(C) 2013 Tiina Särkinen. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Särkinen T, Gonzáles P, Knapp S (2013) Distribution models and species discovery: the story of a new Solanum species from the Peruvian Andes. PhytoKeys 31: 1–20. doi: 10.3897/phytokeys.31.6312
A new species of Solanum sect. Solanum from Peru is described here. Solanum pseudoamericanum Särkinen, Gonzáles & S.Knapp sp. nov. is a member of the Morelloid clade of Solanum, and is characterized by the combination of mostly forked inflorescences, flowers with small stamens 2.5 mm long including the filament, and strongly exerted styles with capitate stigmas. The species was first thought to be restricted to the seasonally dry tropical forests of southern Peru along the dry valleys of Río Pampas and Río Apurímac. Results from species distribution modelling (SDM) analysis with climatic predictors identified further potential suitable habitat areas in northern and central Peru. These areas were visited during field work in 2013. A total of 17 new populations across the predicted distribution were discovered using the model-based sampling method, and five further collections were identified amongst herbarium loans. Although still endemic to Peru, Solanum pseudoamericanum is now known from across northern, central and southern Peru. Our study demonstrates the usefulness of SDM for predicting new occurrences of rare plants, especially in the Andes where collection densities are still low in many areas and where many new species remain to be discovered.
ResumenSe describe una nueva especie de Solanum sección Solanum endémica del Perú. Solanum pseudoamericanum Särkinen, P. Gonzáles & S.Knapp sp. nov., es un miembro del clado Morelloide de Solanum. Se caracteriza por presentar inflorescencias bifurcadas, flores con estambres pequeños de 2.5 mm de largo incluyendo el filamento y estilos muy exertos con estigma capitado. En un principio se pensó que la especie estaba restringida a los bosques tropicales estacionalmente secos del sur del Perú, a lo largo de los valles secos de los ríos Pampas y Apurímac. Los resultados del análisis de los modelos de distribución de especies (SDM) con predictores climáticos identificaron posibles áreas con hábitat adecuado en el norte y centro del Perú. Durante el trabajo de campo en el 2013 estas áreas fueron visitadas. Se descubrieron 17 nuevas poblaciones ubicadas dentro de la distribución predicha, y aunque sigue siendo endémica en el Perú, Solanum pseudoamericanum ahora es conocida en todo el norte, centro y sur de Perú. Nuestro estudio demuestra la utilidad de SDM para predecir nuevos registros de plantas raras, sobre todo en los Andes, donde la cantidad de colecciones sigue siendo baja en muchos areas y donde muchas especies nuevas aún no se han descubierto.
Tropical Andes, Solanaceae, species distribution modelling, Peru, endemism, rare species, Morelloid Clade, Solanum section Solanum
The tropical Andean hotspot is one of the most species rich but data poor areas of the world (
Many more species remain to be discovered, however, especially in Peru and Ecuador, where the number of new discoveries per year shows no sign of diminishing (
In an effort to speed up the process of cataloguing species diversity and recording accurate distributions, an approach referred to as Model-Based Sampling (MBS) has been developed (
Here we present a case study of MBS from Solanum, one of the most species rich vascular plant genera in the Andes. In Peru alone Solanum includes 299 species, of which 102 are endemic (
We examined 26 herbarium specimens in the herbaria listed in the text. These were combined with our field observations from Peru in the identification and description of the new taxon (see Taxonomy below). All specimens are cited in the text and full data is provided in the supplemental file and on Solanaceae Source (www.solanaceaesource.org ). We included all specimens examined in the model-based analysis.
Following the MBS approach by
We first ran MAXENT based on the four observed collections from 2012 from southern Peru to identify potential suitable habitat areas for the target species (Model 1). The model was run with default settings (allowing for transformations of the covariates with the default thresholds for conversion, removing duplicate presence records, maximum number of background points = 10, 000, maximum number of iterations = 500; convergence threshold = 0.00001; fit regulization parameter = 1; default prevalence = 0.5). To evaluate model performance, we ran it with cross-validation, where the occurrence data is randomly split into two equal-sized groups and one of them is then used for creating the model whilst the other is used for validating the model. We chose cross-validation approach because it uses all of the data for validation, unlike a single training/test split, and is hence more suitable when working with small numbers of occurrence points across a complex landscape (
The model was run with 11 bioclimatic variables at 30 arc second spatial resolution (c. 1 km2) (
Principal components analysis (PCA) results of the climatic variables (http://www.worldclim.org ) used to generate distribution models for Solanum pseudoamericanum. Variables with Pearson correlation coefficients equal or greater than 0.75 were removed.
