(C) 2013 Mitchell C. Provance. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Provance MC, Sanders AC (2013) Lucky morning glory, Calystegia felix (Convolvulaceae): a new species from Southern California, with notes on the historical ecology of the Chino ciénega belt. PhytoKeys 32: 1–26. doi: 10.3897/phytokeys.32.6020
A new morning glory, Calystegia felixProvance & A.C. Sanders sp. nov. (Convolvulaceae), is described from the Los Angeles, San Gabriel, and Santa Ana River basins. Historical collections of the species, which prior to 2011 had not been seen alive in 94 years, have been misidentified as Calystegia sepium (L.) R. Br. subsp. binghamiae (Greene) Brummitt. The undescribed species was rediscovered in the City of Chino in April of 2011, a few miles north of the location where the most recent previous collection had been made by I. M. Johnston in 1917. The plants were found just prior to their likely destruction by grading and trenching for an underground power line. Intensive searches have resulted in the discovery of five additional occurrences, all of them in the City of Chino. Calystegia felix is at high risk of soon becoming extinct in the wild. All of the known extant occurrences are associated with well-watered landscaping on recently completed industrial, commercial, and residential developments. Every known living occurrence is within the limits of a ciénega belt, which is now mostly historical. Otherwise, the new species is only known only from collections made around the turn of the 20th century in what are now heavily urbanized areas, including one from South Los Angeles and another from Pico Rivera in Los Angeles County. Calystegia felix lacks the large bracts that immediately subtend, and enclose the calyx, which are always present in members of the Calystegia sepium complex. Affinities to Calystegia felix are found among other western US species with graduated sepals and small, often somewhat remote bracts. We discuss the enduring confusion between Calystegia felix and Calystegia sepium subsp. binghamiae, and differentiate the new species from some of its more likely relatives. The taxonomic treatment is supplemented by photos of herbarium specimens and living plants. We also discuss the ecological setting of Chino’s ciénega belt, which was a mosaic of palustrine wetlands.
alkali meadow, Calystegia felix, Chino Basin, ciénega, Convolvulaceae, new species, seep, Southern California, spring, urban landscape, wet meadows
Calystegia R.Br. is a genus of about twenty-five species, having a worldwide distribution in temperate zones. Its center of diversity is California, where twelve native species, and thirteen additional native subtaxa, are recognized in the latest Jepson Manual (
In 2011, the first author discovered a morning glory in Chino, California, that could not be differentiated from collections determined by the late R. K. Brummitt as Calystegia sepium (L.) R. Br. subsp. binghamiae (Greene) Brummitt, or “Calystegia binghamiae” (an unpublished name that he had written on one of the specimens years before). Until rediscovered in 2011, this taxon had been widely considered extinct (
The rediscovery was followed by elevation of Calystegia sepium subsp. binghamiae to species rank under Calystegia (
The six recent Calystegia locations from Chino are a new species. This new species is also known from three historical collections, including one each from South Los Angeles, Pico Rivera, and Chino. Calystegia felix Provance & A.C. Sanders, sp. nov., lacks the large bracts that immediately subtend, and clasp the calyx, as are always present in members of the Calystegia sepium complex (
Recent Calystegia specimens from Chino were compared with Calystegia and Convolvulus collections at RSA-POM and UCR, as well as a selection of specimens from CAS and UC-JEPS, including the lectotype of Convolvulus binghamiae. We also obtained high-resolution digital images of Convolvulus binghamiae original material held at G-NDG. Scores of Convolvulus and Calystegia specimen images were evaluated for their relevance to the present study. We obtained images of relevant specimens held at the following herbaria: CAS, DS, E, K, LSU, NA, NY, P, SOC, UC-JEPS, US and WWB. Specimens with little immediate relevance are not listed in the appendices; however, regardless of ultimate relevance, all image sources and their source herbaria, are provided in Appendix I. Calystegia felix specimens that were examined are cited in the taxonomic discussion. All specimens of Calystegia sepium examined are cited in Appendix II. Species of Calystegia that we think could be most easily confused with Calystegia felix are compared across a number of characters (Table 1). Specimens that we examined of the species included in that table are cited in Appendix III. Clarifications added to specimen citations appear in brackets. The precisions of the reported geographical coordinates were reduced to ± 300 m for the Calystegia felix collections. Only specimens and specimen images that were seen by the authors are cited in the appendices, except in the case of a putative sheet of Convolvulus binghamiae at F (Mrs. R.F. Bingham s.n.) cited in the original description, and one specimen of Calystegia felix (J.M. Wood et al. 4092). The herbarium code is followed by the word “image” when only an image was examined. Measurements were obtained from specimens conventionally, or from images using Meander V 2.3 (Dixon and Coventry 2008, available at http://www.fastforwardsw .com/products/meander/). Cultivated plants were grown in UC mix outdoors in partial shade, at about 250 m elevation, in Riverside, California. The historical ecological setting in Chino was compiled from historical maps, early literature, herbarium specimens, and field observations.
