al. http://2012.botanyconference.org/engine/search/index.php?func=detail&aid=536 and unpubl. data). Here we report on analyses of a total of 160 ribotypes (Table 3). A recombination test was performed using RDP4 Beta 4.14 (
Flow cytometry. Flow-cytometric methods for quantifying nuclear DNA in embryo and endosperm followed
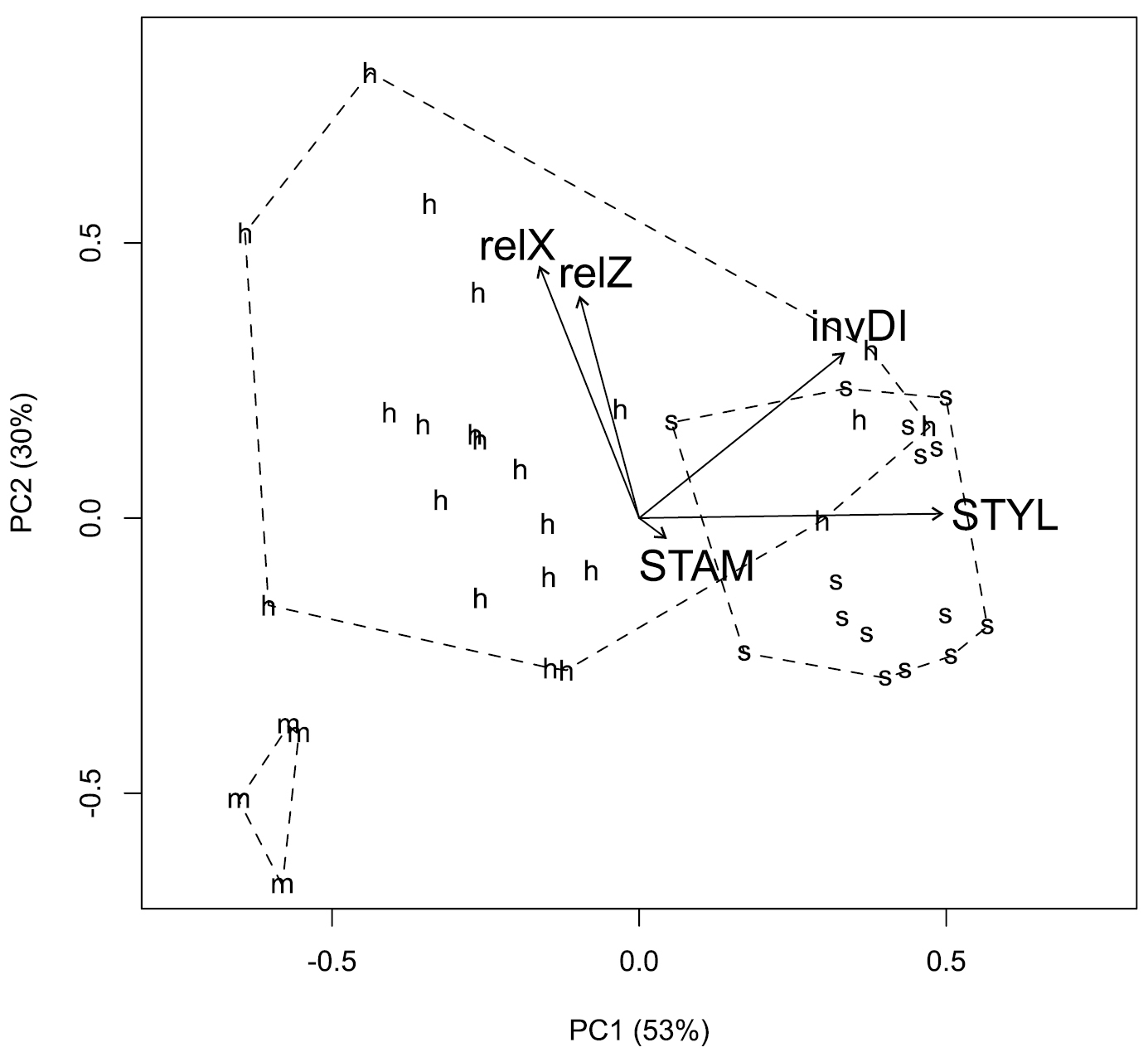
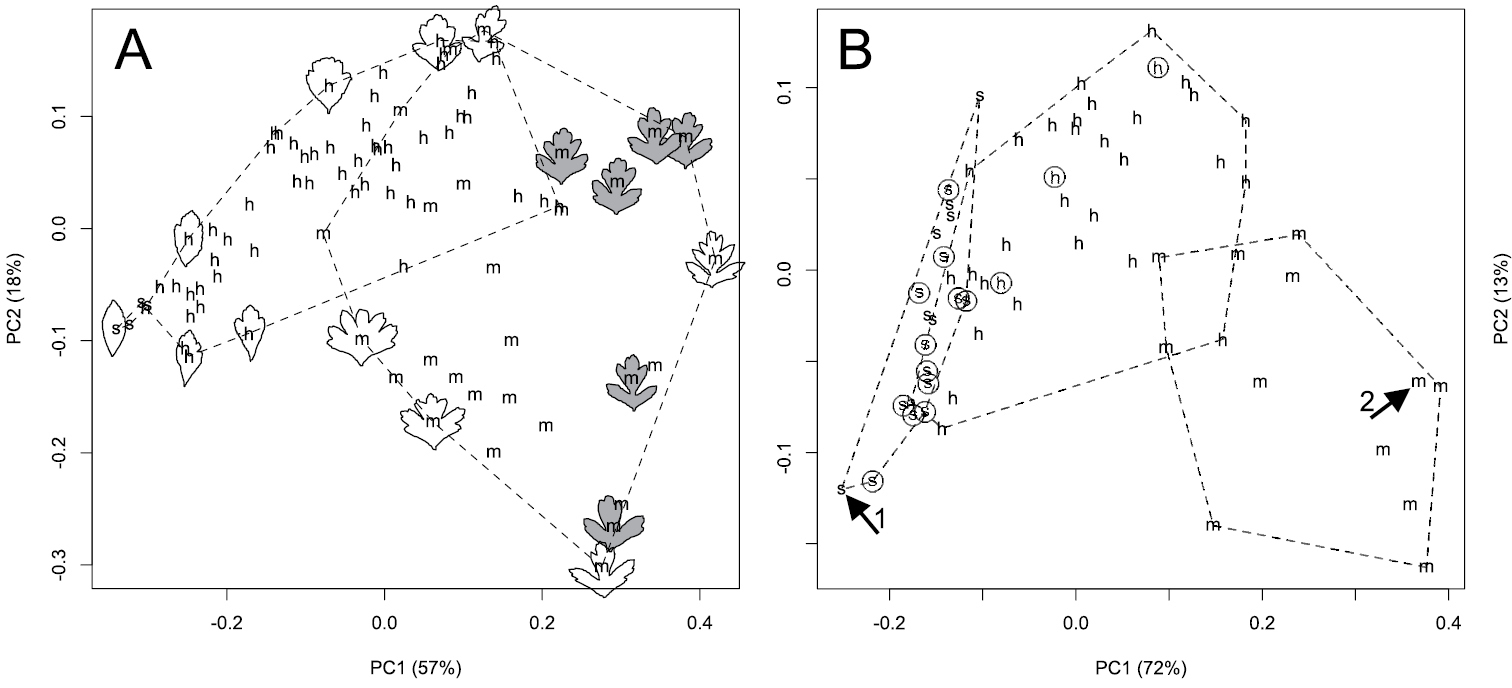
Morphology. Despite differences in sample size, the Pacific Northwest hybrid, Crataegus ×cogswellii, appears more variable than either of its putative parents, Crataegus monogyna or Crataegus suksdorfii (Fig. 1). The hybrid is clearly intermediate with respect to both leaf lobing (the inverse Dissection Index; Fig. 1) and style number (STYLE; Fig. 1). Principal components analyses of leaf outlines from Pacific Northwest Crataegus monogyna, Crataegus suksdorfii, and their putative hybrid, demonstrate variation in leaf shape both within and between these three entities (Fig. 2A, B). The first principal component reflects the contrast between the unlobed leaves of Crataegus suksdorfii and the markedly lobed ones of Crataegus monogyna, as well as the intermediacy of the hybrid (Fig. 2A, B), much as illustrated earlier by
Principal components analysis biplot for five morphometric descriptors averaged for each of 41 Crataegus herbarium specimens from the Cogswell-Foster Preserve and other locations in the Pacific Northwest (Crataegus suksdorfii (s), Crataegus monogyna (m), and the putative hybrid, Crataegus ×cogswellii (h)): relX, leaf length above the widest point, scaled by the width; relZ, leaf length below the widest