| PC1 | PC2 | |
|---|---|---|
| Cumulative variation explained | 47% | 70% |
| BIOCLIM VARIABLES USED | Eigenvectors | |
| Mean Diurnal Range (BIO2) | 0.085 | -0.117 |
| Isothermality (BIO3) | -0.095 | -0.001 |
| Temperature Seasonality (BIO4) | 0.044 | 0.175 |
| Max Temperature of Warmest Month (BIO5) | -0.277 | 0.121 |
| Min Temperature of Coldest Month (BIO6) | -0.304 | 0.160 |
| Temperature Annual Range (BIO7) | 0.097 | -0.094 |
| Mean Temperature of Driest Quarter (BIO9) | -0.300 | 0.148 |
| Precipitation Seasonality (BIO15) | 0.070 | 0.346 |
| Precipitation of Wettest Quarter (BIO16) | -0.224 | -0.254 |
| Precipitation of Driest Quarter (BIO17) | -0.235 | -0.278 |
| Precipitation of Warmest Quarter (BIO18) | -0.109 | -0.253 |
The Model 1 output was ground-truthed with additional field work. To target areas where potential new populations of Solanum pseudoamericanum could be encountered, we chose to interpret the Model 1 cumulative output. We chose not to use a threshold approach, where the prediction is divided into a binary map of presence or absence, due to the fact that only four records were used for building the model and hence using a threshold approach would discard valuable data. The cumulative output indicates relative suitability, not probability, of occurrence with values ranging from 0 to 100. Grid cell values are calculated as the sum of the cells with equal or lower probability, multiplied by 100 to give a percentage (
We ran a second model after the second field season, where all new localities identified through field work and herbarium visits and loans were included. Model 2 was run using a total of 26 records, of which four were from our first field trip in 2012, 17 were from our second field trip in 2013, and five from herbarium records (SI File 1 Occurrence data). The same 11 climatic predictors and MAXENT parameters were used as in Model 1 (see above). The model was trained using Peru as the study extent, and results were projected to an area that covered the whole of Ecuador and northern Bolivia (-81.0, 65.6, -19.5, 0). A final potential distribution map for Solanum pseudoamericanum was produced based on the cumulative output of Model 2, where all areas with relative suitability above 0.4 (logistic output) were considered as potential areas of occurrence for the species.
