(C) 2013 Larry R. Noblick. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Noblick LR (2013) Leaflet anatomy verifies relationships within Syagrus (Arecaceae) and aids in identification. PhytoKeys 26: 75–99. doi: 10.3897/phytokeys.26.5436
The current investigation was carried out to examine how palm anatomy may coincide with the current molecular analysis including the three recognized clades of Syagrus Mart. and to justify the splitting of acaulescent Syagrus species (e.g. Syagrus petraea (Mart.) Becc.) into several species. Free-hand cross-sections of leaflets were made and the comparison of these verifies the relationships suggested by the molecular data. Free-hand leaflet sections were also found to be useful in the identification of otherwise difficult-to-identify acaulescent Syagrus species. The result and conclusion is that anatomical data is valuable in helping to verify molecular data and that splitting the acaulescent species of Syagrus is justified by the differences discovered in their field habit and anatomy. These differences were used to produce an identification key that is based on the anatomy.
leaflet anatomy, identification, Arecaceae, Syagrus, acaulescent
Syagrus is part of the largest subfamily of palms, Arecoideae (
Palm leaflet anatomy has been useful in identification and has been used to suggest systematic relationships.
The first part of this paper investigates leaflet anatomy to see how it coincides and possibly even verifies the relationships supported by the molecular analysis of
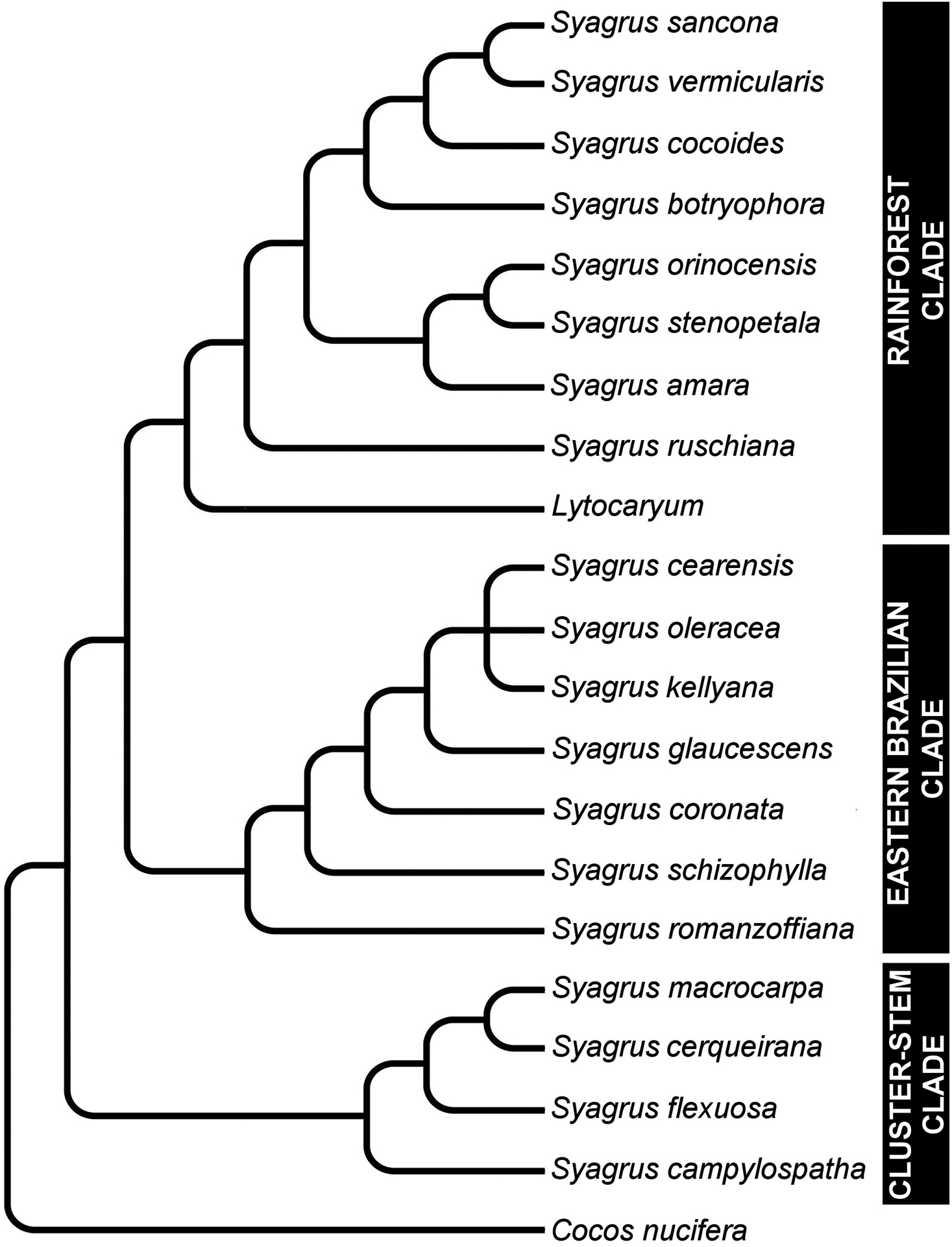
Cladogram showing major relationships in Syagrus (adapted from
The second part of this paper deals with the problem of identifying the “acaulescent” species of Syagrus. Most of these palms grow in Brazilian savannas (cerrados) and high altitude rocky fields (campo rupestre). Many species of Syagrus are described as acaulescent and
Visible morphological field characters of “acaulescent” Syagrus species. x = normally present and s = sometimes present.
| # | Name | Petiole deflexed | Pinnae deflexed | Pinnae regular | Pinnae clustered | Pinnae silvery blue | Pinnae whitish/silver beneath | Inflorescence a spike | Inflorescence branched |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Syagrus allagopteroides Noblick & Lorenzi | x | x | ||||||
| 2 | Syagrus angustifolia Noblick & Lorenzi | x | x | x | |||||
| 3 | Syagrus caerulescens Noblick & Lorenzi | x | x | x | x | ||||
| 4 | Syagrus campylospatha (Barb.Rodr.) Becc. | x | x | ||||||
| 5 | Syagrus cerqueirana Noblick & Lorenzi | x | x | ||||||
| 6 | Syagrus duartei Glassman | x | x | ||||||
| 7 | Syagrus evansiana Noblick | x | x | x | x | ||||
| 8 | Syagrus glazioviana (Dammer) Becc. | s | x | x | |||||
| 9 | Syagrus gouveiana Noblick & Lorenzi | x | x | ||||||
| 10 | Syagrus graminifolia (Drude) Becc. | s | x | x | x | ||||
| 11 | Syagrus graminifolia var. glazioviana (Dammer) Becc. | s | x | x | |||||
| 12 | Syagrus harleyi Glassman | s | x | x | |||||
| 13 | Syagrus itacambirana Noblick & Lorenzi | s | x | x | |||||
| 14 | Syagrus lilliputiana (Barb.Rodr.) Becc. | x | x | x | |||||
| 15 | Syagrus loefgrenii Glassman | x | x | x | x | ||||
| 16 | Syagrus longipedunculata Noblick & Lorenzi | x | x | x | |||||
| 17 | Syagrus mendanhensis Glassman | x | x | ||||||
| 18 | Syagrus microphylla Burret | x | x | x | x | ||||
| 19 | Syagrus minor Noblick & Lorenzi | x | x | x | x | ||||
| 20 | Syagrus petraea (Mart.) Becc. | x | x | x | |||||
| 21 | Syagrus pleioclada Burret | x | x | x | |||||
| 21 | Syagrus pleiocladoides Noblick & Lorenzi | x | x | x | |||||
| 23 | Syagrus procumbens Noblick & Lorenzi | x | x | x | |||||
| 24 | Syagrus rupicola Noblick & Lorenzi | x | x | x | x | ||||
| 25 | Syagrus vagans (Bondar) A. Hawkes | x | x | ||||||
| 26 | Syagrus werdermannii Burret | x | x |
List of anatomical descriptors or characters for each of the species of acaulescent Syagrus. X = present, S = sometimes.