point, scaled by the width; invDI, inverse dissection index = \(2(A\pi)^{1/2}/P\), where \(A\) is the leaf area and \(P\) is the leaf perimeter; STAM, number of stamens per flower; STYL, number of styles per flower. Both axes shown account for significant portions of the total variance according to the broken-stick criterion (
A Principal components analysis of 39 Fourier amplitudes for 86 subterminal short shoot leaves from 20 Crataegus individuals at the Cogswell-Foster Preserve in Linn Co., Oregon (one Crataegus suksdorfii (s), seven Crataegus monogyna (m), and 12 putative hybrids (h), Crataegus ×cogswellii). Leaf outlines illustrate the shape contrasts responsible for the ordination: in grey, six subterminal leaves from short shoots of a single individual (OR1–8) B Principal components analysis of 39 Fourier amplitudes averaged for leaves sampled regardless of position on short shoots of 64 herbarium specimens from the Cogswell-Foster Preserve and (circled points) other locations in the Pacific Northwest (Table 1). In both A and B the two PCA axes shown are significant according to the broken-stick criterion (
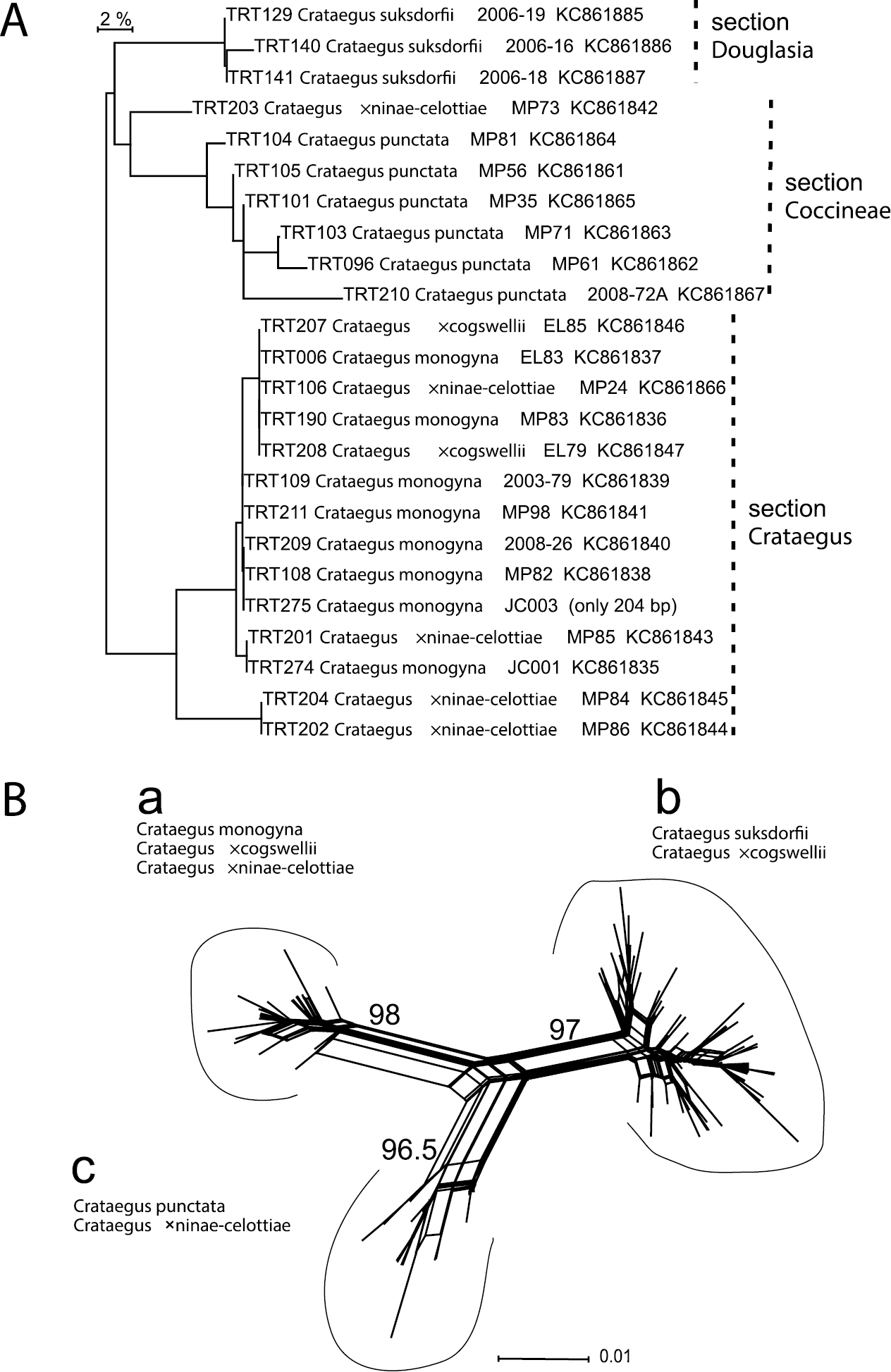
A Neighbor-joining tree calculated by BOLD for ITS2 DNA barcode sequences amplified directly from genomic DNA (labels include corresponding collector and GenBank number; see dx.doi.org/10.5883/DS-CRATMONO and Table 1 for details). Dashed lines indicate the sectional affinity of the sequences B The corresponding Neighbor-Net network for the cloned ITS2 sequences has three branches representing: (a) ribotypes from individuals of Crataegus monogyna, and from its