We evaluated the relative success of our SDM model predictions based on the mean area under curve (AUC) values of the receiver operating characteristic (ROC) curve of the cross-validation replicates. AUC values close to 1 indicate optimal performance, whilst values close to 0.5 indicate performance equal to random. Both models yielded AUC values > 0.98 indicating good model performance (Table 2). The two most important climatic variables included in Models 1 and 2 were precipitation of the driest quarter, temperature seasonality, and minimum temperature of the coldest month based on jacknife analyses of variable importance. Other important variables included isothermality (mean diurnal range coupled with annual temperature range) and maximum temperature of warmest month.
Model performance values for the two models run to detect suitable habitat areas for Solanum pseudoamericanum.<br/>
| Model | No. of records | AUC score (mean) | Standard deviation |
|---|---|---|---|
| Model 1 | 4 | 0.987 | 0.009 |
| Model 2 | 26 | 0.984 | 0.014 |
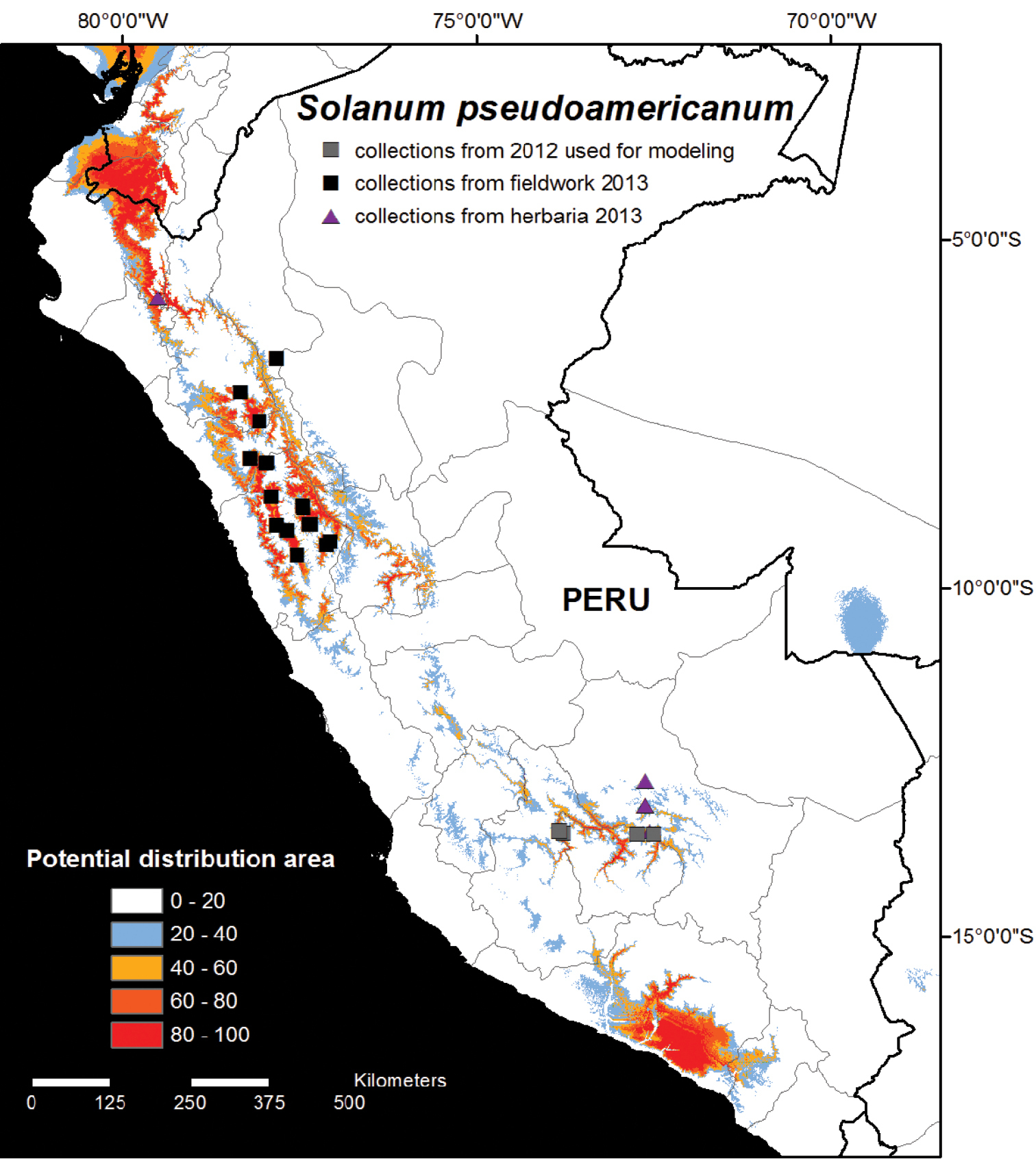
The results of Model 1, using only the first four records from 2012, showed highly suitable climatic conditions in northern and central Peru in the Departments of Cajamarca, La Libertad, Ancash and Huánuco, as well as in northernmost Piura and Loja, El Oro and Azuay provinces of Ecuador (Fig. 1). The core suitable areas were visited in Cajamarca, La Libertad, and Ancash during the second field season, and 17 new populations were identified (Fig. 1). Five specimens were identified amongst herbarium loans from NY and MO, collected from Piura and Cusco (Fig. 1; Appendix). Surprisingly, no collections of Solanum pseudoamericanum were found in local herbaria in Peru. Model 1 also identified highly suitable habitat areas in southern Moquegua and Arequipa (Fig. 1). These areas were visited in 2012 during our first field season and whilst many Solanum collections were made, no specimens of Solanum pseudoamericanum were observed.
Potential habitat distribution map of Solanum pseudoamericanum. The potential habitat areas reflect the cumulative output of the MAXENT model produced using 11 climatic variables with the original four collection localities from 2012 from southern Peru shown as grey squares on the map (see Methods for details). Areas identified as highly suitable (above 40% cumulative probability) in central and northern Peru were visited in 2013 during the second field season, and 17 new collection localities were found as a result (black squares). Five additional collections were identified amongst herbarium loans (purple triangles).