| Descriptors or Characters | Syagrus campylospatha | Syagrus harleyi | Syagrus cerqueirana | Syagrus allagopteroides | Syagrus lilliputiana | Syagrus minor | Syagrus loefgrenii | Syagrus longipedunculata | Syagrus angustifolia | Syagrus itacambirana | Syagrus procumbens ‘emasensis’ | Syagrus procumbens | Syagrus petraea | Syagrus gouveiana | Syagrus duartei | Syagrus caerulescens | Syagrus rupicola | Syagrus evansiana | Syagrus glazioviana | Syagrus microphylla | Syagrus vagans | Syagrus werdermannii | Syagrus pleiocladoides | Syagrus pleioclada | Syagrus mendanhensis | Syagrus graminifolia | Syagrus graminifolia var. glazioviana |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major veins | |||||||||||||||||||||||||||
| Huge major vein adjacent to or nearly adjacent to leaflet margin | X | ||||||||||||||||||||||||||
| Normal-sized major vein near the margin but not adjacent to it (separated by a fiber bundle adaxially and/or a minor vein abaxially. | X | X | X | ||||||||||||||||||||||||
| Major vein usually unattached separated from the abaxial and adaxial hypodermis by another cell layer or more | X | X | ? | X | X | X | X | ||||||||||||||||||||
| Major veins attached to the adaxial hypodermis but separated from the abaxial hypodermis by an additional cell layer or layers | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||
| Major veins mostly attached to the adaxial surface often by a short or long fibrous sheath extension (girder) | X | X | X | X | X | X | X | X | X | ||||||||||||||||||
| Major veins are usually attached to both the adaxial and abaxial hypdermis. | X | X | |||||||||||||||||||||||||
| Intermediate veins | |||||||||||||||||||||||||||
| Intermediate veins unattached | X | X | X | X | X | X | |||||||||||||||||||||
| Most intermediate veins attached adaxially by a fibrous sheath extension | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Intermediate veins attached to both surfaces by a fibrous sheath extension | X | ||||||||||||||||||||||||||
| Minor veins | |||||||||||||||||||||||||||
| Minor veins on both surfaces often nearly pairing up acoss from each other | X | X | X | X | S | ||||||||||||||||||||||
| Minor veins near the middle of the mesophyll or slightly below it (closer to the middle than to the abaxial) | X | X | X | S | S | X | X | X | X | X | |||||||||||||||||
| Many minor veins adjacent to the abaxial surface or in the lower third of the lamina (closer to the abaxial surface than to the middle) | X | X | X | X | X | X | X | X | X | X | X | S | X | X | X | X | X | X | |||||||||
| Veins with exaggerated fiberous sheath | |||||||||||||||||||||||||||
| Vein with large exaggerated fibrous sheath running along the margin | S | S | X | X | X | X | X | X | X | ||||||||||||||||||
| One minor vein with exaggerated fibrous sheath running along the margin | X | X | S | X | X | S | X | X | X | S | S | X | X | ||||||||||||||
| Two or more minor veins with exaggerated fibrous sheath running along the margin | X | X | X | S | X | X | X | X | |||||||||||||||||||
| Fiber bundles | |||||||||||||||||||||||||||
| Major fiber bundle running along or very near the leaflet margin | X | S | X | S | |||||||||||||||||||||||
| First or second fiber bundle on adaxial surface larger than rest | X | X | X | X | X | S | X | X | X | X | X | S | |||||||||||||||
| Most large adaxial fiber bundles reach ca. 1/3 to 1/2 across the mesophyll | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Most small adaxial fiber bundles reach 1/5 to 1/4 across the mesophyll | X | X | X | X | F | X | X | X | X | ||||||||||||||||||
| Adaxial fiber bundles mostly long and skinny | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||
| Adaxial fiber bundles mostly long and thick | X | X | X | X | X | X | X | X | X | ||||||||||||||||||
| Adaxial fiber bundles mostly short and thick | X | X | X | X | X | X | S | ||||||||||||||||||||
| Minor fiber bundles present among or between larger adaxial fiber bundles and veins | S | X | X | X | X | X | X | X | X | X | S | X | X | X | X | X | X | X | X | ||||||||
| Minor fibers or fiber bundles scattered in the mesophyll | X | X | X | ||||||||||||||||||||||||
| Minor fiber bundles along the abaxial surface absent | X | X | X | X | X | X | X | X | X | S | X | ||||||||||||||||
| Few minor fiber bundles along the abaxial surface | X | S | X | X | X | S | |||||||||||||||||||||
| Many minor fiber bundles adjacent to the abaxial surface | X | S | X | X | X | S | X | X | X | X | X | X | |||||||||||||||
| Abaxial minor fiber bundles occasionally alternating with abaxial minor veins | X | S | X | X | X | S | |||||||||||||||||||||
| Sometimes minor fiber bundle adjacent to the margin | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Thick-walled fiber-like hypodermal cells protecting the margin | X |
Both live material and preserved herbarium material (Table 3) were used in this project. The living material used in this study came from the collections at Montgomery Botanical Center (MBC, Miami, FL). The dried material was from collections made while doing fieldwork in Brazil, from the garden and herbarium at Jardim Instituto Plantarum (HPL, Novo Odessa, São Paulo, Brazil) and from dried collections at the Fairchild Tropical Botanic Garden herbarium (FTG, Miami, FL) and a few specimens from the following herbaria: G, IBGE, IPA, K, MO, NY and US.
Selected specimens examined.
| SYAGRUS Martius |
|---|
| Syagrus allagopteroides Noblick & Lorenzi, Lorenzi et al. 6792(HPL, FTG, NY, K, CEPEC, R, SP). |
| Syagrus angustifolia Noblick & Lorenzi, Lorenzi et al. 6636 (HPL, R, SP, BHCB, NY, K); Oliveira et al. 1082 (IBGE). |
| Syagrus caerulescens Noblick & Lorenzi, Lorenzi et al. 6649 (HPL, NY, K, R, SP); Tsuji & Franco 2622 (HPL). |
| Syagrus campylospatha (Barb. Rodr.) Becc., Hassler 1733 (G [holotype]), K, NY); Pedersen 14638 (G); Noblick et al. 5128 (FTG, PY, MBC96103). |
| Syagrus cearensis Noblick, Noblick et al. 4951 (EAC, FTG, RB, MBC94652); Noblick et al. 4953 (EAC, FTG, TEPB, MBC94654); Noblick et al. 5132 (IPA, MBC97262, MBC97263). |
| Syagrus cerqueirana Noblick & Lorenzi, Noblick et al. 5126(FTG, NY, PY, MBC96100); Schinini & Bordas 20288 (MO); Schinini & Bordas 20291 (CTES); Swallen 9520 (US). |
| Syagrus cocoides Mart., Fischer s.n. (MBC96363); Froes 11622 (GH, MO, NY); Henderson et al. 337 (NY); Henderson & Pardini 1503 (NY); Krukoff 1221 (F); Noblick 4954 (FTG, MBC94795); Plowman et al. 8267 (NY); Taylor et al.E1049 (NY). |