A comparison of floral and vegetative structures in Calystegia felix with four similar species of Calystegia.
| Character | Calystegia felix Provance & A.C. Sanders | Calystegia occidentalis (A.Gray) Brummitt subsp. occidentalis | Calystegia occidentalis subsp. fulcrata (A.Gray) Brummitt | Calystegia peirsonii (Abrams) Brummitt | Calystegia subacaulis subsp. episcopalis Brummitt |
|---|---|---|---|---|---|
| habit | clambering to climbing | clambering to climbing | trailing to clambering | tangling subshrub | decumbent to trailing |
| number of flowers per inflorescence | 1, or rarely 2–4 | 1–4 | 1 | 1–2 | 1 |
| corolla tube width, most proximal visible point (mm) | 4–5.9 | 6–9 | 5 | 5.2–6.5 | 3.3–5.7 |
| sepal shape | narrowly oblong | oblong to oblong-ovate | lanceovate to obovate | oblong to oblong ovate | lorate to narrowly lanceolate |
| sepal width (mm) | 2.5–5 | 6–9 | 4–6 | 57 | 2–4 |
| ovary internal vestiture | glabrous | silky hairy | silky hairy | silky hairy | glabrous |
| ovary external vestiture | glabrous or obscurely minutely pubescent apically | subglabrous | glabrous | glabrous | glabrous |
| inflorescence bract length (mm) | 5–14 | 4–16 | 6.5–18 | 5–8 | 5.5–18.5 |
| inflorescence bract shape | entire; narrowly elliptic to obelliptic | entire; narrowly elliptic to elliptic | lobed; triangular-hastate | entire; ovate, oval, or elliptic | entire; ovate to narrowly elliptic |
| lamina subtending flower, main lobe shape | broadly ovate to oblong ovate | narrowly triangular to broadly ovate | narrowly triangular to ovate | linear lanceolate to triangular | lance-ovate to broadly ovate |
| lamina subtending flower, basal lobe shape | short, rounded to truncate | short or not, 2-lobed to bipartite, | long, acute to truncate | long | short, somewhat rounded to narrowly lanceolate |
| lamina subtending flower, basal lobe orientation | barely divergent to parallel | divergent | divergent to parallel | divergent to parallel | strongly divergent |
| lamina subtending flower, length along midrib (mm) | 45–122 | 32–51 | 27–46 | 14–16 | 23–28 |
| lamina subtending flower, greatest width (mm) | 30–96 | 33–66 | 35–54 | 19–22 | 16–34 |
| lamina subtending flower, margin contour | flat to laxly involvute, or with inverted basal lobes | flat | flat, or sometimes slightly wavy (esp. in the San Gabriel Mtns.) | wavy to grotesquely curled, rarely flat | flat |
urn:lsid:ipni.org:names:77134775-1
http://species-id.net/wiki/Calystegia_felix
Fig. 1Differs from Calystegia subacaulis Hook. & Arn. subsp. episcopalis Brummitt, by its clambering to strongly climbing stems (versus decumbent to trailing stems in Calystegia subacaulis subsp. episcopalis), larger leaves, 45–122 mm long, 30–96 mm wide mm long, subtending the peduncle (versus 23–28 mm long, 16–34 mm wide in Calystegia subacaulis subsp. episcopalis), with short, rounded, barely divergent to parallel basal lobes, or sometimes nearly without basal lobes, and essentially truncate (versus the basal lobes somewhat rounded to narrowly lanceolate and strongly divergent in Calystegia subacaulis subsp. episcopalis); Differs from Calystegia occidentalis (Gray) Brummitt subsp. occidentalis by its narrowly oblong, 2.5–5 mm wide sepals (versus oblong to oblong-ovate, 6–9 mm wide sepals in Calystegia occidentalis subsp. occidentalis), narrower corolla tube (basally) 4–5.9 mm wide measured at the most proximal visible point (versus 6–9 mm in Calystegia occidentalis subsp. occidentalis), an ovary that is glabrous on inside walls (versus a silky hairy vestiture inside of the ovary in Calystegia occidentalis subsp. occidentalis), and larger, 45–122 mm long, 30–96 mm wide, oblong-ovate to broadly ovate leaves subtending the peduncles (versus smaller leaves subtending the peduncle, 32–51 mm long, 33–66 mm wide, narrowly triangular to broadly ovate), and short, rounded, barely divergent to parallel basal lobes, or leaves that are nearly truncate at the base (versus leaves with divergent basal lobes of varying length that are 2-lobed to bipartite).
Calystegia felix Provance & A.C. Sanders, sp. nov. The holotype, A.C. Sanders & M.C. and T.A. Provance 40174 (UCR [UCR-246125]). The flowering branchlets are from a single ramet (Photo M. C. Provance, 2011).
USA. California: San Bernardino County, City of Chino, SE of intersection of Edison Ave. and Oaks Ave., edge of Chaffey College Chino Campus, public right-of-way along powerlines. 33°59.822'N, 117°40.518'W, 206 m, 19 May 2012, A.C. Sanders, M.C. Provance, & T.A. Provance 40174 (holotype: UCR! [UCR-246125]; isotypes: ARIZ!, CAS!, K!, MO!, NDG!, NY!, RSA!, SBBG!, SD!).