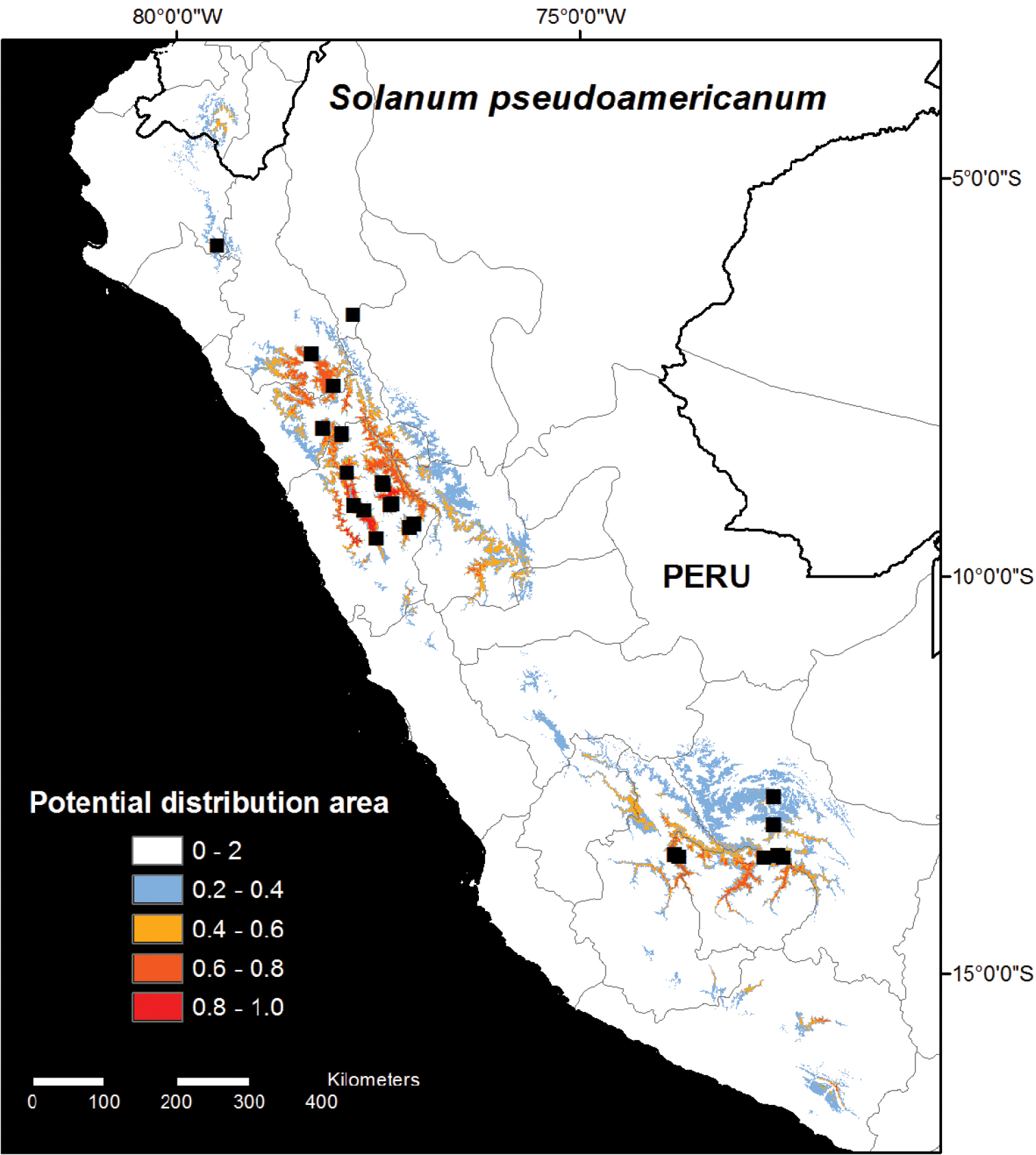
Model 2 was run with all collection data from 2012 and 2013, including all herbarium collections (Fig. 2). The Model 2 prediction was generally similar to Model 1, but Model 2 predicted a smaller range size to the species where no suitable habitat areas are predicted to occur outside Peru except in Loja, Ecuador, and only small areas of likely habitat area are found in Arequipa (Fig. 2). The smaller predicted distribution area in Model 2 was despite the fact that the results were projected over larger area covering both Ecuador and northernmost Bolivia. Areas identified in Model 2 as likely habitat areas but which remain unconfirmed include southwest San Martín, Huánuco, northern Pasco, Huancavelica, Junín, and Arequipa, as well as Loja, Ecuador (Fig. 2).
Distribution map of Solanum pseudoamericanum. The potential habitat areas reflect the logistic output of the MAXENT model produced using 11 climatic variables with all current known occurrence records (N=26; Model 2).
Previous studies have clearly demonstrated how the use of SDM can dramatically increase detection rates of rare species in the field (
Here we describe a new species and provide both an observed distribution map as well as a modelled distribution range for the species. With increasing ease of SDM through publicly supported online portals such as BioVel (http://www.biovel.eu ), the tools are now available for non-specialists to analyse models prior to species publication. Generally, SDMs are still created by GIS specialists rather than taxonomic specialists, but the availability of online portals will hopefully increase the use of SDM amongst taxonomists who are best informed to run such models because of their expert knowledge of species’ ecology.
Modelled distribution maps have large benefits over observed distribution maps. Modelled maps, although still incomplete, can be argued to provide a more realistic picture of the actual species’ distribution area. This is because modelled maps are less biased by collection densities, and although nowhere near complete, provide a step towards representing species distributions in a more realistic manner. Such maps will also aid in targeting field collecting efforts and provide additional information for planning conservation areas compared to traditional maps.