hybrids with both Crataegus suksdorfii and Crataegus punctata; (b) ribotypes from individuals of Crataegus suksdorfii and Crataegus ×cogswellii; and (c) ribotypes from individuals of Crataegus punctata and Crataegus ×ninae-celottiae (Table 3). The numbers shown are the % bootstrap support for each of the three branches.
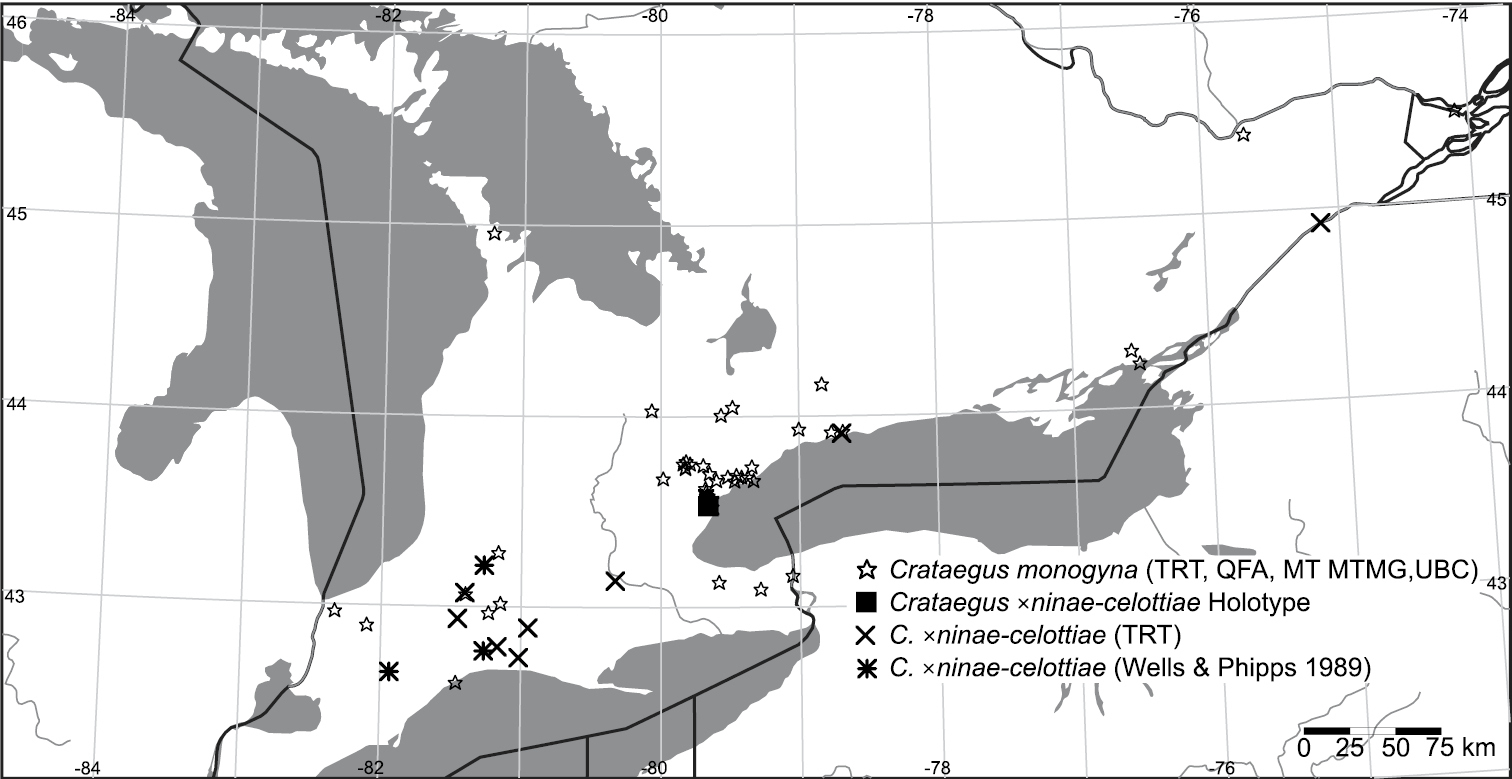
Geographic distribution of Crataegus ×ninae-celottiae K.I. Chr. & T.A. Dickinson nothosp. nov. and Crataegus monogyna in Ontario. Filled square, holotype of Crataegus ×ninae-celottiae; Crosses, TRT specimens of Crataegus ×ninae-celottiae; asterisks, Crataegus ×ninae-celottiae specimens cited by
DNA barcode loci. Analyses of both the directly sequenced and the cloned ITS2 ribotypes demonstrate the parentage of both putative hybrids (Fig. 3; Table 3); no signs of recombination were detected in the cloned ITS2 dataset. ITS2 sequences from the hybrids resemble either Crataegus monogyna or one of the native North American species. The way in which both parental ribotypes are maintained in each of the hybrids examined here is probably due to how recently the hybrids have been formed: less than 200 years ago in the case of the Ontario hybrids (
Flow-cytometric results from seeds of the two described Crataegus nothospecies. The ratios shown for endosperm and embryo nuclear DNA contents are well within the ranges observed for sexually reproducing Crataegus monogyna (Talent unpubl. data) and diploid Crataegus suksdorfii (
| Taxon / TRT accession / site / collection | Total number seeds | Mean embryo DNA | Mean endosperm:embryo ratio (number of seeds) |
|---|---|---|---|