Semi-herbaceous perennial vines, senescing in October, though with some stems and leaves persisting through winter. Aerial stems 1–3 m long, from shallow, creeping rhizomes and stolons (Fig. 2), climbing and twining, or clambering across shrubs, branching frequently, terete, with nonobvious longitudinal ridges, slender, tough and wiry, glabrous to sparsely hairy, in life dull grayish pink to light green with a rosy cast. Leaves alternate, membranaceous to chartaceus, glabrous to sparsely hairy, bicolored when mature green above, paler below, relatively flat and not folding along the midrib, but sometimes the basal half of the lamina slightly involute, and often having the basal lobes abruptly turned upward. Petioles on climbing stems 0.3–0.5 × length of lamina, e.g. about 14–61 mm long, but often longer relative to lamina length on emergent leaves; lamina of climbing stems 45–115(–122) mm long, 30–80(–96) mm wide, oblong-ovate to broadly ovate, but narrowly oblong on the sterile branchlets and on stems distal to the flowering axils, base cordate, with short, rounded, parallel or barely diverging basal lobes, sometimes essentially without basal lobes and nearly truncate, apex obtusely rounded, sometimes subacute, minutely apiculate; emergent leaves from rhizomes and on trailing stems variable in shape, but usually broadly oblong to oval or orbicular, sagittate, with short lobes, or lobeless and rounded to the petiole, apex broadly rounded; lamina venation obscurely pinnate, but with 2–4 lateral veins from the base. Inflorescences axillary, flowers usually solitary, rarely 2–3(–4)-flowered; pedicels 1–30 mm long, peduncles 18–63 mm long; bracts 2, attached (1–)2–3(–4) mm below the calyx, ascending, subopposite, 5–14 mm long, 1–2.5(–3.5) mm wide, narrowly elliptic to narrowly oblanceolate, obtusely pointed, ± flat, with a raised midvein, glabrous to scantly puberulent. Flowers perfect; sepals 5, entire, graduated, narrowly oblong to lanceovate, green with a rosy blush, short-ciliate, inner sepals 11–15 mm long, 3.5–4 mm wide, the lower portion tightly appressed to mature fruit, outer sepals 8–11 mm long, 2.5–5 mm wide, apices ± acutely rounded, mucronulate; corolla funnelform, 27–45 mm long, base of visible tube 4–5.9 mm wide, white (sometimes appearing light yellow in herbarium specimens), with 5 externally pigmented interplicae (midpetaline bands or longitudinal stripes), these very light-yellow, more rarely reddish-purple (Fig. 3), glabrous externally, or rarely, conspicuously hairy adjacent to pleats in the basal third of the corolla, the hairs yellowish, lobes 5, very short, each with a concentrated area of minute hairs along the margin; stamens 5, equal; filaments 18–21 mm long, fused to the corolla tube ± 7-9 mm of that length, glandular hairy along the proximal margins; anthers 4–4.5 mm long, white, barely reaching the base of the stigmas; pistil glabrous both internally an externally; style 16–21 mm long, glabrous, or with a few glandular hairs near the base; stigmas 2, cylindrical, ± 3 mm long, asymmetric, with one axially oriented, and the other ascending; nectary crenate-coronoid. Pollen white to cream, with circular perforations discernible at 60 X. Fruit dry capsule, indehiscent to tardily dehiscent from tip to base, globose, 9–10 mm in diameter, glabrous or obscurely minutely pubescent apically. Seeds 1–4 per capsule, ca. 4 mm in height and 3.5–4 mm in width, ± angular-ovoid, and depending on the number of developing seeds, nearly black to dark brown and tan-speckled, hilar region purplish, finely granular.
Calystegia felix stolons and creeping rootstock. The narrow emergent leaves of this specimen may be atypical; the relatively long petioles are normal (Photo M. C. Provance, 2012).
Calystegia felix in bloom at the type locality, in a planter bed in Chino. This plant, discovered in 2011, is the only one we have seen that produces corollas with reddish-purple midpetaline stripes. Also, note the blushed sepals (Photo M. C. Provance, 2011).
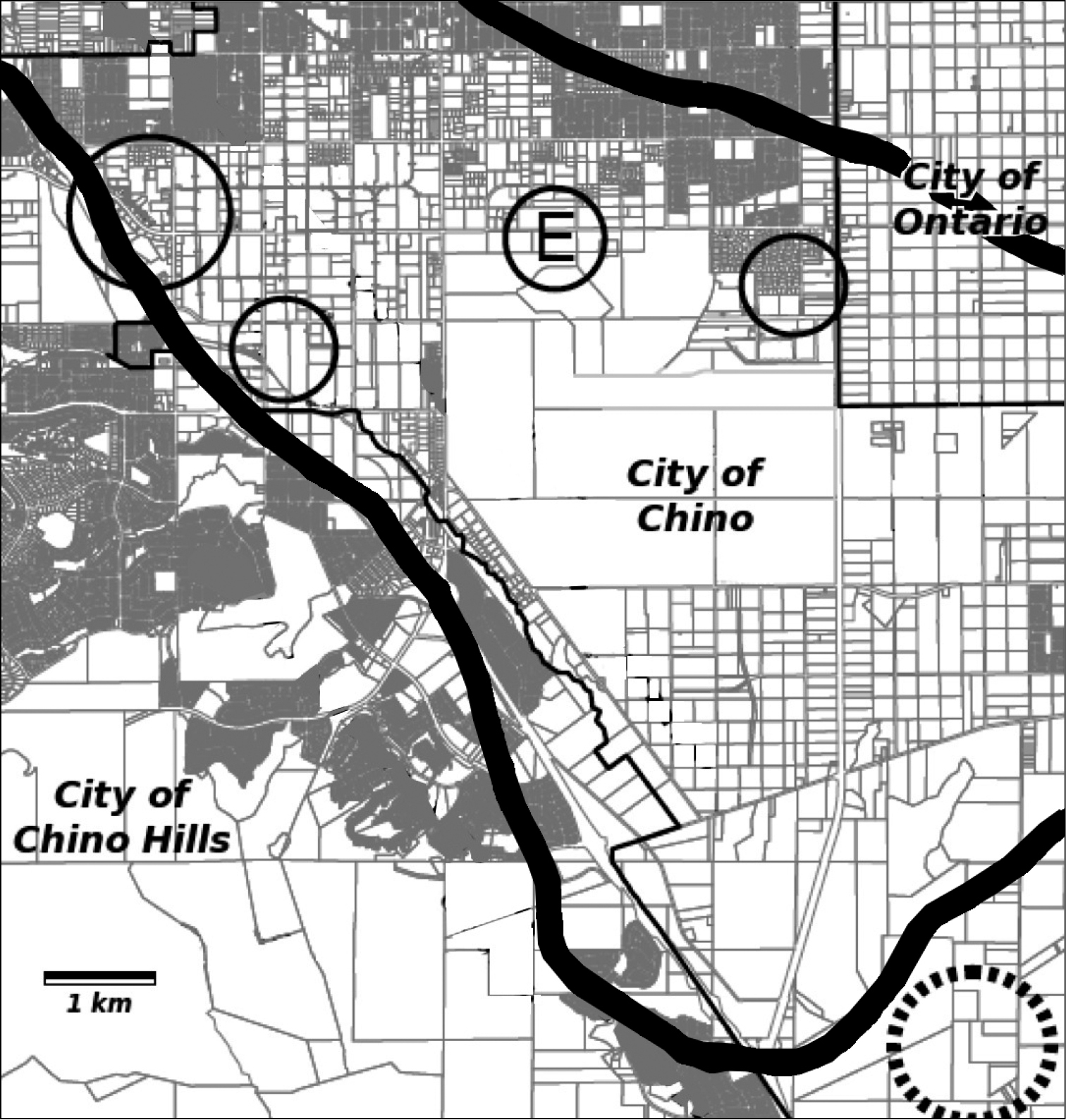
Calystegia felix is endemic to the inland basins of the Los Angeles, San Gabriel, and Santa Ana river watersheds in Southern California, at between 40 and 208 meters elevation. The species has not been seen in Los Angeles County since 1902. Six occurrences are known, all of them in the City of Chino, in San Bernardino County (Fig. 4). The occurrences have a spatial separation ranging from 0.3–2 km. The easternmost occurrence is just west of Euclid Avenue, close to Chino’s border with Ontario and Eastvale. The westernmost occurrences are on alluvial terraces above Chino Creek, coming within several meters of the City of Chino Hills.