Whilst advocating the publication of modelled distributions for new species, we fully acknowledge that species distributions are not guided by simple factors such as climate alone. Many factors govern range size, including dispersal limitation, competitive exclusion, habitat destruction, urbanisation and agriculture, as well as species interactions. These complex factors are often dismissed in simplistic SDMs where only bioclimatic predictors are included. Simple SDMs can, however, be used as a starting point for evaluating rare species (e.g.,
In the case of Solanum pseudoamericanum, the MBS approach helped us to extent the range size of the newly described species, changing our view of the target taxon from a narrow endemic species restricted to only two river valleys in southern Peru to a relatively widespread species that is distributed across Peru. The large increase in the actual observed distribution range of the new species demonstrates not only how poorly collected the Peruvian Andes is for vascular plants, but also how MBS can work with extremely low number of collection records across a complex landscape. This extension of the observed occurrence area of the newly described species was despite the relatively large model training area that was used, where the whole of Peru was considered. The use of relatively large training areas in model training leads to model overfitting and underprediction of distribution areas (
Our null hypothesis was that MBS approach cannot be used in such a highly variable landscape as the Andes with as few records as we had available. Our expectations were low for two reasons. Firstly, the climate data available for the Andes through WorldClim suffers from high uncertainty because only a few weather stations were used to interpolate the data (
Results from our case study indicate that both assumptions might not be correct. The high AUC scores shown by our models indicate that informative models can be run with as little data as used here and with climate predictors alone. The climate data appears to be of high enough quality to reveal broad patterns that can be used to identify suitable habitats across poorly explored regions. Variation in climate, and the associated elevational gradients, seem to explain large parts of plant distribution patterns in the Andes (
Another question is the minimum number of occurrence records required for building accurate distribution models. While it is well established that more data produce better, more accurate models (
The new species described here belongs to Solanum section Solanum within the Morelloid clade (sensu
urn:lsid:ipni.org:names:77134672-1
http://species-id.net/wiki/Solanum_pseudoamericanum
Figs 2–4Like Solanum americanum L. but differing in branched inflorescences with flowers spaced along the rachis (not umbellate), rounded calyx lobes that are not reflexed in fruit, style exserted beyond the anther tube for more than 1 mm, stigma globose and capitate, and fruit with the surface not markedly shiny.
Peru: Cajamarca: Prov. Cajabamba, in town of Cajabamba, 7°36'43"S, 78°03'28"W, 2649 m, 9 May 2013 (fl, fr), S. Knapp, T. Särkinen, H.M. Baden, P. Gonzáles & E. Perales 10575 (holotype: USM!; isotypes: BM!, HUT!, CPUN!).
Herb with woody base, 20–50 cm tall, the individual stems to 1 m long and sprawling. Stems terete or somewhat angled with ridges, pubescent with simple uniseriate 1–4-celled trichomes often clustered along the stem angles; new growth densely pubescent with appressed 1–4-celled simple uniseriate trichomes 0.2–0.8 mm long. Sympodial units difoliate, not geminate. Leaves simple, 4.5–12(–15) cm long, 1.8–8 cm wide, ovate to elliptic; adaxial surface sparsely pubescent with more or less appressed 1-4-celled translucent simple uniseriate trichomes, these denser along the veins; abaxial surface more densely pubescent with simple uniseriate trichomes like those of the upper surface; primary veins 5–8 pairs; base acute and decurrent on the petiole; margins entire or occasionally with shallow lobes in the basal third; apex acute; petiole 0.5–2.5(–5) cm long, occasionally narrowly winged, sparsely pubescent with simple uniseriate trichomes like those of the stems and leaves. Inflorescences lateral and intermodal, 1–2.5 cm long, simple or once-branched, with 3–5(9) flowers, sparsely pubescent with appressed 1–2-celled simple uniseriate trichomes; peduncle 0.4–1.6 cm long, if the inflorescence branched then the peduncle rachis 0.4–0.6 cm long; pedicels 0.6–0.7 cm long, ca. 0.3 mm in diameter at the base and apex, straight and spreading, articulated at the base; pedicel scars spaced ca. 1 mm apart. Buds globose, the corolla only exserted from the calyx tube just before anthesis. Flowers 5-merous, all perfect; calyx tube ca. 1 mm long, the lobes 0.5–0.7 mm long with rounded apices, sparsely pubescent with 1–4-celled translucent simple uniseriate trichomes; corolla 5–6 mm in diameter, stellate, white with a yellow central portion near the base, lobed slightly less than halfway to the base, the lobes ca. 1.5 mm long, 2 mm wide, strongly reflexed at anthesis, later spreading, densely pubescent abaxially with 1–4-celled simple uniseriate trichomes, these usually shorter than the trichomes of the stems and leaves; filament tube minute, pubescent with tangled uniseriate trichomes adaxially; free portion of the filaments ca. 1 mm long, pubescent like the tube; anthers ellipsoid, yellow, ca. 1.5 mm long, 0.7–0.8 mm wide; ovary conical, glabrous; style 3–4 mm long, exserted (0.5)1–2 mm beyond the anther cone, densely pubescent with 2–3-celled simple uniseriate trichomes at the base; stigma globose and capitate, minutely papillate, bright green in live plants. Fruit a globose berry, 4–9 mm in diameter, green at maturity or green and turning purplish black when ripe, the surface not markedly shiny, lacking stone cells aggregates; fruiting pedicels 4–7 mm long, ca. 1 mm in diameter at the base, spreading and becoming somewhat more woody in fruit, usually remaining on the plant after fruit drops; fruiting calyx lobes spreading or appressed to the berry, not reflexed. Seeds 35–45 per berry, 1.2–1.5 mm long, 0.9–1 mm wide, flattened-reniform, yellowish, the surfaces minutely pitted, the testal cells pentagonal in outline.