| Syagrus coronata (Mart.) Becc., Noblick & Soeiro 4694 (CEPEC, F, FTG, HRB, NY); Noblick & Soeiro 4704 (CEPEC, F, FTG, HRB); Noblick & Queiroz 4833 (FTG, HUEFS, MBC92146, MBC92196); Noblick et al. 4975 (FTG. IPA, MBC94473). |
| Syagrus duartei Glassman, Glassman & Gomes 8033 (F); Glassman 8035 (FTG); Hatschbach & Ferreira 35324 (MBM, F); Noblick 4854 (BHCB, FTG, MO, NY). |
| S. evansiana Noblick, Tsujiet et al. 2703 (HPL, R, BHCB, FTG, K, NY); Lorenzi 4269 (HPL); Lorenzi 4276 (FTG, HPL). |
| Syagrus flexuosa (Mart.) Becc., Noblick & Lima 4632 (BAH, CEPEC, CPATSA, F, FTG, NY, RB); Noblick & Lima 4633 (CEPEC, CPATSA, F, FTG); Noblick & Lima 4661 (CEPEC, CPATSA, F, FTG, NY); Noblick 4850 (BHCB, FTG, K); Noblick 4852 (BHCB, F, FTG, K, NY, US); Noblick & Ferreira 4869 (FTG, UFG); Noblick & Cropper 5108 (CEN, FTG, MBC96136); Noblick & Behr 5165 (IPA, MBC97800); Noblick & Behr 5166 (IPA, MBC97801); Noblick 5166 (IPA, MBC97801, MBC971463). |
| Syagrus glaucescens Glaziou ex Becc., Brown s.n. (MBC20030758); Glassman & Gomes 8112 (SP); Glassman 13002 (F, FTG); Noblick 4843 (BHCB, F, FTG, K, NY, US); Noblick 4845 (BHCB, FTG). |
| Syagrus glazioviana (Dammer) Becc., Noblick & Lobo 4527 (CEPEC, F, FTG, HRB, HUEFS, K, NY, RB, SP); 4617 (BAH, CEN, CEPEC, CPATSA, F, K, MICH, MO); Noblick & Lima 4643 (CEN, CEPEC, CPATSA, F, NY); Noblick & Lima 4659 (CPATSA, F, FTG), 4662 (CEPEC, CPATSA, F, FTG); Tsuji et al. 2681 (HPL) |
| Syagrus gouveiana Noblick & Lorenzi, Lorenzi 6537 (HPL, R, SP, BHCB, NY, K). |
| Syagrus graminifolia (Drude) Becc., Belem 2029 (UB); Burchell 5956 (K), holotype for Cocos graminifolia Drude; Davis & Shepherd 60024 (NY); Glassman 13093 (F); Noblick 5164 (FTG). |
| Syagrus graminifolia var. glazioviana (Dammer) Becc., Glaziou 22252 (G, K); Glaziou 22253 (G, K), isotype for Cocos graminifolia var. glazioviana Dammer; Lorenzi et al. 6791; Tsugi et al. 2682 (HPL). |
| Syagrus harleyi Glassman, Noblick 2867 (CEPEC, F, HUEFS, MO); Noblick & Lima 4380 (CEPEC, F, GH, HUEFS, MBM, SP); Noblick 4387 (BH, CEPEC, F, HUEFS, NY); Noblick 4389 (CEN, CEPEC, F, FTG, HRB, HUEFS, IPA, K, NY, RB, SP, US); Noblick & Lobo 4517 (AAU, ALCB, CEN, CEPEC, F, FTG, HRB, HUEFS, K, U). |
| Syagrus itacambirana Noblick & Lorenzi, Andrade-Lima 68-5425 (IPA); Tsuji et al. 2706 (HPL, R, SP, BHCB, NY, K). |
| Syagrus kellyana Noblick & Lorenzi, Noblick & Cline 5156(IPA, FTG, MBC97289, MBC97290). |
| Syagrus lilliputiana (Barb. Rodr.) Becc., Hassler 9519 (G); Lorenzi et al. 2805 (HPL). |
| Syagrus loefgrenii Glassman, Noblick & Lima 4634 (AAU, BAH, BH, CEPEC, CPATSA, F, FTG, K); Noblick & Lima 4660 (CPATSA, F, FTG); Noblick & Lima 4669 (ALCB, CEPEC, CPATSA, F, K, U); Lorenzi 6642 (HPL); Noblick & Buzeiro 4888 (BHCB, FTG, K, MO, US). |
| Syagrus longipedunculata Noblick & Lorenzi, Lorenzi et al. 6790 (HPL, R, SP, BHCB, NY, K); Oliveira et al. 588 (IBGE). |
| Syagrus macrocarpa Barb. Rodr., [No Collector] (MBC20080848, MBC20080849, MBC20080850); Noblick & Abrahao 4841 (BHCB, FTG, NY); Noblick & Abrahao 4842 (BHCB, FTG); Noblick 4857 (CESJ, F, FTG, IPA, NY, US). |
| Syagrus mendanhensis Glassman, Archer 4086 (BH [holotype], US); Glassman 13003 (FTG); Noblick 4844 (BHCB, F, FTG, MO, NY, K, US); Noblick 4846 (BHCB, FTG, NY, US); Noblick 4847 (BHCB, FTG). |
| Syagrus microphylla Burret, Glassman 13018031 (F, SP); Noblick & Clodoaldo 3508 (F, FTG, GH, HUEFS, MO, RB, SP); Noblick 4534 (ALCB, CEPEC, F, FTG, HUEFS, RB); Noblick & Lima 4612 (BAH, BH, CEPEC, CPATSA, F, FTG, K, NY, US); Noblick 4835 (FTG, MO). |
| Syagrus minor Noblick & Lorenzi, Lorenzi et al. 6639 (HPL, R, SP, BHCB, NY, K). |
| Syagrus orinocensis (Spruce) Burret, Balick et al. 1192 (NY); Betancur 1315 (NY); Bomm & Wentzel 6616 (NY); Davidse & Huber 15286 (BH); Mejia et al. 1258 (NY); Noblick et al. 4946 (FTG, MBC94586); Noblick et al. 4948 (FTG, PORT, MBC94588). |
| Syagrus petraea (Mart.) Becc., H. Lorenzi et al 6835 (HPL); Moreno 246 (JBSC); Saldias et al. 953 (NY). |
| Syagrus pleioclada Burret, Glassman & Gomes 8037042 (F, FTG [8037], SP [8041, 8042]); Hatschbach 35313 (F, MBM); Heringer & Castellanos SP80005 (SP); Martinelli & Smith 6333 (MO); Noblick 4853 (BHCB, FTG, MO, NY); Smith 6699 (US). |
| Syagrus pleiocladoides Noblick & Lorenzi, Lorenzi et. al. 6583 (HPL, R, SP, UB, UFMT, NY, FTG, K, AAU, CTES). |
| Syagrus procumbens Noblick & Lorenzi, Lorenzi et al. 6583 (HPL, R, SP, UB, UFMT, NY, FTG, K, AAU, CTES); Lorenzi 4752 (HPL); “emasensis” Noblick & Ferreira 4868 (FTG, UFG); Tsuji et al. 974 (HPL); Lorenzi et al. 6787 (HPL). |
| Syagrus rupicola Noblick & Lorenzi, Lorenzi et al. 6647 (HPL, R, SP, UB, NY, K). |
| Syagrus stenopetala Burret, Liesner & Gonzalez 11928 (NY); Noblick & Smith 4936 (FTG, PORTO, MBC94576); Noblick & Smith 4938 (MBC94577); Pittier 9154 (NY, US); Steyermark et al. 102432 (MO); Steyermark & Manara 110614 (BH). |
| Syagrus vagans (Bondar) A. Hawkes, Carvalho 2409 (CEPEC); Glassman & MedeirosCosta 8725726 (F); Lima & Noblick 140147 (CPATSA); Mori 10066 (CEPEC, NY); Noblick 3161 (HUEFS); Noblick et al. 3253 (HUEFS); Noblick & Clodoaldo 3537 (HUEFS); Noblick 3609 (HUEFS); Noblick 3846 (F, HUEFS). |
| Syagrus vermicularis Noblick, Fischer s.n. (MBC96364); Noblick & Feitosa 4971 (FTG, IPA); Noblick & Feitosa 4974 (FTG; MBC94690). |
| Syagrus werdermannii Burret, Carvalho 1790 (CEPEC, US); Glassman & MedeirosCosta 8728739 (F); Noblick & Clodoaldo 3769 (BH, F, HRB, HUEFS); Noblick & Lobo 4519 (BAH, CEN, CEPEC, F, FTG, HUEFS, K, MO, NY, RB). |
Two methods were employed for expedient identification. First, one side of the middle section of a middle leaflet was folded back and forth on itself in accordion fashion; the folded leaflet was then held down on a cutting board, while using a double-edged razor blade to cut thin cross-sections. The sections were rinsed into a watch glass with water and a thin brush was used to select the thinnest sections under a dissecting scope and then placed on a microscope slide in a droplet of 1:1 glycerin/water solution. A cover glass was placed over the specimen and the slide was placed under a compound light microscope and photographed under the 10× objective (100× magnification). Most of the sections were unstained, but in rare cases toluline blue (0.01%) was tested to see if it made it easier to view certain characters, which it did not (Fig. 3C, 6D).