Map of historical and recently discovered Calystegia felix occurrences in the Chino Basin. The circled E marks the plant discovered in 2011; normal circles are 2013 occurrences, the largest a cluster of three populations. The broken circle is the approximate location of Johnston’s collection in 1917. The perimeter of the Chino artesian belt and “moist land” prior to 1904 is delineated by the thick black line. The street map was adapted from the City of Chino draft general plan EIR, July, 2010, and the artesian belt is based on
Flowering begins in late March, and is heavy until early August, with flowering thereafter decreasing through late September. In 2011 and early 2012, inflorescences on the only plants known at that time had solitary flowers. During mid-May of 2012, inflorescences at that site were observed to be two or three-flowered, and rarely solitary. It is not certain whether flower number increased as the season progressed, or if flowers during the later visit were originating on vines of a different genet. Only solitary flowers were seen at the sites discovered in 2013. Ripe seeds have been collected from early June until late October. Fruit with small holes indicative of seed predation by bruchid beetles have been found (Provance, pers. obs.). Small, senesced, nodding, sterile, apetalous flowers, mostly near ground level, have recently been noticed on some plants. We observed similar flowers on herbarium specimens of a few other species of Calystegia. It is unknown if these flowers are apetalous developmentally, or if the corollas were lost to insect predation. More in-depth study of this condition is needed.
USA. California. San Bernardino County: Chino Creek south of Ontario, climbing in trees, 500 ft., 30 May 1917 (fl), I.M. Johnston 1274 (RSA, POM, UC); City of Chino, 33°59.823'N, -117°40.537'W, 206 m, SCE right-of-way, just northeast of Chaffey College, southeast corner of the intersection of Edison Rd. & Oaks Ave., 11 May 2011, M.C. Provance 17214 (UCR); same location, 14 May 2011, M.C. Provance 17351 (UCR, UC, DAV, NDG); same location, 25 Mar 2012, M.C., J.M., & T.A. Provance 17430 (UCR, WIS); West Chino, east of Chino Creek, 33°59.17'N, 117°42.38'W (± 300 m), 190 m, planter bed in public parking area, 31 May 2013, M.C. Provance 17525 (UCR, to be distributed); West Chino, east of Chino Creek, 34°00.18'N, -117°43.31'W (± 300 m), 208 m, planter bed in public parking area, 3 June 2013, M.C. Provance 17526 (UCR, to be distributed); West Chino, Highway 71 – Grand Avenue ramp, 33°59.44'N, -117°43.30'W (± 300 m), 205 m, Highway 71 and an adjacent planter bed in a public parking area, 11 June 2013, M.C. Provance 17527 (UCR, to be distributed); West Chino, 33°59.63'N, -117°43.18'W (± 300 m), 200 m, planter bed in a public parking area, 11 June 2013, M.C. Provance 17528 (UCR, to be distributed); East Chino, planter bed along public sidewalk near southeast corner of Buckeye Street and Fern Avenue, 33°59.65'N, -117°39.24'W (± 300 m), 206 m, 11 June 2013, M.C. Provance 17529 (UCR, to be distributed); City of Chino, SE corner of Edison Ave. and Oaks Ave., near entrance to Chaffey college campus, ca. 2.5 mi N of Chino Creek (Prado Basin), irrigated landscaped area adjacent to ruderal grasslands, 17 May 2011, J.M. Wood et al. 4092 (K not seen, RSA not seen). Los Angeles County: Rivera [historic town, later part of Pico Rivera, misspelled “Riveria” on Davidson’s herbarium label, and misspelled “Riviera” in
Taxonomy: Although arguments to maintain Calystegia have been weakened by recent molecular studies, we describe this new species as such, pending molecular phylogenetic studies that sample more thoroughly across both Calystegia and Convolvulus. Although their flowers are not similar, it is noteworthy that few vegetative characters seem to separate Calystegia felix from the weed Convolvulus arvensis L. The only vegetative feature we currently know that can reliably be used to tell these taxa apart is the cross section of the stem, which is angular in Convolvulus arvensis, and terete with weak longitudinal ridges in Calystegia felix. There may be differences in leaf venation, but that will require additional study. Unfortunately, Convolvulus arvensis is abundant throughout the Chino area, and occurs at several of the Calystegia felix sites.