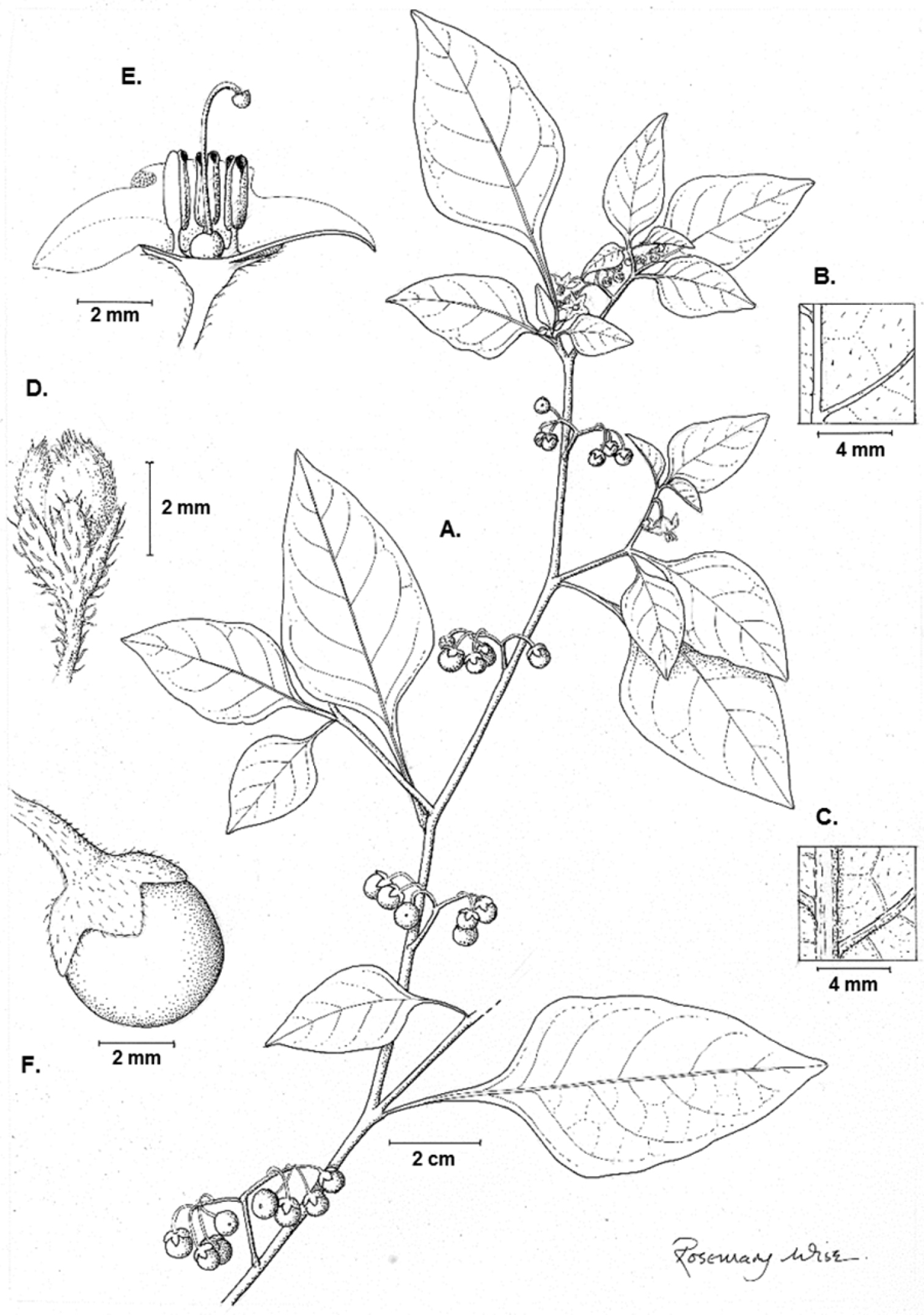
Illustration of Solanum pseudoamericanum. A Habit B Adaxial leaf surface C Abaxial leaf surface D Bud E Half flower F Fruit (A–F Knapp 10351). Illustration by Rosemary Wise.
Photos of Solanum pseudoamericanum. A Habit B Ridged stem C Flowers with small anthers c. 1.5 mm long, strongly exserted styles and with capitate stigmas D Developing fruits which turn purple-black when fully ripe with calyx appressed to the fruit. (A Särkinen et al. 4640; B Knapp et al. 10357; C, D Knapp et al. 10300) Scale bars = 1 mm.
