(C) 2012 Alex K. Monro. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Three hitherto undescribed species of Pilea (Urticaceae) from limestone karst in China are described and illustrated. Affinities of the species are discussed and Global Species Conservation Assessments presented. The new species are Pilea cavernicola A.K. Monro, C.J. Chen & Y.G. Wei, sp. nov. (Vulnerable) which most closely resembles Pilea scripta (Buch.-Ham. ex D.Don) Wedd. and Pilea gracilis Handel-Mazzetti, Pilea shizongensis A.K. Monro, C.J. Chen & Y.G. Wei, sp. nov. (Endangered) which is most similar to Pilea aquarum Dunn and Pilea guizhouensis A.K. Monro, C.J. Chen & Y.G. Wei, sp. nov. (Vulnerable) which resembles Pilea boniana Gagnep. and Pilea rubriflora C. Wright mostclosely.
Urticaceae, Pilea, China, Guizhou, Yunnan, limestone karst, caves
Pilea
As part of ongoing research into the diversity of cave-dwelling Urticaceaethe authors undertook four field trips to SW China in 2009 and 2010. Collecting wasfocused on the karst area in Guangxi, Guizhou and Yunnan and caves, stoneforests (karst cockpit formations), gorges and natural forest were sampled. During the course of these collecting trips seven collections (not all in caves) corresponded to unknown species of Pilea, three of which are described here. Their affinities are discussed and position within
Collecting in Karst is difficult as areas away from roads are sparsely populated and the terrain is steeply dissected and difficult to travel across. A consequence of this is that there are relatively few collections from such areas and undescribed species are frequently represented by very little material. Describing species based on a single or only two collections is problematic as there is no estimate of variation within the species and so the risk of over recognising species is greater. This is compounded in Floras such as China’s where there has been a tradition of describing species from single collections resulting in a situation whereby the most closely related species may also be known from a single collection. Despite the above we have decided to describe one of the species in this manuscript based on a single collection as we feel that, given China’s fast changing landscape and the fragility of many of the localities, to describe the species now affords the best hope for their conservation.
Herbarium specimens were compared with collections at IBK, BM and K and with scanned images of the collections at PE (http://pe.ibcas.ac.cn/herbinfor/vhtypequery.aspx ) and HK (http://www.hkherbarium.net/Herbarium/tcc_pop.aspx ) using the Flora of China. A morphological species concept developed during the course of previous taxonomic research on Pilea (
In the case of cave habitats, photosynthetically active radiation (PAR) was recorded. Observations of PAR were made at several points in the caves associated with living plantsand expressed as micro moles per m2 per second (mmol/m2/sec) using a Skye instruments 180° panorama light meter for each point. Where available these observations are included in the Distribution section of the species descriptions.
Conservation assessments were undertaken using
urn:lsid:ipni.org:names:77123888-1
http://species-id.net/wiki/Pilea_cavernicola