In the second method, better suited when material is limited, a small square of carrot of the appropriate size is cut to fit in an inexpensive hand-held student microtome. I purchase my hometrainingtools hand-held microtome online. A vertical slit is cut in the carrot and a small piece of leaflet is inserted in the appropriate orientation. The carrot is clamped into the hand-held microtome. The microtome is screwed to the appropriate level and an ordinary folding straight edge razor, the kind used for shaving, is utilized to cut the cross-sections and honed occasionally to keep it sharp. Sections are handled the same way as above. Scale was later added using a stage micrometer. Dried material can also be sectioned and photographed after rehydrating in a 5% solution of Contrad 70® (Decon Labs, King of Prussia, Pennsylvania) for a period of 24 hours (
This paper is focused mainly on characters of the more easily sectioned marginal and laminal portions of the leaflet and not so much on the harder to section midrib. Trichomes, epidermis and dark staining bodies were also not looked at.
Characters examined during this study follow some of Glassman’s 4, 5 and 10 characters listed above and Tomlinson’s characters 4, 5, 6, and 7 listed above. Figure 2 will clarify much of the terminology and characters used in this paper. In each leaf cross-section the upper or superior side of the lamina is called the adaxial surface, meaning “towards the axis”, since this side of the leaf faces towards the axis or center of the plant as it grows out. The lower or inferior side is called the abaxial, meaning “away from the axis”, since this side faces away from the center of the plant (
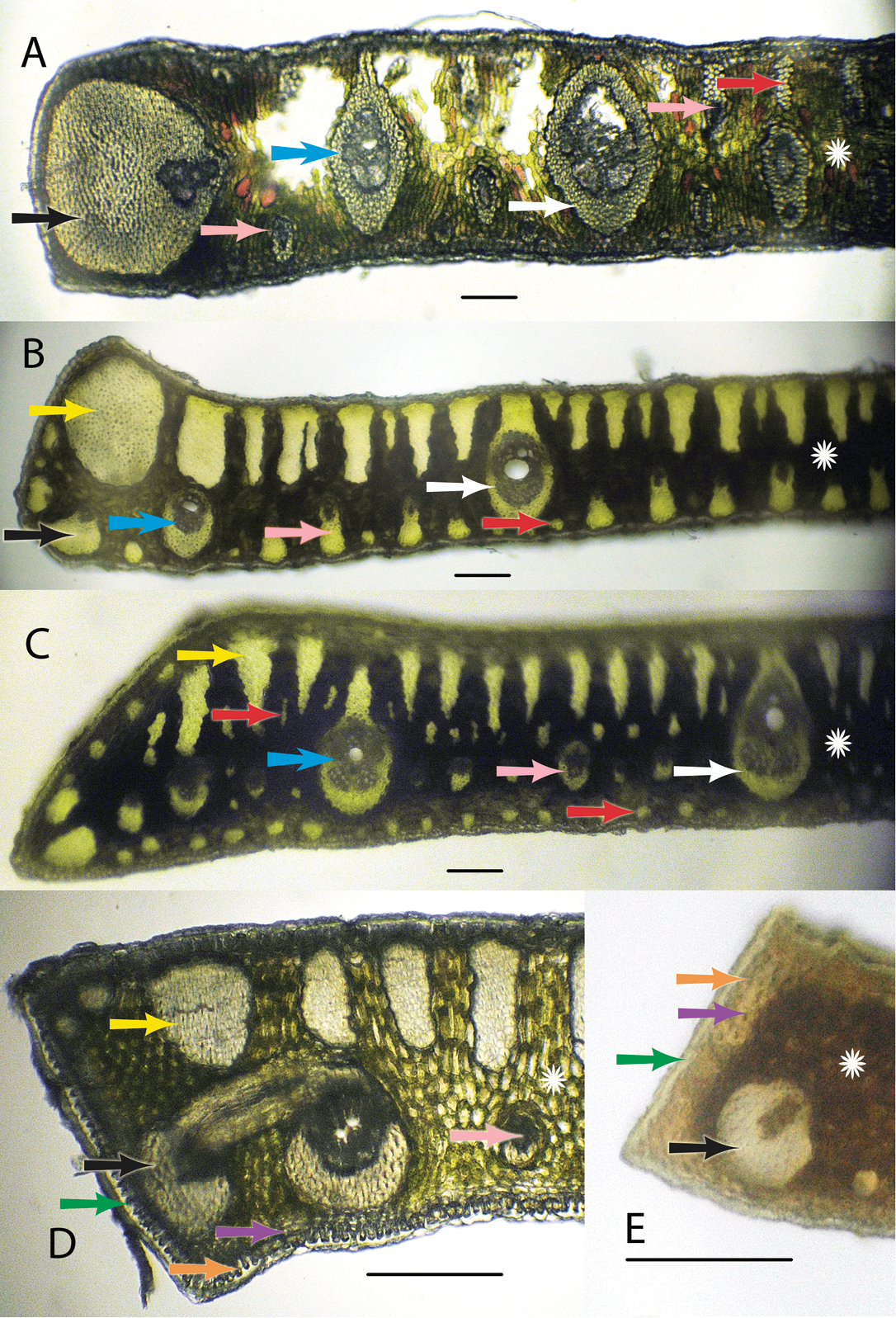
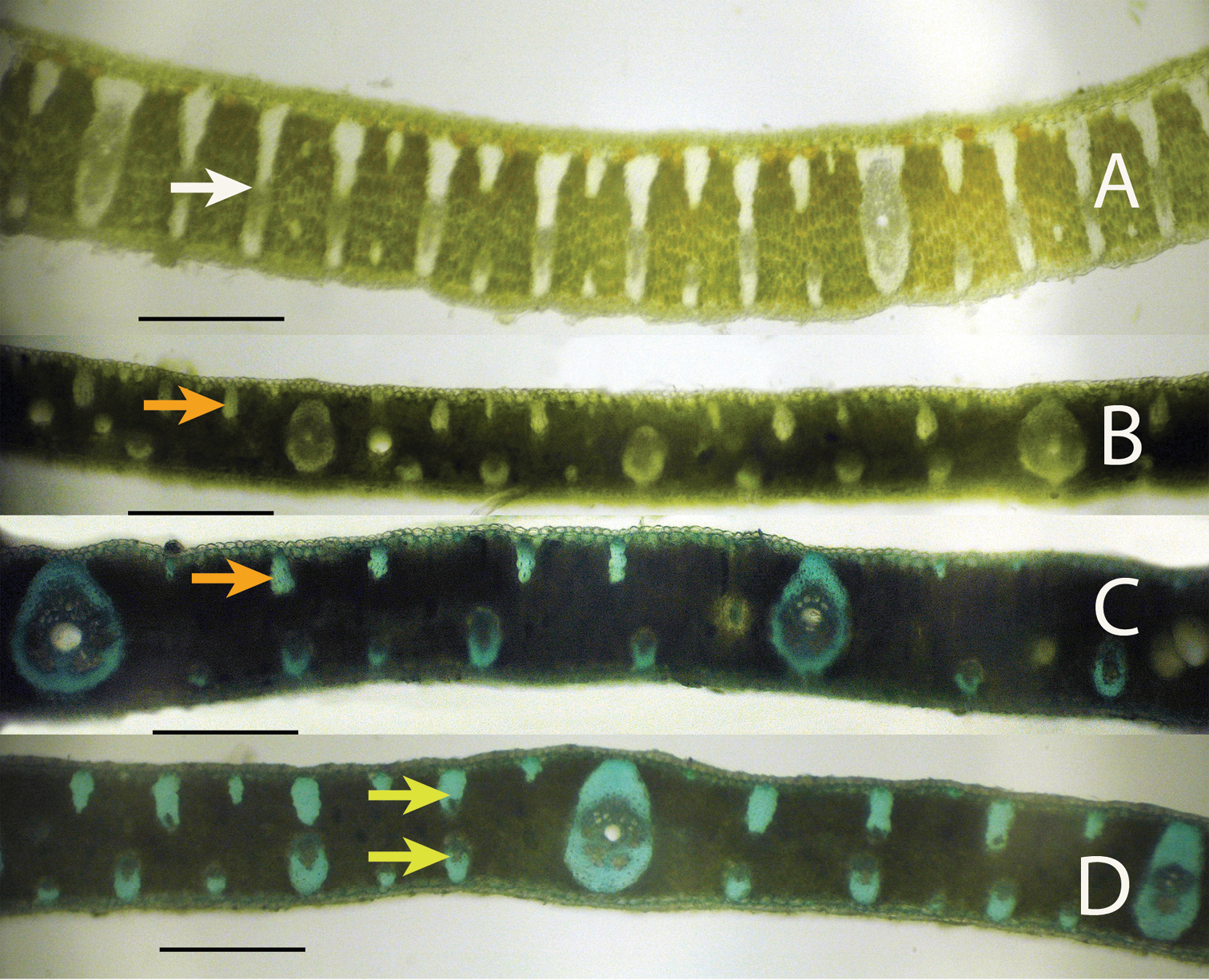
Anatomical characters. White arrows = major veins; Blue arrows = intermediate veins; Pink arrows = minor veins; Black arrows = vein with an exaggerated fibrous sheath; Yellow arrows = major fiber bundles; Red arrows = minor fiber bundles; Green arrows = Cuticle; Orange arrows = epidermis; Purple arrows = hypodermis; White star = mesophyll A Syagrus allagopteroides illustrates a large marginal vein with an exaggerated fibrous sheath (black), an unattached major vein (white), the presence of minor veins on both the adaxial and abaxial surfaces (pink) and an occasional minor adaxial fiber bundle (red) B Syagrus caerulescens illustrates a large marginal fiber bundle (yellow), a major vein attached to the adaxial surface by a fibrous extension (white), a small vein with an exaggerated fibrous sheath (black), minor veins (pink) sometimes alternating with minor fiber bundles (red) along the abaxial surface, and adaxial fiber bundles reaching nearly 1/2 the distance across the mesopyll (white star) C Syagrus vagans illustrates the first or second fiber bundle as being the largest along the adaxil surface (yellow) and minor fiber bundles (red) scattered throughout the mesophyll (white star), minor veins located near the middle or just slightly below D Syagrus gouveiana illustrates the cuticle (green), epidermis (orange), hypodermis (purple) E Syagrus harleyi illustrates a protective layer of thick-walled hypodermal cells (purple) on the margin, which is characteristic of this species. A, B, C Scale = 0.1 mm; D, E scale = 0.2 mm.