| Crataegus ×ninae-celottiae | |||
| ON45 2002-13 (TRT00000406) | 2 | 1.58 pg | 1.56 (2) |
| ON31 EH52 (TRT00002256) | 1 | 1.67 pg | 1.53 (1) |
| Crataegus ×cogswellii | |||
| OR1 EL-79 (TRT00002657) | 3 | 2.08 pg | 1.58 (1) |
| OR1 2009-36 (TRT00002568) | 1 | 1.87 pg | 1.60 (1) |
Only two of the three chloroplast genome barcode loci showed sufficient variation for individuals from Crataegus section Crataegus to be distinguished from ones belonging to either Crataegus section Coccineae or Crataegus section Douglasia (Table 2). Sequence data from both rbcL-a and the trnH-psbA spacer region showed the same two clusters, Crataegus sections Coccineae and Douglasia (Cluster 1), and Crataegus sect. Crataegus (Cluster 2; Table 2). The way in which the hybrids fell into one of these clusters or the other demonstrates that, with one exception, Crataegus monogyna is the female parent of the Ontario hybrids with Crataegus punctata studied here, while Crataegus suksdorfii is the female parent of the Pacific Northwest hybrids.
These results corroborate earlier observations based on seed-set in artificial crosses between the parent species (
Our use of data from DNA barcoding is not a test of the value of DNA barcoding in Crataegus, as this is discussed elsewhere (Dickinson et al. http://2011.botanyconference.org/engine/search/720.html; Zarrei et al. unpubl. data). Rather, we have taken advantage of our barcode sequence data from individuals unequivocally identifiable as Crataegus monogyna, Crataegus punctata, Crataegus suksdorfii and their hybrids in order to use sequence similarity to inform us about the hybridization process.
