(C) 2013 Pedro Lage Viana. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of Axonopus ser. Suffulti from Minas Gerais, Brazil, is described and illustrated. Comparison with morphologically related species, as well as comments on the ecology and the conservation status are provided.
ResumoUma nova espécie de Axonopus ser. Suffulti é descrita e ilustrada para o estado de Minas Gerais, Brasil. São fornecidas comparações com espécies morfologicamente relacionadas, assim como comentários sobre ecologia e estado de conservação.
Atlantic Forest, inselbergs, Grasses, new taxon, Gramineae
Axonopus P.Beauv. is an American genus of Poaceae comprising approximately 110 species (
Species of the Axonopus ser. Suffulti are perennial plants, with the upper glume and lower lemma lacking a central nerve, and fertile florets characteristically shiny brown to dark brown (
A floristic survey in an overlooked granitic outcrop, or inselberg, in northeastern Minas Gerais, Brazil (de Paula et al. in prep.), revealed at least five new species of flowering plants. One of those, belonging to the Axonopus ser. Suffulti, is herein presented, illustrated and compared with putatively related species. SEM images of the fertile floret, as well as comments on its ecology and the conservation status are provided. For SEM images, samples were mounted on stubs, coated with gold palladium in a Hummer 6.2 (Anatech, Union City, CA, USA) sputtering system and viewed with a JSM-541OLV (JEOL, Tokyo, Japan) at 10kV.
Axonopus graniticola is distinguished from all other species of the Axonopus ser. Suffulti by its mostly caulinar leaves, distichous laterally compressed leaf sheaths, 1.5–2.5 cm wide leaf blades, deciduous with a subcordate base, and multi-racemose inflorescences of 26–75 racemes, with the basal ones re-branched.
BRAZIL: Minas Gerais: Município Teófilo Otoni, Afloramento rochoso ao lado esquerdo da MG 418, cerca de 30 km norte de Teófilo Otoni, 17°51'21.5"S, 41°15'39.4"W, 560 m alt., 8 Jan 2011, L.F.A. de Paula, N.F.O. Mota, P.L. Viana, T.B. Jorge, P.M. Burkowski 145 (Holotype: BHCB! Isotypes: RB! NY!) (Figures 1–3).