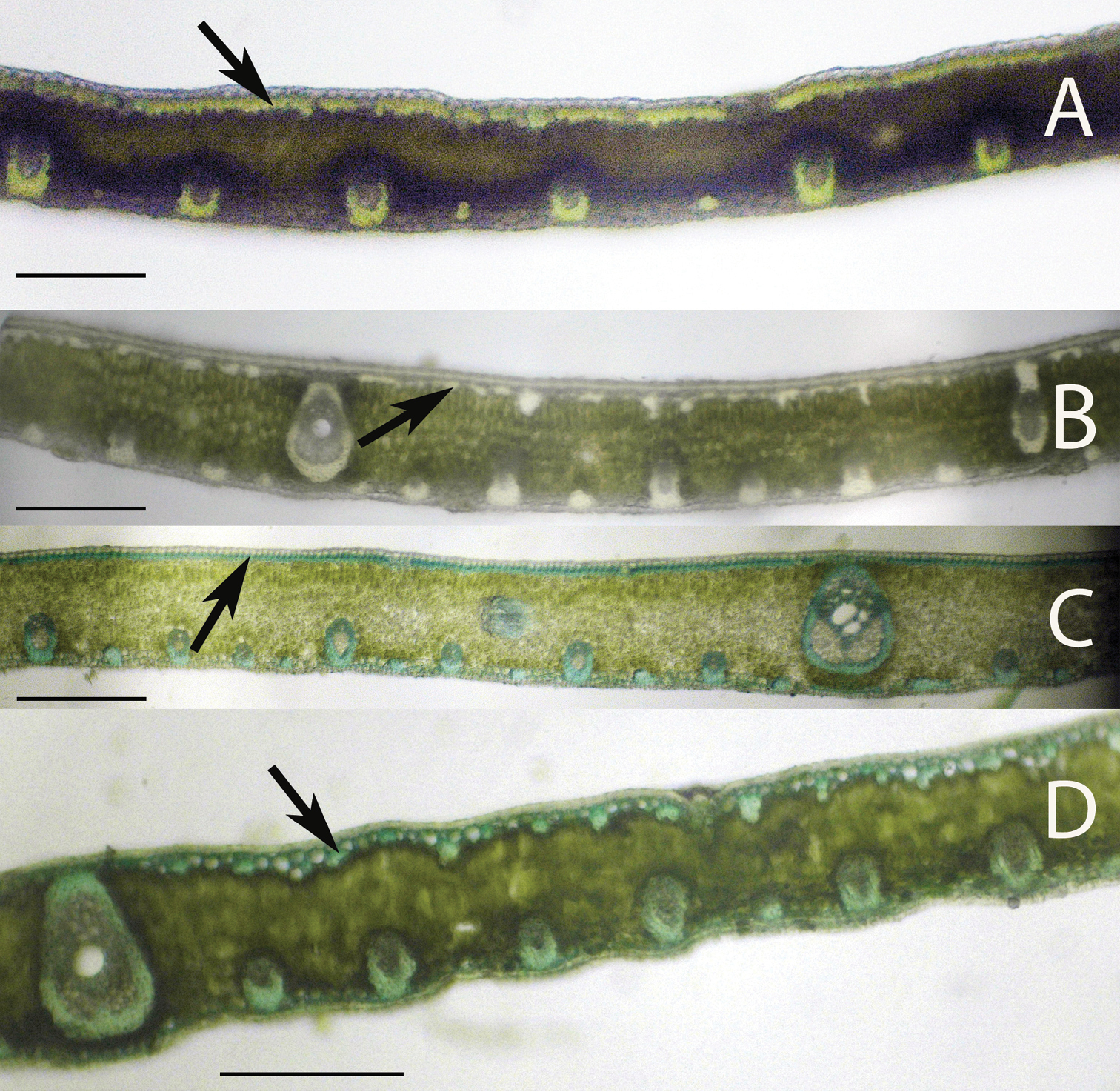
Leaflet cross-sections of the Rain Forest Clade of Syagrus species with arrows pointing out the 1–2 cell thick fibrous sheet just below the epidermis that is a defining character of species of this clade: A Syagrus vermicularis Noblick B Syagrus stenopetala Burret C Syagrus sancona (Mart.) Becc D Syagrus cocoides (Mart.) Mart. Scale = 0.2 mm.
To keep things simple for field examination, the following qualitative characters were examined: (1) location, attachment or lack of attachment of the major veins to one or both surfaces and method of attachment (fiber sheath extension or not); (2) location, attachment or unattachment of intermediate veins to one or both surfaces and method of attachment (e.g. sheath extensions, formation of girders); (3) location of the minor veins (e.g. adaxial, abaxial, abaxial and adaxial, middle, marginal); (4) presence, size and location of veins with an exaggerated fibrous sheath (large ones often located on the leaflet margin); (5) presence, location, size and sometimes cross-sectional shape of fiber bundles and the extent they reach across the mesophyll. These characters can also be further summarized as follows:
- Major vein location {adjacent to the margin; near the margin but not adjacent to it (this means that along a horizontal plane there is a maximum of one minor vein or one fiber bundle separating it from the actual margin); not adjacent to nor near the margin}
- Major vein attached where {unattached; attached to adaxial hypodermal surface only; attached to both adaxial and abaxial hypodermal surfaces}
- Major vein attachment how{attached by a short or long fibrous sheath extension; attachment not by a fibrous sheath extension}
- Intermediate veins attached {unattached; attached to adaxial surface only; attached to both surfaces}
- Intermediate vein attachment {to both surfaces by fibrous sheath extension (girders); attached to adaxial surface only by fibrous sheath extension; attached but without fibrous sheath extension}
- Minor vein location {adjacent to both the adaxial and abaxial surface; a few adjacent to the adaxial but most on the abaxial surface; near the middle of the mesophyll; adjacent to the abaxial surface or at least in the lower third of the mesophyll; only adjacent to the abaxial surface}
- Presence of major marginal vein with large exaggerated fibrous sheath {absent; present}
- Presence of minor marginal vein with exaggerated fibrous sheath {absent; one present; two or more present}
- One major rounded fiber bundle adjacent to the margin {absent; present}
- First fiber bundle on the adaxial surface the largest {absent; present}
- Adaxial fiber bundles size if present {reach 1/3 to 1/2 across the mesophyll; reach 1/5 to 1/4 across the mesophyll}
- Fiber bundles shape {mostly long and skinny: mostly long and thick: mostly short and thick}
- Fibers or minor fiber bundle locations {adaxial only; adaxial and abaxial only; adaxial, abaxial and scattered in the mesophyll}
- Minor fiber bundles adjacent to the margin {absent; present}.