Hybridization. Since its introduction to North America during the late 18th and the 19th centuries (
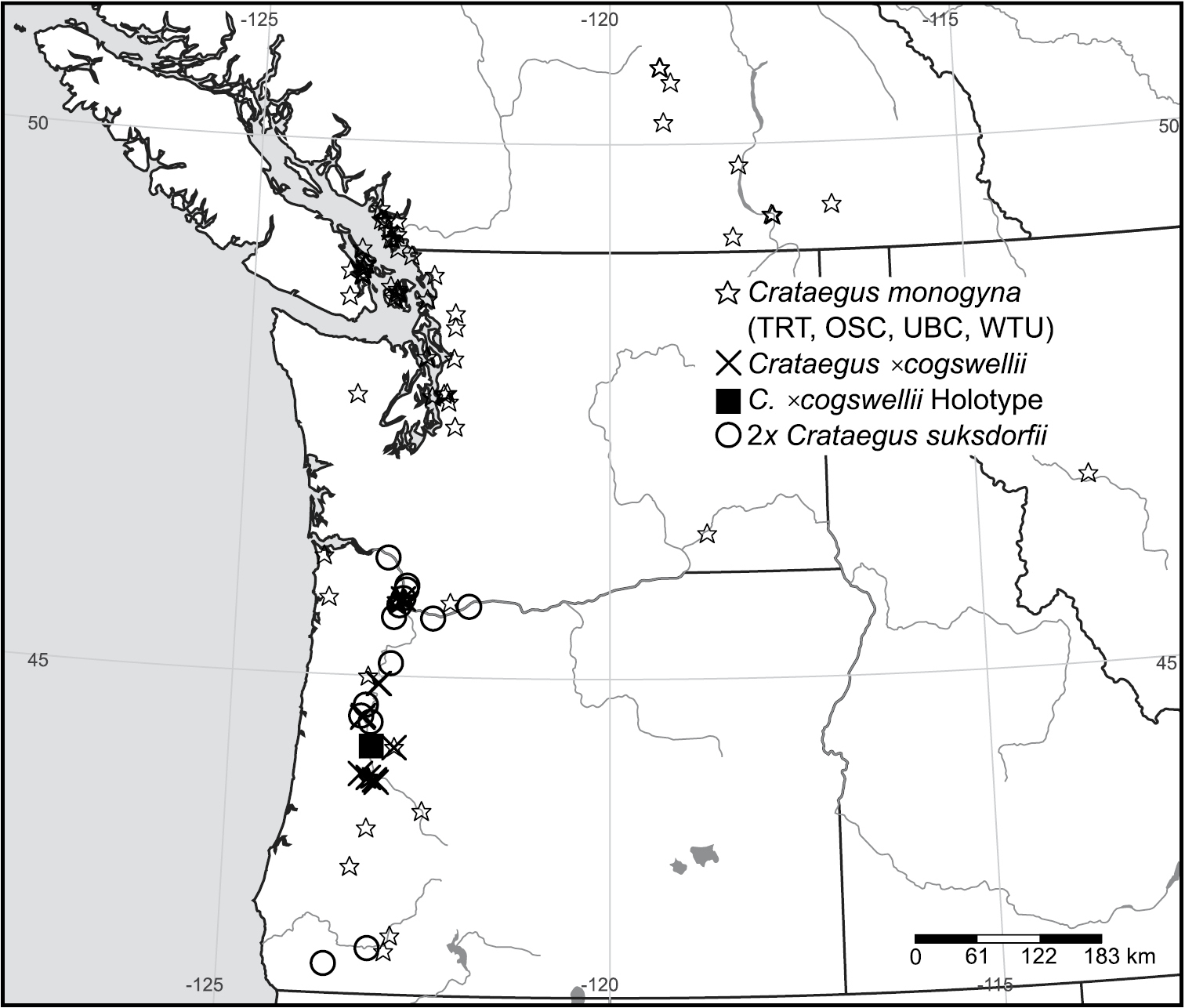
Geographic distribution of Crataegus ×cogswellii K.I. Chr. & T.A. Dickinson nothosp. nov. and its parental species in the Pacific Northwest. Filled square, holotype of Crataegus ×cogswellii; crosses, TRT specimens of Crataegus ×cogswellii; circles, diploid Crataegus suksdorfii; stars, Crataegus monogyna (specimens in OSC, TRT, UBC, and WTU).
Crataegus monogyna may never have been commonly planted in boundary hedges in Canada as it was in Europe. Fences and hedges appear to have been only rarely constructed in 17th Century Canada by European settlers to confine ruminant animals (
Flow cytometry of seeds from both hybrids was consistent with diploid embryos and triploid endosperm, except that the embryos from Crataegus ×cogswellii show slightly higher than diploid measurements, higher than the 1.39–1.66 pg measurements previously obtained from leaf data (Table 4;
In her examination of hybridization between Crataegus punctata and Crataegus monogyna in Ontario,
Crataegus nothoser. Punctaegus K.I. Chr. & T.A. Dickinson nothoser. nov. (Crataegus ser. Crataegus × ser. Punctatae)
Crataegus ×ninae-celottiae K.I. Chr. & T.A. Dickinson nothosp. nov. (Fig. 6). – Type: CANADA, Ontario, Peel R M, Don Gould Park and E side of Erin Mills Parkway (ON22), 43°31.960'N, 79°39.591'W, woodlot and fields with extensive hawthorn colonization, 2 Jun 1989, Dickinson D1492 (holotype TRT00002197!; isotype S!) (♀Crataegus monogyna × ♂Crataegus punctata)
Holotype of Crataegus ×ninae-celottiae K.I. Chr. & T.A. Dickinson nothosp. nov. (♀Crataegus monogyna × ♂Crataegus punctata): TRT00002197, CANADA, Ontario, Peel R M, loc. ON22, Don Gould Park and E side of Erin Mills Parkway, 43°35'N, 79°40'W, abandoned fields with extensive hawthorn colonization, 2 Jun 1989, Dickinson D1492.
Ramunculi pubescenti vel glabri. Folia distalia ramorum fertilium non profunde quinque-undecim-partita, 30–55 mm longa, 16–38 mm lata, nervi supra profunde impressi; stipulae caducae, 3–4 mm longae, plusminusve denticulatae. Inflorescentiae 5–17-florae, laxae, pubescentae; bracteae caducae, plusminusve denticulatae. Sepala integra, rarius sparsim glandulosa, post anthesin reflexa. Fructus 9–12 mm longus, 12–14 mm latus, ruber vel aurantiacus; pulpa lutea, mitis et succida; pyrenae 2–3, ventraliter sulcatae vel foveatae.