While a definitive treatment of the entire Calystegia sepium complex has not been published, the best defining features of this group are the large bracts which immediately subtend, and often enclose the calyx, and have conspicuously netted venation. It is a taxonomically difficult complex that may include over twenty Calystegia sepium subtaxa, some additional closely related species, and their subtaxa (
Lectotype of Convolvulus binghamiae Greene, Santa Barbara, July, 1886, R.F. Bingham s.n. (UC335392), with one small bract, and a broad-based corolla.
The leaves shown here from R.F. Bingham s.n. (UC335392) are consistent with many specimens of Calystegia sepium.
Original material of Convolvulus binghamiae Greene, Santa Barbara, 1886, E.L. Greene s.n. (NDG [NDG-66275]). Flower buds with large clasping bracts typical of the Calystegia sepium complex are noteworthy (a), as is the solitary flower (b) with a broad calyx, broad corolla tube, and a smallish, but otherwise typical clasping bract for the Calystegia sepium complex (Image courtesy of Barbara Hellenthal at the Notre Dame Herbarium).
Specimens of Calystegia felix were included in Convolvulus binghamiae by Davidson in his list of new records for Los Angeles (1909) and by Davidson & Moxley in their flora of Southern California (1923). When Jepson made the combination Convolvulus sepium var. binghamiae (Greene) Jepson, he was very particular in his application of the name, stating, “Santa Barbara; a distinct localized variety, rarely collected”. Under Convolvulus sepium var. pubescens,
In 1945,
Philip A. Munz annotated a Johnston collection at RSA as Convolvulus purpuratus Greene in 1931, thus clearly including Calystegia felix in his concept of Convolvulus purpuratus. In his Southern California Manual (
The number of flowers per inflorescence, corolla pigmentation, external corolla vestiture, and the vestiture of leaves and stems vary in Calystegia felix. Whether this variation is influenced more by genetics or environmental factors remains to be studied. Heterophylly is profound in Calystegia felix, and generally manifests as narrower lamina on sterile twining stems, instead of the larger ovate to oblong-ovate leaves of reproductively active stems. There seems to be a tendency towards rounder leaves with longer petioles on emergent stems and sometimes trailing stems. Calystegia felix is similar to other species in the genus with small, somewhat remote bracts, and graduated sepals. Several morphological characters are used to compare four of those species with Calystegia felix (Table 1). Leaf parameters alone are often insufficient for the identification of Calystegia, but fortunately, several other characters in addition to leaf shape, differentiate Calystegia felix from other species of Calystegia.
At first glance, Calystegia felix looks most similar to Calystegia occidentalis (Gray) Brummitt subsp. occidentalis, since both taxa have a similar clambering or climbing habit, similar bracts inserted approximately the same distance below the calyx, and potentially produce multiple flowers in inflorescences. However, Calystegia felix differs from Calystegia occidentalis by its narrower sepals, narrower corolla tube, internally glabrous ovary, and larger oblong-ovate to broadly ovate leaves. The leaves subtending peduncles of Calystegia felix have short, rounded, barely diverging to parallel basal lobes. Sometimes, Calystegia felix leaves are nearly truncate at the base. This easily differentiates Calystegia felix from Calystegia occidentalis, which has lamina basal lobes that are of varying length, but divergent, and usually 2-lobed or bipartite. Calystegia felix also looks like Calystegia subacaulis Hook. & Arn subsp. episcopalis Brummitt. Both taxa have slender, but tough and wiry stems, corolla tubes that narrow toward the base, narrow sepals, and an ovary that is glabrous both internally and externally. It differs from Calystegia subacaulis subsp. episcopalis by its strong climbing habit, and much larger leaves that differ considerably in basal lobe morphology.
The similarities between Calystegia felix and Calystegia subacaulis subsp. episcopalis tend to be less readily apparent than the similarities between Calystegia felix and Calystegia occidentalis subsp. occidentalis. However, the characters shared seem not to be widespread in the genus. For example, while ovaries of Calystegia felix sometimes have a small number of minute hairs toward the apex, they are essentially glabrous externally. They are also glabrous internally. Though we have had only one specimen of Calystegia subacaulis subsp. episcopalis upon which we have been able to conduct detailed flower dissections (F. Bowcutt 2163 [UCR]), we are especially intrigued by the ovaries of this collection, which are glabrous both internally and externally. We have seen this combination of characters only in Calystegia felix, and similarities such as these might indicate that the two taxa are more closely related than their superficial appearances suggest.