Endemic to Peru in the upper zones of seasonally dry tropical forests or in mid-elevation montane forests, usually above 2, 000 m elevation, with only some overlap between the closely related Solanum americanum that occurs from sea level to 2, 200 m in elevation; commonly growing in sandy soils in full sun or partial shade in disturbed sites such as landslides and roadsides or cultivated areas, often in moist depressions in otherwise dry areas, associated with Schinus molle L., Aspidosperma polyneuron Müll. Arg., Eriotheca sp., Vachellia macracantha (Humb. & Bonpl.) Seigler & Ebinger, Alnus acuminata Kunth, Solanum probolospermum Bitter, and Calceolaria spp.; (930-)1700–3200(-3735) m in elevation. Based on field and herbarium collections Solanum pseudoamericanum occurs in the Departments of Amazonas, Ancash, Apurímac, Cajamarca, Cusco, La Libertad, and Piura, but based on the modelled habitat suitability map (Fig. 2) it is also likely to also occur in the Departments of Lambayeque, Huánuco, Huancavelica, Ayacucho, Junín, southwestern San Martín, northernmost areas of Lima, and in the Province of Loja in Ecuador.
Flowering January–July, fruiting March–July.
The name Solanum pseudoamericanum refers to the fact the new species greatly resembles Solanum americanum in general form and has been commonly identified under the name of the more common pantropical weed.
The IUCN threat status ofSolanum pseudoamericanum is here considered of least concern (LC) based on the relatively large extent of the species occurrence (c. 159, 000 km2), although the actual area of occupancy is small (96 km2). The species grows readily in disturbed sites and combined with the fact that the currently known populations are spread across Peru, it appears to have relatively low threat status despite the generally increasing human pressure and habitat destruction in the Andes.
PERU. Amazonas: Chachapoyas, 8 km along road from Leimebamba to Celendín, between km 417–416, 6°42'48"S, 77°49'05"W, 2634 m, 21 Apr 2013 (fl, fr), T. Särkinen et al. 4624 (USM, BM). Ancash: Pallasca, Puente Chucusvalle over Río Tablachaca, left bank of river (other side of bridge in La Libertad), 8°12'10"S, 77°57'06"W, 2148 m, 11 May 2013 (fl, fr), S. Knapp et al. 10604 (USM, BM); Pallasca, ca. 10 km above Puente Chucusvalle over Río Tablachaca on rd to Pallasca, 8°13'25"S, 77°57'23"W, 2148 m, 11 May 2013 (fl, fr), S. Knapp et al. 10616 (USM, BM); Huaylas, Dist. Pueblo Libre, just beyond Carapampa village, a few km above bridge over Río Santa, 9°06'28"S, 77°48'42"W, 2637 m, 13 May 2013 (fl, fr), S. Knapp et al. 10650 (USM, BM); Huaraz, Huaraz, in city, 9°31'51"S, 77°31'27"W, 3003 m, 15 May 2013 (fl, fr), T. Särkinen et al. 4670 (USM, BM); Carhuaz, on rd from Mancos to Musho, before Puente Apachico, 9°10'35"S, 77°40'31"W, 2886 m, 16 May 2013 (fl, fr), T. Särkinen et al. 4678 (USM, BM); Corongo, km1-3 on rd to Corongo, a side road from Chimbote-Huaraz main rd, 8°41'38"S, 77°53'51"W, 2334 m, 18 May 2013 (fl, fr), T. Särkinen et al. 4686 (USM, BM); Pomabamba, just in the outskirts of Pomabamba on rd leading to Piscobamba, 8°49'27"S, 77°27'12"W, 3008 m, 21 May 2013 (fl, fr), T. Särkinen et al. 4730 (USM, BM); Pomabamba, 2-3km from Pomabamba towards Lucma, 8°51'13"S, 77°26'12"W, 2837 m, 22 May 2013 (fl, fr), T. Särkinen et al. 4737 (USM, BM); Yungay, ribera del Río, 20 Jul 1977, Luna, A., 70 (USM); Carlos F. Fitzcarrald, on rd between Sapcha and San Luis, 9°05'50"S, 77°21'05"W, 3133 m, 24 May 2013 (fl, fr), T. Särkinen et al. 4778 (USM, BM); Carlos F. Fitzcarrald, in San Luis, outskirts of town, 9°05'35"S, 77°19'42"W, 3147 m, 24 May 2013 (fl, fr), T. Särkinen et al. 4780 (USM, BM); Huari, c. 5km from Pomachaca on road to Llamellín, 9°23'05"S, 77°06'59"W, 2605 m, 26 May 2013 (fl, fr), T. Särkinen et al. 4791 (USM, BM); Huari, on rd from Pomachaca to Llamellín, 9°20'24"S, 77°03'22"W, 2571 m, 26 May 2013 (fl, fr), T. Särkinen et al. 4794 (USM, BM). Apurímac: ca. 11 km from Chincheros descending to Río Pampa on Ayacucho-Andahuaylas rd (RN3), 13°31'27"S, 73°46'15"W, 2215 m, 7 Mar 2012 (fl, fr), S. Knapp et al. 10300 (USM, BM); Chincheros, along Río Pampa on Ayacucho-Andahuaylas rd (RN3), ca. 3-4 km from Puente Pampa on Apurímac