Remarks. Shrub or tree up to ca. 6 m tall. Twigs of the current year densely to sparsely hairy or glabrous, hairs appressed to patent, straight or slightly curly; twigs of the previous year pale grey or ash-grey; aphyllous thorns 0.5–2 cm long, stout, straight; spine-tipped, leaf- and dwarf-shoot-bearing branchlets lacking. Leaf blades ovate, obovate or elliptical, acute at apex, attenuate, cuneate or rounded at base, shallowly or deeply and regularly lobed, lobes with an acute apex, basal pair of veins convergent, straight or slightly divergent, intercalary veins running to the sinuses partly present, upper surface with ± deeply impressed veins at maturity, dull or lustrous bright or dark green, sparsely hairy and often becoming glabrous except along the veins, hairs appressed or semi-patent; lower surface dull, pale green, sparsely hairy throughout or only along the major veins and in the vein axils, hairs appressed or semi-patent; margin regularly crenate-serrate or serrate, teeth minutely glandular, glands less than 0.1 mm; petiole eglandular, narrowly winged in upper part. Subterminal leaf blade of flowering shoots 30–55 mm long, 16–38 mm wide, shallowly and regularly lobed, lobes 2–5 pairs, basal pair extending 0.2–0.4 times the width of lamina to midrib, each lobe with 6–11 teeth, basal pair of sinuses in apical 1/4 to basal 1/3 of lamina; petiole 6–20 mm long; stipules caducous, membranous or herbaceous, 4–8 mm long, irregularly or regularly glandular-denticulate, with 20–30 teeth. Leaf blades of elongate shoots 35–45 mm long, 25–35 mm wide, shallowly or deeply and regularly lobed, lobes 3–5 pairs, basal pair extending 0.2–0.6 times the width of lamina to midrib, each lobe with 4–11 teeth, basal pair of sinuses in basal 1/2–1/3 of lamina; petiole 8–12 mm; stipules caducous, herbaceous, ca. 6 mm long, regularly glandular denticulate-serrate, with ca. 15 teeth. Inflorescence 3–4 cm long, lax, corymbose, 5–17-flowered, densely to sparsely hairy, hairs appressed, semi-patent or patent, straight or slightly curly; pedicels 3–18 mm, densely to sparsely hairy, hairs appressed, semi-patent or patent, straight or slightly curly; bracts caducous, membranous or herbaceous, 3–4 mm long, 0.2–0.4 mm wide, linear-lanceolate, 10–15 times as long as wide, irregularly glandular-denticulate, with 5–7 teeth. Hypanthium 3–4 mm long, densely to sparsely hairy, hairs appressed, semi-patent or patent, straight or slightly curly; sepals 2–4 mm long, 1.5–2 mm wide, triangular-lanceolate or triangular, 1–2.7 times as long as wide, entire or rarely irregularly and minutely glandular-serrate, teeth 0–2, apex acute or obtuse; petals 6–7 mm long and wide; stamens 18–20, anthers 1–1.2 mm long, pink or purple; styles 2–3; hypostyle pilose. Fruit 9–12 mm long, 8–12 mm in diameter, 1.0–1.1 times as long as wide, globose, broadly ellipsoidal or obovoid, ± lustrous, red or orange, punctate with small, pale brown lenticels, up to ca. 0.2 mm in diameter, sparsely hairy, crowned by the persistent, reflexed sepals; calyx tube indistinct, ca. 0.5 mm long, 3–4 mm wide; flesh yellowish, hard and mealy; pyrenes 2–3, ventro-laterally smooth; hypostyle pilose.
Phenology. Flowering in May–June. Fruiting in August–September.
Reproductive biology. Sexual. \(2n = 2x\) (\(2n = 34?\)
Distribution. Eastern Canada. Ontario (Fig. 4).
Etymology. Crataegus ×ninae-celottiae honors Nina Celotti (1971–1995), who studied the pollination pathway of the two parent species, Crataegus punctata and Crataegus monogyna.
Similar taxa. Crataegus ×ninae-celottiae differs from Crataegus monogyna in: spine-tipped, leaf- and dwarf-shoot-bearing branchlets lacking; leaf blades with ± deeply impressed veins above; subterminal leaf blade of flowering shoots shallowly lobed, lobes 2–5 pairs (not ± deeply lobed and lobes 1–3 pairs); stipules caducous, often membranous, irregularly or regularly glandular-denticulate, with 20–30 teeth (not ± persistent, herbaceous and ± entire); styles and pyrenes 2–3 (not 1–(2)); fruit often orange, punctate with pale brown lenticels up to ca. 0.2 mm in diameter.
Crataegus ×ninae-celottiae differs from Crataegus punctata in: aphyllous thorns shorter, 0.5–2 cm long (not 2–5 cm long); leaf blades regularly lobed almost to the base (not unlobed or shallowly lobed towards apex), intercalary veins running to the sinuses sometimes present; subterminal leaf blade of flowering shoots usually smaller, up to ca. 55 mm long, and veins 2–5 pairs (not up to ca. 85 mm and veins 6–10 pairs); stipules often herbaceous and irregularly glandular-denticulate; sepals shorter, 2–4 mm long, and wider, 1–2.7 times as long as wide (not 3–7 mm long and 2–4.7 times as long as wide); styles and pyrenes 2–3 (not 3–5); fruit usually smaller, up to ca. 12 mm long and in diameter (not up to ca. 15 mm long and in diameter) and less distinctly punctate with smaller lenticels up to ca. 0.2 mm in diameter (not up to ca. 0.4 mm in diameter).