Ecology: The six known occurrences are associated with somewhat poorly drained alkali silt loam (
Historically, the water table in the vicinity of the artesian spring belt was 6–35 feet below ground (
On Edison Rd., Calystegia felix was discovered in a sidewalk tree basin on Chino silt loam. In that area, the soil is pale gray, with occasional small patches of fluffy salt crust. Disturbed alkali playa habitat was observed nearby, with Heliotropium curassivicum L., Heliotropium europaeum L., Cynodon dactylon (L.) Pers., Chenopodium berlandieri Moq., Malvella leprosa (Ortega) Krapov., Convolvulus arvensis L., and Amaranthus palmeri S. Watson. Also nearby was a sparsely vegetated earth-bottom ditch with Conyza and Lepidium strictum (S. Watson) Rattan, and old fields with Secale cereale and a diverse group of weedy native and introduced forbs. Native plant species documented within 400 m of the Calystegia site include: Amaranthus palmeri, Ambrosia psilostachya DC., Amsinckia sp., Atriplex serenana Abrams, Baccharis salicifolia (Ruiz and Pav.) Pers., Chenopodium berlandieri, Epilobium brachycarpum C. Presl., Epilobium ciliatum Raf., Fraxinus velutina Torr., Pseudognaphalium californicum (DC.) Anderb., Heliotropium curassivicum, Heterotheca grandiflora Nutt., Malacothrix saxatilis (Nutt.) Torr. & A. Gray, Malvella leprosa, and Solanum americanum Mill.
Although seeds and rhizomes can be moved around in many ways, we contend that invoking accidental transport of stem fragments or seed by humans is not the most parsimonious explanation for the presence of Calystegia felix in the City of Chino, since the species is known nowhere else. While we have no direct proof, we think the recently discovered Calystegia felix populations represent plants that have emerged from latent, long-lived seed banks or roots following a return to “moist soil” conditions (Figs 8, 9), similar to those in the historical record. Buried seeds of Calystegia sepium have retained high levels of viability after 39 years (
Calystegia felix climbing upward through urban landscaping in the City of Chino (Photo M. C. Provance, 2013).
Emergent Calystegia felix on crusted, moist, Chino silt loam, amongst urban landscaping. It is unknown if large groups of emergent plants such as these represent one to just a few clones, or many genotypes (Photo M. C. Provance, 2013).
Historical information and early herbarium collections suggest that the Chino Basin originally had vegetation of wet meadow and alkali meadows dominated by Anemopsis californica (Nutt.) Hook. & Arn., with perennial grasses, such as Elymus triticoides Buckley, Sporobolus airoides (Torr.) Torr., and Distichlis spicata (L.) Greene, and herbs such as Trifolium willdenovii Spreng., Trifolium wormskioldii Lehm., and Helianthus annuus L. In addition, there were small bodies of open water, alkali and freshwater marshes, alkali scrub, alkali grassland, alkali playa, moist stream banks, and willow thickets. There were also phreatophytic woodland communities of Salix, Populus, and Platanus racemosa Nutt. (
“The lands just above this [above the ciénega-lands] are flat and often ill drained. The waters rising and evaporating here, under the influence of the effective southern sun, leave behind them their salt content, and thus alkali lands may result”
Thus, much of the landscape represented a mosaic of ciénega and ciénega-creek associated palustrine communities. The historical natural vegetation of the City of Chino cannot easily be envisioned because of past and current development. For example, a satellite of the University of California Agricultural Experiment Station called the “Ten Acre Tract” used to be in Chino and experiments related to growing crops on alkali soil were conducted there. This property was described as being dominated by Anemopsis californica (Hilgard & Loughridge 1896), which indicates that it was likely alkali marsh. The Ten Acre Tract is now occupied by industrial buildings and offices. However, taxa highly indicative of alkaline marsh and alkaline meadow have persisted in unusual places. For instance, we documented a number of Anemopsis californica persisting in plantings of Hedera helix along a sidewalk in northeast Chino near the Ontario border, just within the mapped historical limits of moist ground. The following year we found Calystegia felix growing in a sidewalk planter across the street from the Anemopsis site, in similar urban landscaping. We think other remnants of the ciénega flora may persist in Chino.
Conservation: Calystegia felix is endemic to Southern California, extirpated in Los Angeles County, and now likely confined to the Chino Basin in San Bernardino County. It is doubtless at high risk of soon becoming extinct in the wild. This is due to hydrological changes in the Chino Basin, including the paving of streams, lowering of the water table, and loss of ciénega habitat, including vegetation associated with marshes, meadows, grasslands and alkaline playas; and encroaching commercial, industrial, residential developments, and public works projects. Large areas of habitat have already been transformed. Six extant occurrences are now known, with an estimated 200 ramets emerging in 2013 at a single location near Chino Creek. However, those plants likely represent clones, as do the about 50 ramets at the other sites. Based on there being few known populations, a limited overall distribution, and a small number of individuals in existence, we suggest a conservation status of Critically Endangered (CR). Upon discovering the plants along Edison Road, it was obvious they were in imminent danger of being destroyed by impending grading and trenching for the burial of high-voltage power lines. We initially thought that these plants represented a single clone, but two ramet-specific flower color morphs, seed production, and spatial separation of clusters of emergent stems, suggest that two or more genotypes are present. Over the short term, Rancho Santa Ana Botanical Gardens has been contracted to conduct ex-situ propagation of rhizomes from the Edison Road population, and a few other plants are being cultivated in private and institutional gardens.
We are grateful for the help of Barbara Hellenthal at the Greene Herbarium, who sent us high-resolution images of original material. Special thanks are extended given to our colleagues Scott White and Justin Wood (Aspen Environmental Group), Sandra Namoff (Rancho Santa Ana Botanic Garden), and the late Richard K. Brummitt (Herbarium, Royal Botanic Gardens, Kew). We thank RSA, CAS and UC for the use of their collections, and the numerous herbaria contributing specimen images online, especially CAS, DS, E, K, LSU, NA, NY, P, SOC, UC-JEPS, US and WWB. We were very pleased to have received useful comments on the manuscript from Brett Provance, Sandra Namoff, Dan Austin, Sandra Knapp, and two other anonymous reviewers.