- Minor fiber bundles abundance {none; few along the adaxial and abaxial surface; only a few along the abaxial surface alternating with the minor veins; many along the abaxial surface}
- Thick walled hypodermis protecting the margin {absent; present}
The key was designed for field use, which means minimal equipment, no staining, and low magnification and the use of simple characters. Refer to the characters in the methods for clarification of terminology. By using the methods listed above and following many of the simple techniques mentioned by
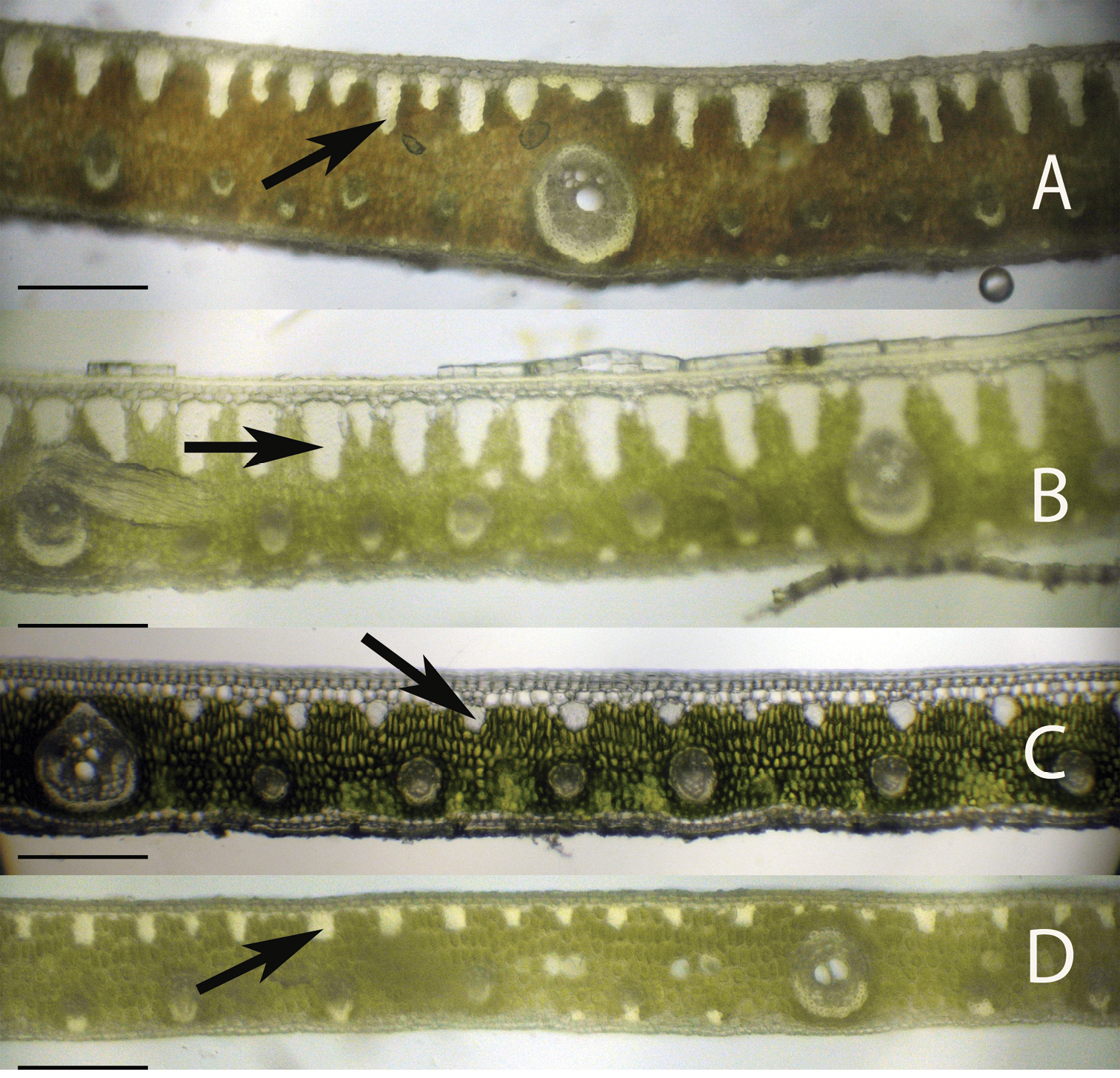
Anatomical characters observed on marginal palm leaflet cross-sections have been found to verify the Rain Forest and Eastern Brazilian clades and to some extent the Cluster-stem clade found within Syagrus (Fig. 1). In the Rain Forest clade, there is a continuous hypodermal layer of fibrous to thick-walled cells, one to two layers thick just below the adaxial epidermis (Fig. 3A, 3B, 3C, 3D). In the Eastern Brazilian clade, there are many thick, closely-spaced, multicellular fiber bundles running along the adaxial surface of the leaflet (Fig. 4A, 4B, 4C, 4D). Finally the Cluster-stem clade is usually characterized by minor sparsely spaced fiber bundles on the adaxial side and minor veins adjacent to the abaxial surface (Fig. 5B, 5C) or with minor veins on both surfaces (Fig. 5D) that make the anatomy of Syagrus macrocarpa Barb. Rodr. Syagrus flexuosa (Mart.) Becc. and Syagrus cerqueirana look interestingly similar to one another.
Leaflet cross-sections of the Eastern Brazilian Clade of Syagrus species with arrows showing the multicellular fiber bundles that are a defining character of species of this clade: A Syagrus coronata (Mart.) Becc. B Syagrus glaucescens Glaziou & Becc. C Syagrus kellyana Noblick & Lorenzi D Syagrus cearensis Noblick. Scale = 0.2 mm.
Leaflet cross-sections of the Cluster-stem Clade of Syagrus species. (A) Syagrus campylospatha, white arrow pointing at an intermediate vein with fibrous sheath extensions to both surfaces forming a girder type vein B Syagrus macrocarpa Barb. Rodr., orange arrow indicating a minor fiber bundle C Syagrus flexuosa (Mart.) Becc. orange arrow indicating a minor fiber bundle D Syagrus cerqueirana, yellow arrows indicating minor veins on both surfaces of the leaflet. Scale = 0.2 mm.