Crataegus ×ninae-celottiae was studied by
Specimens examined, paratypes (in bold, specimens in Tables 2–4). CANADA, Ontario: Peel Co., City of Mississauga, Don Gould Park and E side of Erin Mills Parkway (ON22), 1989-06-02, Dickinson D1480 (TRT00000408!); 1989-06-02, Dickinson D1482 (TRT00000407!); 1989-05-31, Dickinson D1485 (TRT00000409!); 2000-05-19, Talent NT-03 (TRT00002306!); 2011-05-28, Christensen & Dickinson s.n. (TRT00024869!). Middlesex Co., Denfield Twp., SE corner Denfield Side Road and Ilderton Road (ON31), 2001-05-17, Harris & Dickinson EH-52 (TRT00002256!); 2001-05-17, Harris & Dickinson EH-54 (TRT00002257!); 2002-07-30, Talent & Dickinson EH52 (TRT00000405!). Durham R.M., Bowmanville, floodplain of Bowmanville Creek (ON45), 2002-09-30, Dickinson & Nguyen 2002-13 (TRT00000406!), 2004-06-03, Purich 85 (TRT00002250!), 2004-06-03, Purich 86 (TRT00002251!).
Crataegus nothoser. Crataeglasianae K.I. Chr. & T.A. Dickinson nothoser. nov. (Crataegus ser. Crataegus × ser. Douglasianae)
Crataegus ×cogswellii K.I. Chr. & T.A. Dickinson nothosp. nov. (Fig. 7.). – Type: U.S.A., Oregon, Linn Co., Cogswell-Foster Preserve, 44°19.985'N, 123°7.353'W, 3 Sep 2009, Dickinson & Dickinson 2009-40 (holotype TRT00002574!; isotype TRT). (♀Crataegus suksdorfii × ♂Crataegus monogyna)
Holotype of Crataegus ×cogswellii K.I. Chr. & T.A. Dickinson nothosp. nov. (♀Crataegus suksdorfii × ♂Crataegus monogyna): TRT00002574, U.S.A., Oregon, Linn Co., Cogswell-Foster Preserve, 44.333082°N, 123.122547°W, 3 Sep 2009, Dickinson & Dickinson 2009-40.
Ramunculi glabri vel rarius sparsim villoso-lanati. Folia distalia ramorum fertilium quinque-novem-partita, rarius integra, 25–70 mm longa, 15–50 mm lata; stipulae caducae, 4–8 mm longae, plusminusve denticulatae. Inflorescentiae 4–25-florae, laxae, glabrae vel rarius villoso-lanatae; bracteae caducae, plusminusve denticulatae. Sepala integra vel rarius sparsim glandulosa, post anthesin reflexa. Fructus 9–12 mm longus, 12–14 mm latus, lampro-atro-purpureus vel anthracinus; pulpa lutea, mitis et succida; pyrenae 2–5, ventraliter sulcatae vel foveatae.
Remarks. Shrub or tree up to ca. 12 m tall. Twigs of the current year glabrous, rarely sparsely villous-lanate; twigs of the previous year dark reddish-brown or pale- or dark-grey; aphyllous thorns 0.5–2 cm long, stout, straight or slightly recurved; spine-tipped, leaf- and dwarf-shoot-bearing branchlets lacking, rarely present. Leaf blades broadly or narrowly obovate, ovate, rhombic-ovate or elliptical, acute at apex, attenuate, cuneate or rounded at base, deeply or shallowly and regularly lobed, rarely some leaves unlobed, lobes with an acute or obtuse apex, basal pair of veins divergent or straight, intercalary veins running to the sinuses usually present; upper surface dull, dark green, sparsely hairy especially along the veins, hairs appressed or semi-patent; lower surface dull, pale green, villous in the vein axils and occasionally along the major veins; margin regularly and ± coarsely or finely crenate-serrate or serrate, teeth eglandular or minutely glandular, glands less than 0.1 mm; petiole eglandular or rarely sparsely glandular, narrowly winged in upper part. Subterminal leaf blade of flowering shoots 25–70 mm long, 15–50 mm wide, deeply or shallowly and regularly lobed, rarely unlobed, lobes (0–)2–4 pairs, basal pair extending 0.2–0.8 times the width of lamina to midrib, each lobe with 5–18 teeth, basal pair of sinuses in apical 1/3 to basal 1/3 of lamina; petiole 5–15 mm long; stipules persistent or caducous, herbaceous, 5–12 mm long, irregularly or regularly glandular denticulate-serrate or serrate, with 4–30 teeth. Leaf blades of elongate shoots 40–90 mm long, 30–50 mm wide, deeply or shallowly and regularly lobed, lobes 1–4 pairs, basal pair extending 0.4–0.9 times the width of lamina to midrib, each lobe with 7–20 teeth, basal pair of sinuses in basal 1/2–1/5 of lamina; petiole 10–20 mm; stipules persistent or caducous, herbaceous, 6–14 mm long, regularly glandular denticulate-serrate or serrate, with 15–30 teeth. Inflorescence 2.5–5 cm long, lax, corymbose, 4–25-flowered, glabrous, rarely sparsely villous-lanate; pedicels 4–11 mm, glabrous, rarely sparsely villous-lanate; bracts caducous or very rarely persistent, membranous or herbaceous, 3–10 mm long, 0.2–2.5 mm wide, linear-lanceolate, 4–10 times as long as wide, regularly glandular-serrate or ± irregularly glandular-denticulate, with 4–22 teeth. Hypanthium 2–3 mm long, glabrous or rarely sparsely villous-lanate; sepals 1–2.5 mm long, 1.5–2 mm wide, triangular, 0.5–1.7 times as long as wide, entire or very rarely irregularly and minutely glandular-serrate, teeth 0–2, apex acute or obtuse; petals 4–6 mm long and wide; stamens 18–20, occasionally vestigial, anthers 0.6–1 mm long, purple; styles 2–5; hypostyle pilose. Fruit 6–9 mm long, 6–8 mm in diameter, 1–1.2 times as long as wide, globose-subglobose or broadly ellipsoidal, epruinose, ± lustrous, blackish purple or black, glabrous-subglabrous, crowned by the persistent, reflexed sepals; calyx tube indistinct, 0.4–1 mm long, 3.5–4.5 mm wide; flesh yellowish, soft and juicy; pyrenes 2–5, irregularly ventro-laterally pitted; hypostyle pilose.