Plants perennial, densely caespitose, with very short falciform rhizomes. Culms 95–125 cm long, erect to decumbent, slightly curved at the base, not geniculate, unbranched; nodes various, hidden by leaf sheaths, glabrous; internodes 5–8.5 mm wide, cylindrical to slightly flattened, glabrous, stramineous. Leaves distichous, mostly caulinar; leaf sheaths 5.5–32 cm long, larger than the internodes, conduplicate, strongly keeled, striate, scabrous, glabrescent, persistent; ligule 0.15–0, 20 mm long or absent, ciliate, apparently deciduous, because it is usually absent in older leaves; collar prominent, glabrous; leaf blades (4.5)12–32 × 1.5–2.5 cm, oblong to linear, lanceolate, flat, retrosely scabrous abaxially, antrorselly scabrous adaxially, eventually with sparse hairs on abaxial or adaxial surfaces, deciduous, nerves prominent, margins scabrous, base rounded, subcordate, arising from a constriction of 1–2 mm long in each margin of the ligular region, apex obtuse, asymmetrical, emarginate, slightly folding, reflexed, scabrous. Inflorescences 2 per flowering culm, terminal and axillary; peduncle up to 55 cm long, partially included in the leaf sheaths, cylindrical to angulose, striate, scabrous; pulvinulus pubescent; main axis 8–16.5 × 0.05–0.14 cm, angulose, striate, scabrous; panicles 12–26 cm long, in dense clusters of alternate to verticillate racemes, the lower branches re-branching in 5–18 racemes; racemes (4–)9–16.5 cm long, the apical ones slightly shorter than the basal, 26–75 per panicle; rachis of racemes triquetous, fertile all along, except for the 1–4.5 mm basal portion length, ending in a fertile spikelet, (5)10–15 spikelets per portion of 25 mm long, pubescent, scabrous in the angles; pedicels 0.25–0.5 mm long, scaberulous, sometimes with a few hyaline tuberculate trichomes to 0.8–1.5 mm long. Spikelets 1.8–2.0 × 0.6–0.8 mm, oblong-ellipsoid, dorsiventrally compressed, apex acute; upper glume as long as the spikelet, elliptical, membranous, glabrous or with sparse trichomes, hyaline to stramineous, 2–4(–5)-nerved, nerves prominent, scaberulous in the apex, mid-nerve occasionally present; lower lemma glumiform, 2(–3)-nerved, nerves glabrous; upper lemma 1.8–2.0 × 0.8–0.9 mm, elliptical, stiff, glabrous, except for a discrete tuft of short white hairs at the apex, densely ornamented by diminute papillae, fading in density toward the margins, shiny brown to dark brown, apex acute, brown to pale, base brown to pale; upper palea similar to the upper lemma but slightly shorter, 1.7–1.9 × 0.6–0.8 mm, glabrous. Lodicules 0.2–0.3 mm long, 2, oblong, erose; stamens 3, anthers ca. 0.8 mm, dorsifixed, purplish; stigmas plumose, whitish. Caryopsis not seen.
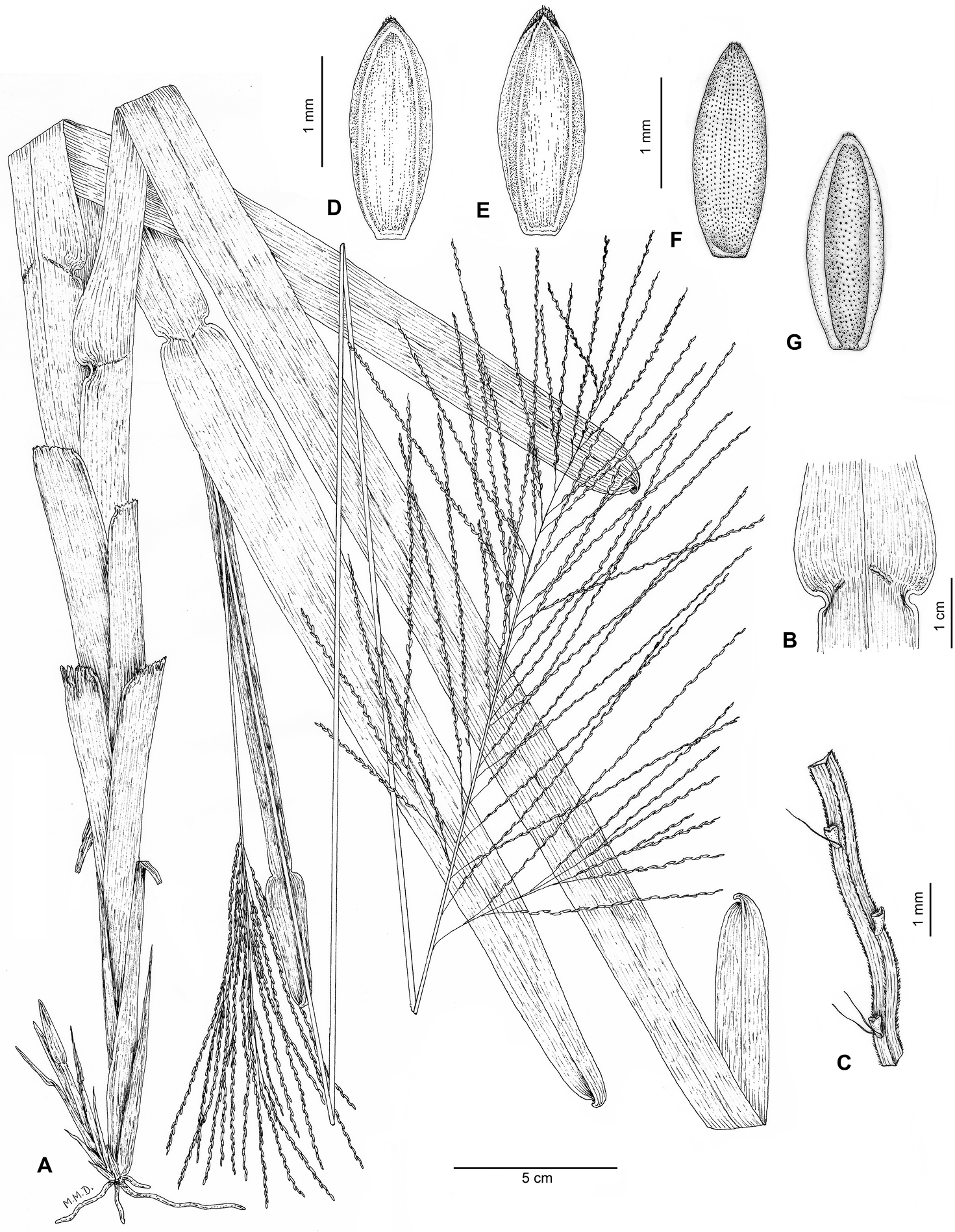