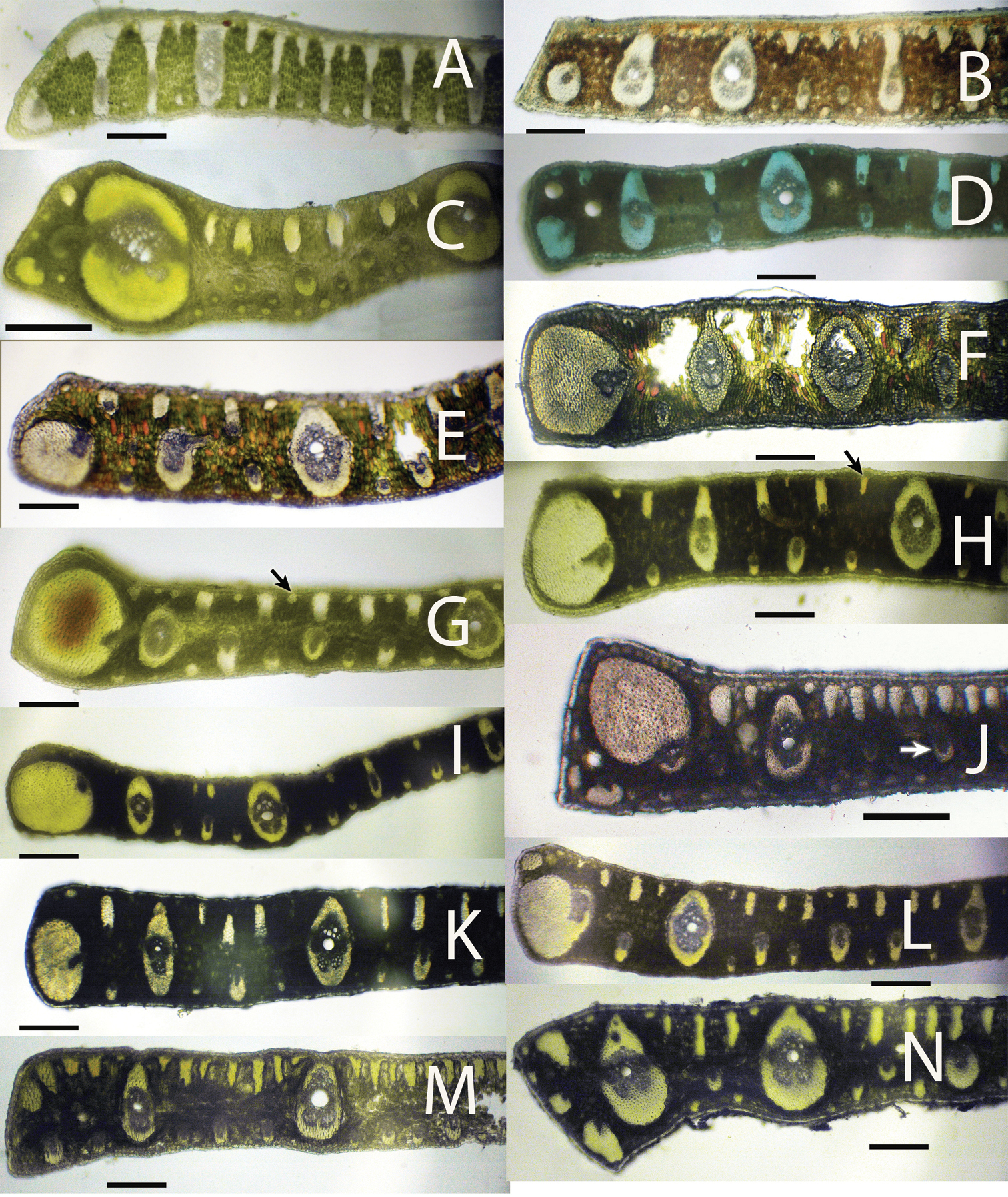
Leaflet cross-sections of acaulescent Syagrus species found in the key: A Syagrus campylospatha B Syagrus harleyi C Syagrus procumbens “emasensis” D Syagrus cerqueirana E Syagrus minor F Syagrus allagopteroides G Syagrus lilliputiana, arrow indicates a rounded minor fiber bundle H Syagrus cerqueirana, arrow indicate an elongated, longer than wide minor fiber bundle I Syagrus loefgrenii J Syagrus longipedunculata, arrow indicates a minor vein located in the middle of the mesophyll K Syagrus angustifolia L Syagrus itacambirana M Syagrus petraea N Syagrus procumbens, note major vein near but not adjacent to the margin. Scale = 0.2 mm.
After examining many leaflet hand sections of various acaulescent palm specimens, it was discovered that many had very different leaflet anatomy. The presence and absence of the anatomical characters in all 25 species is recorded in Table 2. Useful anatomical characters were found to separate the 25 known species of acaulescent and short-stemmed Syagrus and an identification key was developed. Several acaulescent Syagrus specimens frequently identified as Syagrus petraea were found to have distinctive field habits and leaflet anatomies (Table 1 and 2).
Species of the Rain Forest clade (Fig. 1), which includes many Amazonian species, are distinguished anatomically by an almost continuous adaxial fibrous layer, one or a few cells thick just under the epidermis (the hypodermal layer) (Fig. 3A, 3B, 3C, 3D). I speculate that perhaps this nearly continuous fibrous layer strengthens the lamina while maintaining its flexibility (
Most acaulescent Syagrus exhibit the Eastern Brazilian pattern (e.g. Syagrus gouveiana; Fig. 7A) with the large, multicellular fiber bundles running along the adaxial side of the leaflet and the Cluster-stem pattern, similar to that of Syagrus cerqueirana (Fig. 5D, 6D, 6H), with minor veins on both surfaces (e.g. Syagrus lilliputiana, Fig. 6G), each attached to either the adaxial or abaxial surface by short, fibrous extensions. Since most acaulescent palms grow in seasonally dry areas (cerrados) that require stiffer leaflets, it is perhaps understandable why the Rain Forest pattern is not seen among them.
Some of the problems of identifying acaulescent Syagrus species were covered previously in the introduction concerning the lack of good label information in relation to how leaves and leaflets are displayed or arranged on the plant before pressing and drying. Having observed most of these variations personally in the field has led me to the challenging process of trying to straighten out this much neglected complex of species. For me, it started in Bahia, Brazil with the misidentification of the acaulescent cerrado palm, Syagrus glazioviana. Many palm taxonomists, including Glassman and myself (
Many of the Syagrus petraea-types have very different leaflet anatomies. Their visible field characters (Table 1) and their distinctive anatomy has justified splitting up the complex (
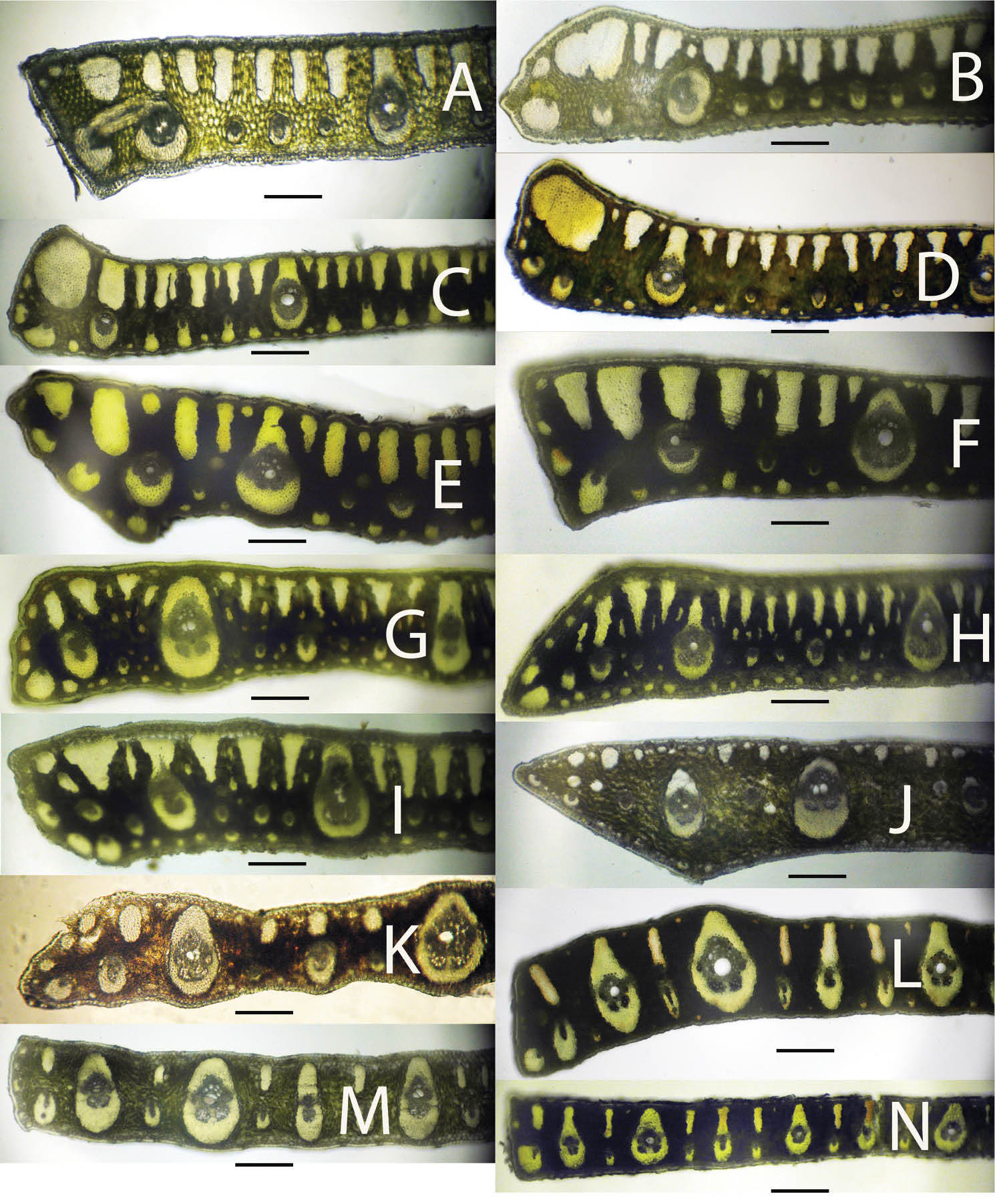
Leaflet cross-sections of acaulescent Syagrus species found in the key: A Syagrus gouveiana B Syagrus drudei C Syagrus caerulescens D Syagrus rupicola E Syagrus glazioviana F Syagrus evansiana G Syagrus werdermanii H Syagrus vagans I Syagrus microphylla J Syagrus pleiocladoides K Syagrus pleioclada L Syagrus graminifolia M Syagrus mendanhensis N Syagrus graminifolia var. glazioviana. Scale = 0.2 mm.