Phenology. Flowering in April–May. Fruiting in September. Some individuals strongly parthenocarpic.
Reproductive biology. Sexual. \(2n = 2x [\approx 34]\) (
Distribution. Northwestern U.S.A.; western Oregon (Figure 5); potentially present in adjacent northwestern California and southwestern Washington where the parent species are sympatric.
Etymology. Crataegus ×cogswellii honours the Cogswell family, and Mr. and Mrs. Lee Foster, of Halsey, Oregon. In 1872 John Cogswell, Mrs. Foster’s grandfather, purchased the land that the Fosters gave to the Oregon Nature Conservancy as the Cogswell-Foster Preserve (
Similar taxa. Crataegus ×cogswellii differs from Crataegus monogyna in: leaf- and dwarf-shoot-bearing branchlets usually lacking; stipules of leaves of flowering shoots irregularly or regularly glandular denticulate-serrate or serrate (not ± entire); styles and pyrenes 2–5 (not 1–(2)); fruit blackish purple or black (not bright or dark red).
Crataegus ×cogswellii differs from Crataegus suksdorfii in: twigs of the current year occasionally sparsely villous-lanate; leaf- and dwarf-shoot-bearing branchlets occasionally present; leaf blades usually deeply or shallowly and regularly lobed, intercalary veins running to the sinuses usually present; inflorescence, pedicels and hypanthia occasionally sparsely villous-lanate; hypostyle pilose (not glabrous or sparsely pilose).
Specimens examined, paratypes (in bold, specimens in Tables 2–4). U.S.A., Oregon: Columbia Co., Sauvie Island (OR11), 2003-06-14, Zika 18482 (TRT00002651!); 2005-08-31, Lo & Dickinson 103.2 (TRT00001918!), Lo 105.2 (TRT00001917!); Lane Co. Eugene, 1993-05-07, Love 9304 (TRT00002644!), 2003-05-13, Love C2003-12 (TRT00002646!), C2003-13 (TRT00002647!); 2003-06-01, Zika 19571 (TRT00001890!); Linn Co., Cogswell-Foster Preserve (OR1), 1987-04-7, 1987-04-27, 1987-09-20, Love 8707 (TRT00001895!, TRT00001907!, TRT00001912!), 8714 (TRT00001897!, TRT00001899!, TRT00002643!), 8715 (TRT00001901!, TRT00001902!, TRT00001910!), 8716 (TRT00001894!, TRT00001913!), 8717 (TRT00001900!, TRT00001909!), 8718 (TRT00002645!), 8719 (TRT00001893!, TRT00001905!, TRT00001906!), 8720 (TRT00001904!), 1993-05-18, Barbour, Evans & Love 93064 (TRT00001896!), 1997-07-27, Love 9726 (TRT00002196!); 2004-06-10, Lo, Dickinson & Nguyen 71 (TRT00002650!), 73 (TRT00002660!), 76 (TRT00002658!), 77 (TRT00002659!), 79 (TRT00002657!), 81 (TRT00002655!), 82 (TRT00002656!), 84 (TRT00002653!), 85 (TRT00002654!); 2009-09-03, Dickinson & Dickinson 2009-22 (TRT00002555!), 2009-23 (TRT00002556!), 2009-24 (TRT00002557!), 2009-28 (TRT00002560!), 2009-33 (TRT00002565!), 2009-34 (TRT00002566!), 2009-36 (TRT00002568!), 2009-38 (TRT00002570!), 2009-39 (TRT00002571!), 2009-41 (TRT00002573!), 2009-42 (TRT00002572!), 2009-43 (TRT00002575!); Marion Co., Salem, 2003-05-01, Zika 18296 (TRT00001889!). Washington: Clark Co., 2003-06-01, Zika 18431 (TRT00001891!).
Dr Peter Wagner, Copenhagen, kindly checked the Latin diagnoses. Diane Celotti gave us biographical information about her daughter, Nina. Rhoda M. Love introduced TAD to Crataegus suksdorfii and its hybrids at the Cogswell-Foster Preserve, and provided unpublished information from her research on these plants; she also arranged with The Nature Conservancy for our access to the Cogswell-Foster Preserve. Ed Alverson provided bibliographic information for the Lopez article. We obtained the Billings reference thanks to Mike Palmer and the adventive species website (FloraS of North America, http://botany.okstate.edu/floras/). Dale Leadbeater told TAD about the Bowmanville site. Saša Stefanović is our collaborator on the Crataegus ITS2 project. Jenn Coughlan, John Dickinson, Rebecca Dotterer, Eric Harris, Eugenia Lo, Rhoda Love, Sophie Nguyen, Melissa Purich, Peter Zika assisted our fieldwork or provided their specimens. Jen Byun and Kathleen Buck helped us collect data from herbarium material. Tara Winterhalt adjusted the contrast in Fig. 6 and Fig. 7. We are grateful to the herbaria mentioned in the figures for lending specimens or making specimen data available to us. Financial support to KIC from the Carlsberg Foundation (Grant 2008_01_0155) and to TAD from the Natural Sciences and Engineering Research Council of Canada (Discovery Grant A3430), the Royal Ontario Museum, the Department of Ecology & Evolutionary Biology (formerly the Botany Department) of the University of Toronto, and the Canada Foundation for Innovation and Ontario Research Fund (funding through Canadensys for equipment and personnel for specimen documentation) are gratefully acknowledged. DNA barcoding was funded by the Government of Canada through Genome Canada and the Ontario Genomics Institute (2008-OGI-ICI-03).