Image databases, internet address, and source herbaria, of specimen images initially examined for this study.
| Image database | Location | Herbaria coverage |
|---|---|---|
| Herbaria@home | http://herbariaunited.org/atHome/ | ABS, BIRM, SLBI, DBC, PTH |
| Digital specimen images at the Herbarium Berolinense. | http://ww2.bgbm.org/herbarium/ | B |
| Appalachian State University | http://vascularflora.appstate.edu/vps | BOON |
| California Academy of Sciences, botany collection CAS-BOT | http://collections.calacademy.org/bot/ | CAS-DS |
| George Safford Torrey Herbarium (CONN) database | http://bgbaseserver.eeb.uconn.edu/databasesimple.html | CONN |
| Royal Botanic Garden Edinburgh herbarium catalogue | http://elmer.rbge.org.uk/bgbase/vherb/bgbasevherb.php | E |
| Botany collections database | http://emuweb.fieldmuseum.org/botany/detailed.php | F |
| Fairchild Tropical Garden, virtual herbarium | http://www.virtualherbarium.org/vh/db/main.php | FTG |
| Geneva Herbaria Catalogue | http://www.ville-ge.ch/musinfo/bd/cjb/chg/?lang=en | G, G-DC, G-BOIS, G-BU |
| Kew Herbarium catalogue | http://apps.kew.org/herbcat/gotoHomePage.do | K |
| Virtual guide to the main collections of the LE Herbarium | http://www.mobot.org/MOBOT/Research/LEguide/index.html | LE |
| The Linnean collections | http://linnean-online.org/view/type/specimen/ | LINN, LINN-HS |
| The Herbarium of Louisiana State University, image gallery | http://www.herbarium.lsu.edu/ | LSU |
| Tropicos Image Search | http://www.tropicos.org/ImageSearch.aspx | MO |
| US National Herbarium: vascular plant types | http://www.usna.usda.gov/Research/Herbarium/BotType.html | NA |
| C. V, Starr virtual herbarium | http://sciweb.nybg.org/science2/vii2.asp | NY |
| Sonnerat – Muséum National d’Histoire Naturelle (Paris, France) Spécimens d’herbier | http://coldb.mnhn.fr/colweb/form.do?model=SONNERAT.wwwsonnerat.wwwsonnerat.wwwsonnerat | P |
| Consortium of Pacific Northwest Herbaria | http://www.pnwherbaria.org/ | SOC, WWB |
| Herbarium, Taiwan Forestry Research Institute, specimen data search | http://taif.tfri.gov.tw/search.php?l=Eng | TAIF |
| Alabama Plant Atlas | http://www.floraofalabama.org/ | TROY |
| Type specimens in UC and JEPS that have high-resolution images | http://ucjeps.berkeley.edu/db/types/imaged_types.html | UC-JEPS |
| Databased vascular plant types at UC/JEPS | http://ucjeps.berkeley.edu/db/types/types_table.html | UC-JEPS |
| US Herbarium, type register search | http://collections.mnh.si.edu/search/botany/?ti=3 | US |
| Atlas of Florida vascular plants, herbarium specimen search | http://florida.plantatlas.usf.edu/specimen.aspx | USF |
| Virtual Herbaria Austria | http://herbarium.univie.ac.at/database/search.php | W, WU, KUFS, HAL |
Specimens examined of Convolvulus binghamiae Greene, Convolvulus limnophilus Greene, and Calystegia sepium L.
Original, and possibly original material of Convolvulus binghamiae Greene. USA. California. Santa Barbara County: Santa Barbara, “Burton’s Mound” (acc. to the protologue), Aug, 1886 (fl), Mrs. R.F. Bingham s.n. (lectotype UC [UC-335392!, originally in the Lemmon Herbarium]; isolectotype F [not seen], according to
Original and probably original material of Convolvulus limnophilus Greene. USA. California. County unknown: Suisun Bay, Aug 1883, E.L. Greene s.n. (probable syntype NDG [NDG-66290] image); “Gxxx Station” [partly illegible], 1883, E.L. Greene (probable syntype NDG [NDG-66293] image).