In conclusion, leaflet anatomy has been found to be useful in helping to confirm or verify relationships discovered through the molecular analysis and in identifying some of the difficult acaulescent Syagrus species.
| 1 | Many large intermediate veins with fibrous extensions to both adaxial (upper) and abaxial (lower) surfaces forming girders across the leaflet (Fig. 5A, 6A) | Syagrus campylospatha |
| – | No such girders formed | 2 |
| 2 | Margin of leaflet protected by a layer of thick-walled cells (Fig. 2E, 6B) | Syagrus harleyi |
| – | Margin of leaf lacking protective layer with few fibers, veins with exaggerated fibrous sheaths, large fibrous bundles | 3 |
| 3 | Margin with a huge fully functional major vein with a somewhat exaggerated fibrous sheath at or near the margin (Fig. 6C) | Syagrus procumbens“emasensis” |
| – | Margin with a vein with an exaggerated fibrous sheath, fiber bundles or anything other than a major vein | 4 |
| 4 | Minor veins adjacent to both the adaxial and abaxial surface (Fig. 6D-H) | 5 |
| – | Minor veins mostly present adjacent to the abaxial surface and few if any on the adaxial surface | 9 |
| 5 | A minor to intermediate vein with an exaggerated fibrous sheath adjacent to the margin and occupying less than half of the margin (Fig. 6D) | Syagrus cerqueirana |
| – | A major vein with an exaggerated fibrous sheath adjacent to the margin and occupying more than half to nearly the entire margin (Fig. 2A) | 6 |
| 6 | Marginal vein with exaggerated fibrous sheath occupies over half of the margin but not the entire margin (Fig. 6E) | Syagrus minor |
| – | Marginal vein with exaggerated fibrous sheath occupies the entire margin | 7 |
| 7 | Major vein usually unattached separated from the hypodermis by another cell layer or two (Fig. 2A, 6F) | Syagrus allagopteroides |
| – | Major vein usually attached to the adaxial hypodermis but separated from the abaxial by an additional cell layer or two | 8 |
| 8 | Minor fiber bundles along the adaxial nearly round in shape (Fig. 6G) | Syagrus lilliputiana |
| – | Minor fiber bundles along the adaxial elongated, longer than wide (Fig. 6H) | Syagrus cerqueirana |
| 9 | Vein with a very large exaggerated fibrous sheath adjacent to the margin | 10 |
| – | Margin without such a vein but with or without minor veins, and/or minor or major large fiber bundles | 13 |
| 10 | Minor veins near the middle of the mesophyll (Fig. 6J) | Syagrus longipedunculata |
| – | Minor veins adjacent to the lower abaxial surface | 11 |
| 11 | Most large adaxial fiber bundles reaching less than 1/4 to 1/5 across the mesophyll (Fig. 6I) | Syagrus loefgrenii |
| – | Most large adaxial fiber bundles reach 1/3 to 1/2 across the mesophyll | 12 |
| 12 | A few minor veins near or attached to the adaxial surface and veins often alternating with the minor fiber bundles adjacent to the abaxial surface (Fig. 6K) | Syagrus angustifolia |
| – | No minor veins near or attached to the adaxial surface and minor veins but no fiber bundles present on the abaxial surface (Fig. 6L) | Syagrus itacambirana |
| 13 | Large major vein near the margin but not adjacent to it (Fig. 6N) | Syagrus procumbens |
| – | Major veins neither near the margin nor adjacent to it | 14 |
| 14 | Margin with one very large fiber bundle or the first or second adaxial fiber bundles are larger than the rest | 15 |
| – | Margin with no significantly large fiber bundles | 24 |
| 15 | Adaxial fiber bundles long and skinny and reaching less than 1/5 to ¼ across the mesophyll (Fig. 6M) | Syagrus petraea |
| – | Adaxial fiber bundles long and usually fat and reaching 1/3 to 1/2 across the mesophyll | 16 |
| 16 | No minor fiber bundles scattered throughout the mesophyll | 17 |
| – | Minor fiber bundles scattered throughout the mesophyll | 22 |
| 17 | Minor fiber bundles usually absent from the abaxial surface | 18 |
| – | Minor fiber bundles usually present either along the abaxial surface and/or margin | 19 |
| 18 | Major veins are usually attached adaxially (Fig. 2D, 7A) | Syagrus gouveiana |
| – | Major veins are usually unattached (Fig. 7B) | Syagrus duartei |
| 19 | One major fiber bundle adjacent to the margin | 20 |
| – | No major fiber bundle adjacent to the margin | 21 |
| 20 | Minor veins all attached to the adaxial hypodermis (Fig. 2B, 7C) | Syagrus caerulescens |
| – | Some adaxial minor veins attached but many unattached (Fig. 7D) | Syagrus rupicola |
| 21 | Major vein attached to adaxial surface by a fibrous sheath extension (Fig. 7E) | Syagrus glazioviana |
| – | Major vein usually unattached (Fig. 7F) | Syagrus evansiana |
| 22 | Major veins surrounded by a very thick fibrous sheath (Fig. 7G) | Syagrus werdermanii |
| – | Major veins surrounded by a thin to medium fibrous sheath | 22 |
| 23 | Adaxial fiber bundles mostly long and skinny in cross-section (Fig. 2C, 7H) | Syagrus vagans |
| – | Adaxial fiber bundles mostly long and thicker (Fig. 7I) | Syagrus microphylla |
| 24 | Most adaxial fiber bundles reach less than 1/5 across the mesophyll (Fig. 7J) | Syagrus pleiocladoides |
| – | Most adaxial fiber bundles reach 1/3 to 1/2 across the mesophyll | 25 |
| 25 | Leaflets deflexed, adaxial fiber bundles more rounded (Fig. 7L) | Syagrus pleioclada |
| – | Leaflets straight or erect, adaxial fiber bundles long and skinny | 26 |
| 26 | Leaflets silvery blue color, very small minor fiber bundles between the veins and intermediate fiber bundles adaxially, and a few minor fibers abaxially (Fig. 7L) | Syagrus graminifolia |
| – | Leaflets green in color, no or few small minor fiber bundles between the veins and intermediate fiber bundles adaxially and none abaxially | 27 |
| 27 | Leaflets with many minor fibers adjacent to the margin (Fig. 7M) | Syagrus mendanhensis |
| – | Leaflets with one to no minor fiber bundles adjacent to the margin (Fig. 7N) | Syagrus graminifolia var. glazioviana |
I would like to acknowledge Dr. Patrick Griffith in helping to prepare Figure 1 and for his other valuable motivational suggestions and special thanks to Dr. Chad Husby and Tracy Magellan for their comments. Thanks also to Dr. Barry Tomlinson for our valuable discussions concerning appropriate palm leaflet anatomical terminology. Thanks to Montgomery Botanical Center (MBC) for their support and use of their living collections. Sincere thanks to Fairchild Tropical Botanic Garden (FTG) and Jardim Instituto Plantarum (HPL) for allowing me to sample small portions of their herbarium material for anatomical study. Thanks to the following herbaria for sending our small loans to answer my questions (G, K, MO, NY, US). Also, thanks to the National Science Foundation since parts of this investigation was done under NSF grant #0212779 in preparing a phylogentic analysis of Syagrus.
Numerical list to Syagrus taxa and numbered collections. (doi: 10.3897/phytokeys.26.5436.app) File format: Microsoft Word file (doc).