Axonopus graniticola P.L. Viana A Habit B Ligular region, adaxial side C Rachis of receme D Spikelet, upper glume view E Spikelet, lower lemma view F Upper floret, lemma view G Upper floret, palea view. Drawn from the holotype (de Paula et al. 145).
The epithet refers to the occurrence of the plants of this species on exposed granite rock outcrops.
The new species has perennial habit, scabrous pubescent rachis, with few scattered trichomes 0.8–1.5 mm long associated with the region of the pedicel insertion, the 2–4(–5)-nerved upper glume and 2(–3)-nerved lower lemma, and typically shiny brown and stiff fertile florets. In accordance with
A mid-nerve in the upper glume and lower lemma is found in some spikelets of the specimen de Paula 237. Although this feature is uncommon in Axonopus ser. Suffulti (
Axonopus flabeliformis shares with Axonopus graniticola the characteristically equitant base, with distichous, laterally compressed and persistent leaf sheaths, disposed along the culm. The compound panicle, occasionally occurring in Axonopus flabeliformis, and spikelets 1.6–2.2 mm long, also suggest affinity, even though superficial, to the new species. Axonopus graniticola can be easily distinguished from Axonopus flabeliformis by its wider leaf blades (1.5–2.5 cm vs. 0.5–0.9 cm in Axonopus flabeliformis), the rounded and subcordate base of the blade arising from a constriction in the ligular region (against blade bases straight and following the sheath apex width in Axonopus flabeliformis), and its multi-flowered panicles (26–75 racemes vs. 6–20(–30) racemes in Axonopus flabeliformis).
The new species also bears slight resemblance to Axonopus pressus (Nees ex Steud.) Parodi, from the Brazilian cerrado, Bolivia and Paraguay, by its strongly conduplicate and keeled leaf sheaths, giving the typical laterally compressed aspect to the plant. However, the leaves of Axonopus pressus are predominantly basal, contrasting with mostly caulinar leaves of Axonopus graniticola, with shorter spikelets (1.8–2 mm long, vs. 2.2–3 mm in Axonopus pressus), wider leaf blades (1.5–2.5 cm, vs. 0.8–1.2 cm in Axonopus pressus) and inflorescences with 26–75 racemes (against less than 35 racemes in Axonopus pressus). Moreover, the panicles of the new species are compound, with the lower branches re-branching in 5–18 racemes, a feature absent in Axonopus pressus, with its panicles with unbranched racemes.