side, 13°29'26"S, 73°49'32"W, 2028 m, 7 Mar 2012 (fl, fr), S. Knapp et al. 10307 (BM, USM); Abancay, village of Tambo, above Curahuasi, on rd from Abancay to Cusco, Dist. Curahuasi, 13°32'21"S, 72°43'02"W, 2673 m, 10 Mar 2012 (fl, fr), S. Knapp et al. 10351 (USM, BM); Abancay, at turn to Santuario Curahuasi, ca. 17 km above Puente Cunyac over Río Apurímac, road Abancay-Cusco towards Curahuasi, Dist. Curahuasi, 13°32'19"S, 72°29'28"W, 2340 m, 11 Mar 2012 (fl, fr), S. Knapp et al. 10357 (USM, BM). Cajamarca: Cajabamba, in town of Cajabamba, 7°36'43"S, 78°03'28"W, 2649 m, 9 May 2013 (fl, fr), S. Knapp et al. 10575 (USM, BM); Cajamarca, km1244 on rd from Cajamarca to San Marcos, just outskirts of Namora village, 7°12'04"S, 78°19'33"W, 2764 m, 24 Apr 2013 (fl, fr), T. Särkinen et al. 4640 (USM, BM). Cusco: La Convención, Dist. Echarate, Papelpata, Alto Echarate, 12°46'37"S, 72°36'39"W, 931 m, 24 May 2007 (fl, fr), G. Calatayud et al. 4062 (NY); Anta, Mollepata, W of Cusco, 13°30'29"S, 72°33'21"W, 3200 m, 10 Jan 1984 (fl), A.H. Gentry et al. 44135 (MO); La Convención, Santa Teresa, Dist. Santa Teresa, Carretera Santa Teresa-Hidroelectrica, Bosque Seco Secundario, 13°07'21"S, 72°36'31"W, 1700 m, 20 Mar 2004 (fl, fr), I. Huamantupa et al. 4280 (MO); La Convención, Santa Teresa, Dist. Santa Teresa, Carretera Santa Teresa-Hidroelectrica, Bosque Seco Secundario, 13°07'21"S, 72°36'31"W, 1700 m, 20 Mar 2004 (fl, fr), I. Huamantupa et al. 4287 (MO, USM). La Libertad: Santiago de Chuco, ca. 1 km outside Santiago de Chuco on rd from Shorey and Shorey Chico, at stream crossing, 8°08'38"S, 78°11'08"W, 3735 m, 10 May 2013 (fl, fr), S. Knapp et al. 10590 (USM, BM); Santiago de Chuco, 6-8 km below Mollepata on rd to river valley of Río Tablachaca, right side of river, 8°12'03"S, 77°57'11"W, 3735 m, 11 May 2013 (fl, fr), S. Knapp et al. 10599 (USM, BM). Piura: Huancabamba, Porculla, km 38, 5°50'25"S, 79°29'38"W, 5°50'25"S, 79°29'38"W, 1800 m, 8 Apr 1989 (fl, fr), S. Llatas Q. 2348 (NY).
Most of the collections of Solanum pseudoamericanum are the result of our intensive collecting of Solanaceae in Peru in the last two years. We suspect that the paucity of earlier collections may in part be due to the resemblance to the widespread and weedy Solanum americanum that has led to botanists regarding this new species as not worth collecting. Widespread species often harbour cryptic diversity (e.g.,
Solanum pseudoamericanum can be distinguished from the similar Solanum americanum by the following suite of characters; berries that are matte or somewhat shiny at maturity, versus very shiny in Solanum americanum, styles that are always exerted to approximately equal to the length of the anther cone, versus styles almost included in the anther cone in Solanum americanum, and globose, bright green stigmas, versus white or pale green stigmas that are merely a widening of the style tip in Solanum americanum. Other members of the Morelloid clade in Peru without glandular trichomes which grow sympatrically with Solanum pseudoamericanum differ from it in being larger in growth form reaching up to 2 m in height, having larger, always violet flowers and fruits that are green at maturity (Solanum probolospermum Bitter and Solanum zahlbruckneri Bitter), or being smaller herbs up to 30 cm high with similar sized flowers but fruits orange or yellow in colour (Solanum corymbosum Jacq and Solanum radicans L.f.).
We thank Maria Baden, Emilio Perales, Diana Percy, Erica McAlister, and Andrew Matthews for assistance in the field, Asunción Cano and Betty Millán for assistance with permits in Peru, Severo Baldeón, Eric Rodríguez, Isidoro Sánchez Vega and the staff of the USM, HUT and CPUN herbaria for assistance during herbarium visits, and the Peruvian authorities of the Ministerio de Agricultura, Dirección General Forestal y de Fauna Silvestre, for granting our collection permit No. 084-2012-AG-DGFFS-DGEFFS under which this study was executed. We thank the herbaria mentioned in the text for specimen loans. This work was supported by NSF grant DEB-0316614 “PBI Solanum: A worldwide treatment” to SK.
Occurrence records of Solanum pseudoamericanum (doi: 10.3897/phytokeys.31.6312.app) File format: Adobe PDF file (PDF).