Specimens examined of Calystegia sepium L. USA. California. Contra Costa County: levee along Bethel Island, 5 Sep 1954, I.L. Wiggins 13122, 13123 (DS); Otto’s Black Bass Resort, 8 Jul 1949, M.A. Nobs & S.G. Smith 941 (DS). Los Angeles County: near Los Angeles, May–June 1904, Pupils of Los Angeles High School s.n. (UC); SE of Huntington Beach, 15 Jun 1932, L.M. Booth 1192 (POM). Riveria [Rivera], 1 May 1902, A. Davidson 1892 [mixed sheet with Calystegia felix] (RSA). Mariposa County: Martinez, 30 Jul 1893, J.W. Congdon s.n. (CAS). Orange County: East of Huntington Beach, 5 Aug 1932, L.M. Booth 1359 (UC); Bolsa Chica, 28 Jun 1932, L.M. Booth 1214 (POM); Wintersberg, 26 Sept 1926, Peirson 7086 (UC, RSA). Riverside County: Hidden Valley Wildlife Refuge, 28 May 1989, M. Braun 39 (UCR); Springbrook, Fairmount Park, Riverside, S.B. Parish 916, 4612, 5334 (DS), 6435 (CAS); same location, Sep 1921, E. Smith s.n. (CAS); same location, 31 May 2004, Clarke s.n. (UCR); same location, Aug 1901, Hall s.n. (UC); same location, 10 Jun 1952, J.C. Roos 5763 (UCR); same location, 14 Sep 1999, M.C. Provance & S. Boyd 1780 (UCR, CAS, RSA, SD, UCD); same location, 29 May 2013, M.C. Provance & R. Richmeier s.n. (UCR). Sacramento County: Sacramento Valley, 1838–1842, Wilkes 1365 (US image). San Bernardino County: San Bernardino, no date, Wright 1727 (NDG image); two miles SE of San Bernardino, 17 Jul 1924, P.A. Munz 8690 (UCR); China Ranch, 7 Aug 1950, J.C. & A.R. Roos 4919 (RSA); San Bernardino Valley, 7 Sep 1907, S.B. Parish 6435 (CAS); San Bernardino, Jul 1881, S.B. & W.F. Parish 916 (DS); E St. Swamp, S.B. Parish 5334 (DS [2 sheets]); Chino, 16 Jul 1908, I.J. Condit s.n. (UC). San Joaquin County: Middle River, 22 Aug 1978, B. Atwater 52 (CAS); Old River, 16 Aug 1978, B. Atwater 37 (CAS); Holt, 21 Aug 1946, H.L. Mason & V. Grant 13067 (CAS). Solano County: Collinsville, Sep 1921, E. Smith s.n. (CAS); Suisun Marshes, 15 Oct 1905, W.R. Dudley s.n. (DS); same location, 15 Oct 1905, W.R. Dudley s.n. (DS); same location, 28 Oct 1938, J.T. Howell 14594 (CAS); same location, 28 Aug 1920, V. Jones s.n. (CAS); same location, Jul 1913, A. Eastwood 3451 (CAS).
Herbarium specimens examined of the Calystegia species included in Table 1.
Specimens examined. Calystegia occidentalis ssp. fulcrata. USA. California. Los Angeles County: Big John Flat, 21 Jun 1999, Swinney 7365 (UCR); Granite Mountain vicinity, 24 July 1991, T. Ross & S. Boyd 5602 (UCR). San Bernardino County: Fredalba, 22 Jul 1902, Abrams 2780 (E image, P image); N end of Fawnskin, at base of Delamar Mountain, 2141 m, 14 Jul 1993, S.D. White 1680 (UCR); San Bernardino Mtns., 1880, J.C. Nevins 417 (P image), Arrowhead Hot Springs and immediate vicinity, 579 m, A.C. Sanders 13805 (UCR). Sonoma County: near Sonoma [according to R. K. Brummitt], J.M. Bigelow s.n. (K image, NY image, PH image). Tuolumne County: Twain-Harte, A. Eastwood & J.T. Howell 8618 (CAS image). Calystegia occidentalis ssp. occidentalis. USA. California. County unknown: near San Francisco, date not known, H. Gibbons (GH54299 image). Amador County: New York Falls, 1500 ft., 3 Jul 1892, G. Hansen 79 (E image). Butte County: no further location provided, 1848, M. Hartweg 1862 (P image). Madera County: Chowchilla, June 1885, J.W. Congdon s.n. (P image). Napa County: S. of Lake Berryessa, 15 May 1971, M.J. Minabe 70 (LSU). Placer County: Forest Route 13, 1.8 mi south of junction with Forest Hill Rd., 28 Jul 2010, G. Helmkamp 16643 (UCR); 0.2 mi north of Forest Hill Rd. on unnamed road to Clementine Lake, 14 Jun 2006, G. & E.A. Helmkamp 10706 (UCR). Plumas County: Meadow Valley, 14 Jul 1963, B. Horn 630714 #10 (UCR). Shasta County: Anderson, 20 April 1968, G.C. Strausbaugh 29 (WWB image); near Middle Creek Station, 3 Jun 1905, A.A. Heller 7955 [4955 on label apparently in error] (E image). Siskiyou County: Little North Fork Public Camp, 1997 ft., C. Miller 56 (SOC image); Yreka, 8 Jun 1905, A.A. Heller 7997 (E image). Oregon. Douglas County: Mt. Nebo, Roseburg, 900 ft., 22 May 1967, Godfrey 49 (SOC image). Jackson County: Jackson City, 22 May 1981, F.A. Lang 1412 (SOC image). Lake County: Mountains near Lakeview, 19 Aug 1901, W.C. Cusick 2771 (E image). Calystegia peirsonii. USA. California. Los Angeles County: Rock Creek, 27 May 1923, F.W. Peirson 3537 (CAS image); same location, 21 May 1923, Peirson 7301 (UC image); near Palmdale, 9–24 May 1896, J.B. Davy 2304 (UC image). Bouquet C. felix Road east of the reservoir, 20 Jun 1979, T. Krantz s.n. (UCR); Castaic Mesa, 28 Apr 2003, A.C. Sanders 26128 (UCR); Mt. Gleason, 20 Aug 1992, O. Mistretta 761 (UCR); Newhall Ranch, 19 Jun 2002, A.C. Sanders & M. Elvin 25200 (UCR). Calystegia subacaulis ssp. episcopalis. USA. California. Acad. du San Francisco, Anonymous s.n. (P4492495 image). San Luis Obispo County: Cambria, 28 Apr 1926, A. Eastwood 13641 (K image, CAS image). San Mateo County: Woodside, 13 May 1932, L.S. Rose 32248a (P image). Above Crystal Springs on the Half Moon Bay road, 28 May 1907, Heller 8557 (P image). Sonoma County: Adobe Canyon, just NW of Sonoma, 1200 ft., 24 May 1996, F. Bowcutt 2163 (UCR).