The flat and characteristically wide leaf blades and the compound panicles of the new species bear a slight resemblance to the widely distributed Axonopus scoparius (Flüggé) Kuhlm. However, the latter species is placed in the Axonopus sect. Axonopus ser. Barbigeri Black (
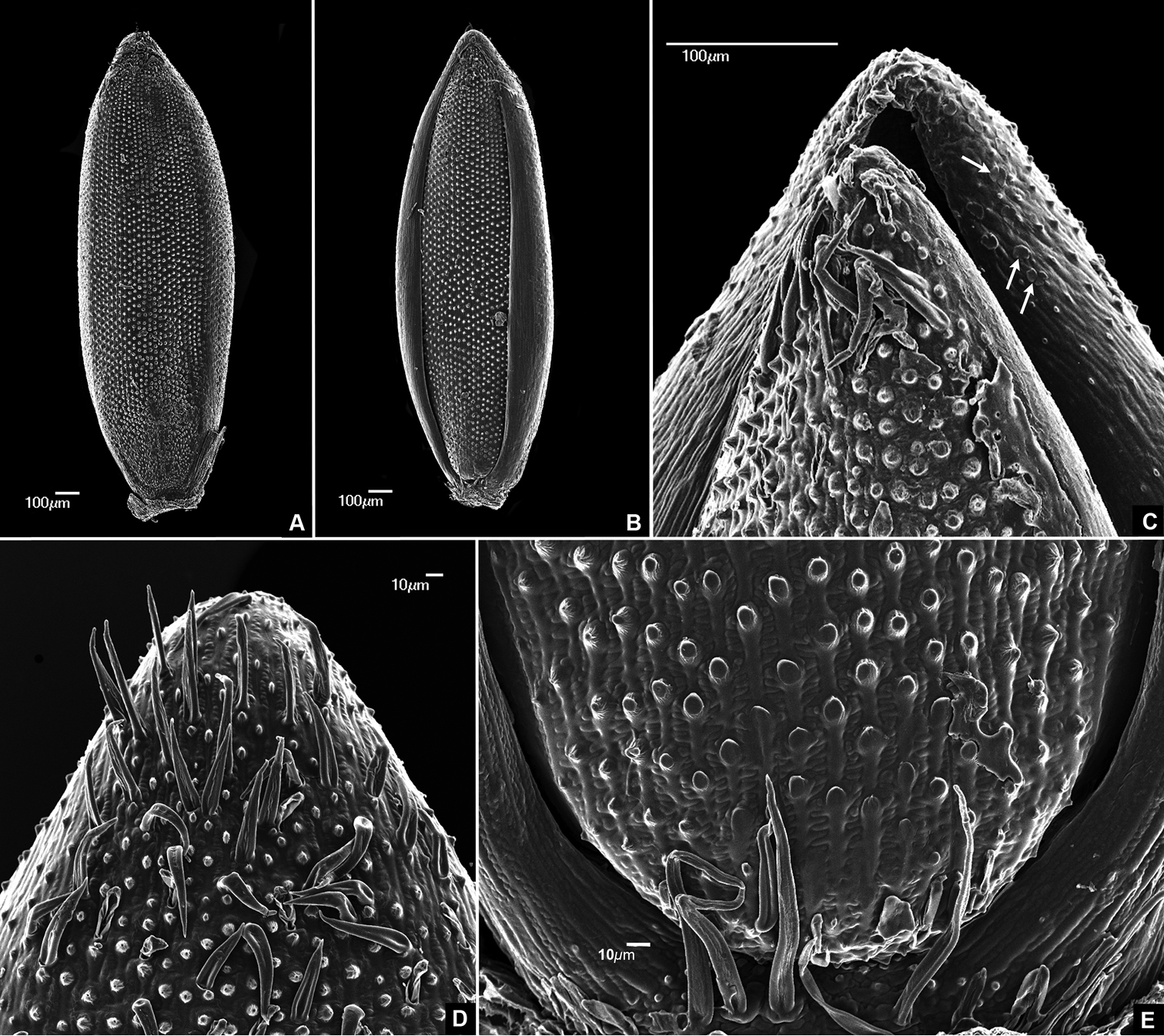
(Figure 2). Abaxial surface of palea and lemma ornamented with papillae, silica bodies, macro-hairs and micro-hairs. Papillae simple, conical, apex acute, one per cell, evenly distributed in longitudinal rows on the floret surface, except in the margins of the lemma, which lack papillae. Silica bodies equidimensional, dumbbell shaped, visible on the apical portion of lemma margins. Macro-hairs unicellular, simple, located in the apex of lemma and palea and in the basal portion of the lemma (Figure 2E). Micro-hairs collapsed in the studied material, probably due to samples preparation process, distributed in the apex of lemma and palea and in the basal portion of the lemma.
The presence of numerous papillae, dumbbell shaped equidimensional silica bodies on the apex of fertile floret and macro- and micro-hairs on the base and apex of floret are typical features of species included in Axonopus ser. Suffulti (
SEM micrographs of upper floret A Lemma view B Palea view C Apical portion of floret showing silica bodies (arrows) D Upper lemma apex, showing conspicuous macrohairs and pappilae E Basal portion of floret. Images taken from the holotype (de Paula et al. 145).
BRAZIL: Minas Gerais: Município Teófilo Otoni, Afloramento rochoso ao lado esquerdo da MG 418, cerca de 30 km norte de Teófilo Otoni, em inselbergue, 17°51'11"S, 41°15'44"W, 650 m alt., 16 Apr 2011, L.F.A. de Paula, M. Augsten 237 (BHCB, RB).
The new species is known only from its type locality, an inselberg in the municipality of Teófilo Otoni, eastern Minas Gerais, Brazil. It occurs on granitic and gneissic rock outcrops, surrounded by the Atlantic Forest matrix (
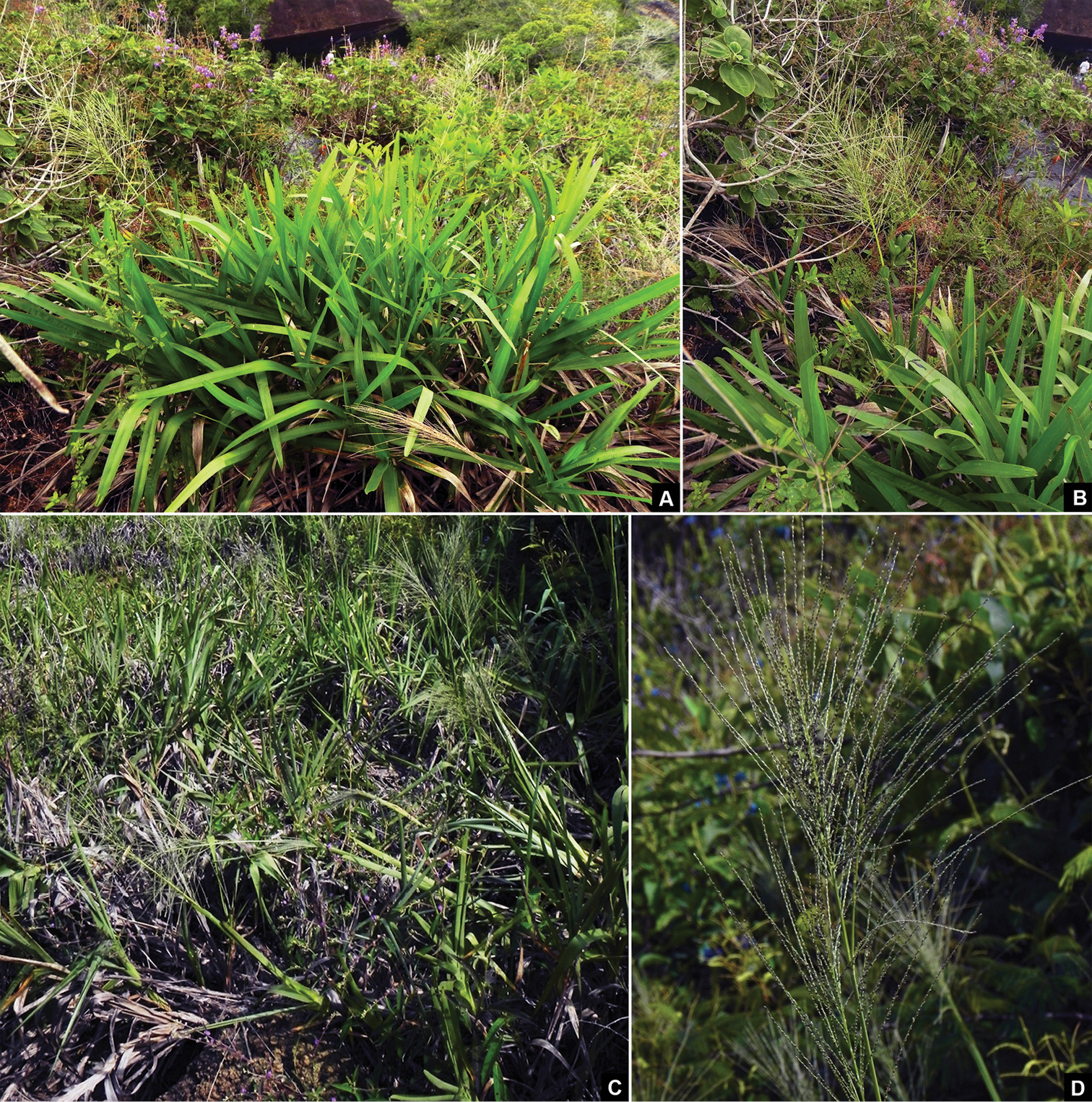
The species is found in depressions filled with thin soil, forming dense clumps, surrounded by rocky surface. During the rainy season, the profuse growth of new leaves with conspicuous flat and wide blades gives a vivid green color to the clumps (Figure 3A), in contrast with the pale brown, almost bladeless, clumps observed during the dry season (Figure 3C). The persistent leaf sheaths covering the culms and the readily deciduous leaf blades may be adaptations to avoid desiccation during the dry season and serve as protection against high temperatures of this extremely seasonal environment. These features are described for other species among monocots families, like Velloziaceae and Cyperaceae, which are usually known as desiccation-tolerant plants (
The vegetation of the inselberg is influenced by the soil (
In situ photographs of Axonopus graniticola P.L. Viana A Dense clump of A. graniticola during the rainy season B Flowering culm C Clumps, in the beginning of the dry season D Detail of panicle. Photographs by L.F.A. de Paula.
The species is known so far from a single granite-gneiss outcrop in the Teófilo Otoni region, Minas Gerais, Brazil. Due to the poor state of knowledge of the flora from that region (
Although it was not possible to assess the precise conservation status of the species, it is important to note that the vegetation of the inselbergs are under threat due to the ever increasing granite and gem exploration, road-building, grazing and illegal plant collection in southeastern Brazil’s inselbergs (
We thank Marcelo de Paula, Ana Lúcia de Paula, Nara F.O. Mota, Felipe Leite, Pablo Burkowski, Mariana Augsten, Tulio Jorge, Creuzo and Cerva Jonka for help in building our foothold lodging on the inselberg. We also want to express our gratitude to Cássio Addiny for kindly providing permission to collect botanical samples on his property. Special thanks go to Nara F.O. Mota and Poliana Cardoso, for providing the SEM images of fertile florets, to Myrian M. Duarte for line drawings, and to Alex Popovkin who improved the English of this article. We are also thankful for Tarciso S. Filgueiras for encouraging us to publish this article and for clarifying questions on the etymology of the new species epithet. Leonardo Versieux and two anonymous reviewers provided crucial suggestions on the final text.