Figs 1 A–E, 2 A–C, 3 A–BMost similar to Pilea scripta from which it can be distinguished by the shorter stems, ovate rather than elliptic or oblong leaves, stipules with auriculate bases rather than deltate ones, the staminate tepals not ribbed and the sub-compressed elliptic rather than ovoid achenes with smooth non verrucose surfaces.
China. Guangxi: Fengshan County, Paoli Town, Yangzi cave, 490 m, 024°23'22.2"N, 107°03'59.1"E (DMS), 9 May 2010, A. K. Monro & Y.G. Wei 6669 (holotype: IBK; isotypes: BM001001214, MO, PE).
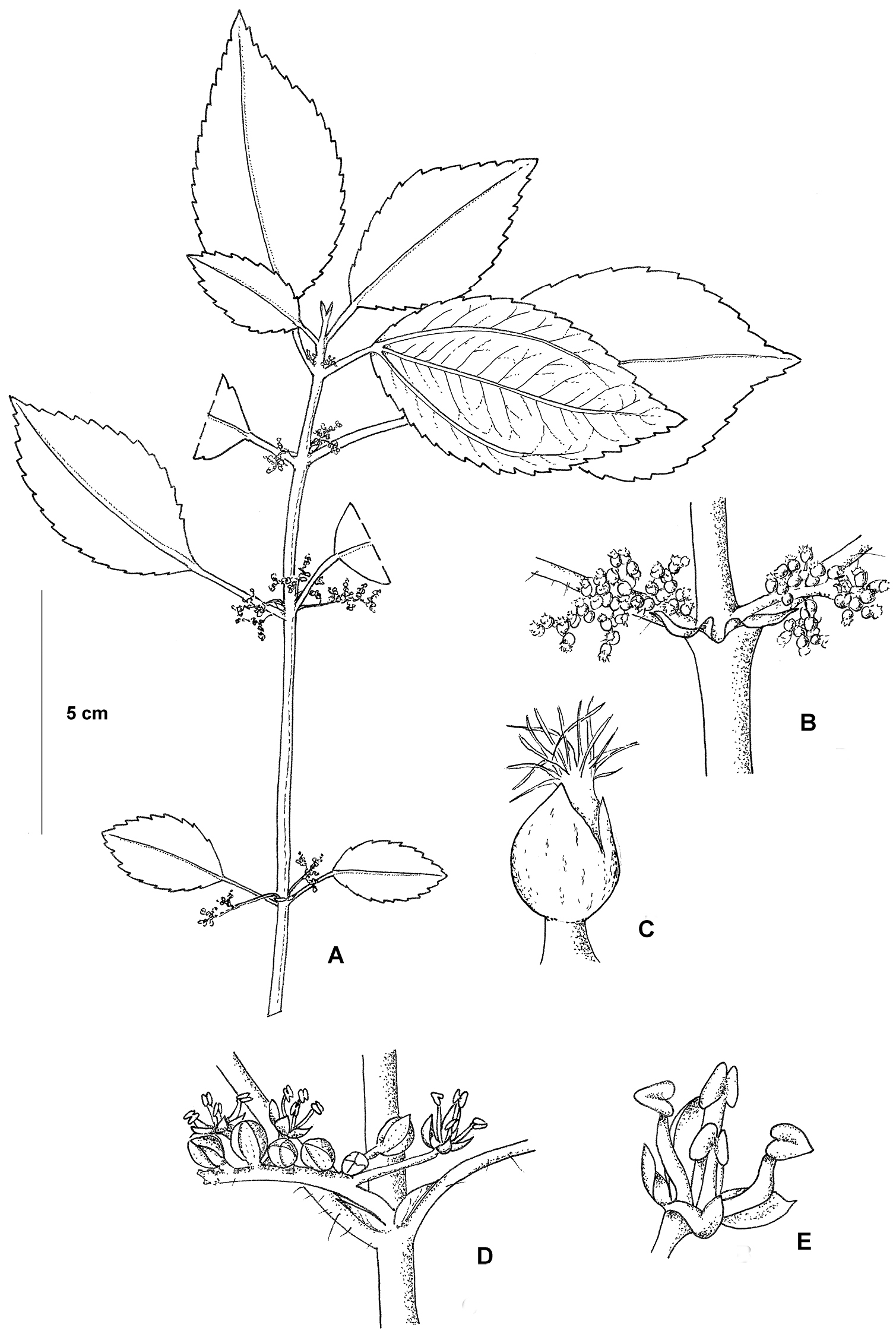
Herb to 50 cm, terrestrial. Stems erect, drying brown, maroon to green when fresh, glabrous or pubescent at the nodes and towards the base, where pubescent the hairs 1.0 mm, erect, crooked, cystoliths fusiform, the internodes 23–300 × 1.5–2.5 mm, angulate to square in cross-section, striate. Stipules 2.5–4.0 mm, auriculate-ovate, drying brown. Leaves petiolate, distichous; petioles at each node subequal or unequal by ratio 1:1.1–2.8, 12–33 mm, pubescent or glabrous, where pubescent the hairs 1.0-1.25 mm, erect, weakly curved or crooked; laminae at each node equal or subequal, 26–90 × 12–46 mm, ovate, subchartaceous; 3-nerved, the lateral nerves visible for 2/3 or more of the lamina length, secondary nerves 7–11 pairs, borne 60–75° to the midrib, weakly curved; upper surface drying dark brown, green or bronze when fresh, glabrous, cystoliths densely scattered, less than 0.125 mm, elliptic and punctiform, midrib and secondary nerves sunken; lower surface drying brown to dark brown, pale green or flushed bronze-purplish when fresh, pubescent, the hairs 0.75–1.0 mm, appressed, weakly curved, eglandular; base symmetrical, cuneate or weakly decurrent; margin serrate, the basal 1/8–1/4 entire; apex symmetrical, cuspidate. Inflorescences 4–8 per stem, unisexual, staminate and pistillate inflorescences synchronous, born on separate stems; bracts 0.75 mm; bracteoles 0.5 mm. Staminate inflorescences 2 per axil, 17–22 mm, bearing 45–90 flowers in a loose cyme; peduncle 1/4 or less inflorescence length, 0.75 mm in diameter, glabrous, occasionally with cystoliths present; pedicels 0.50–1.5 mm, glabrous. Staminate flowers 1.5 × 1.5 mm immediately prior to anthesis, green-brown; tepals 4, 1.75 mm, valvate, fused for their basal 1/3, elliptic, glabrous, the subapical appendage less than 0.25 mm, corniculate, glabrous; stamens 4. Pistillate inflorescences 1 or 2 per axil, 8–13 mm, bearing 150–300 flowers in a loose cyme; peduncle 1/4 to 1/3 inflorescence length, 0.75 mm in diameter, densely covered in cystoliths, cystoliths punctiform, glabrous; pedicels 0.25–0.75 mm, glabrous. Pistillate flowers 0.50–0.75 mm, tepals 3, unequal, glabrous, adaxial tepal 0.50–0.75 mm, oblong or ovate, the dorsal tepal appendage 0.50–0.75 mm, oblong, markedly thickened almost hood-like; the lateral tepals 0.375–0.50 mm, asymmetrically ovate. Infructescences 8–13 mm; peduncle 1/4 to 1/3 infructescence length; achenes 0.75× 0.675 mm, sub compressed, asymmetrically ellipsoid, the abaxial margin very narrowly thickened.
Pilea cavernicola. A Habit B Close up of a pistillate inflorescence C Close up of a pistillate flower D Close up of staminate inflorescence E Close up of staminate flower. Based on Monro & Wei 6669.
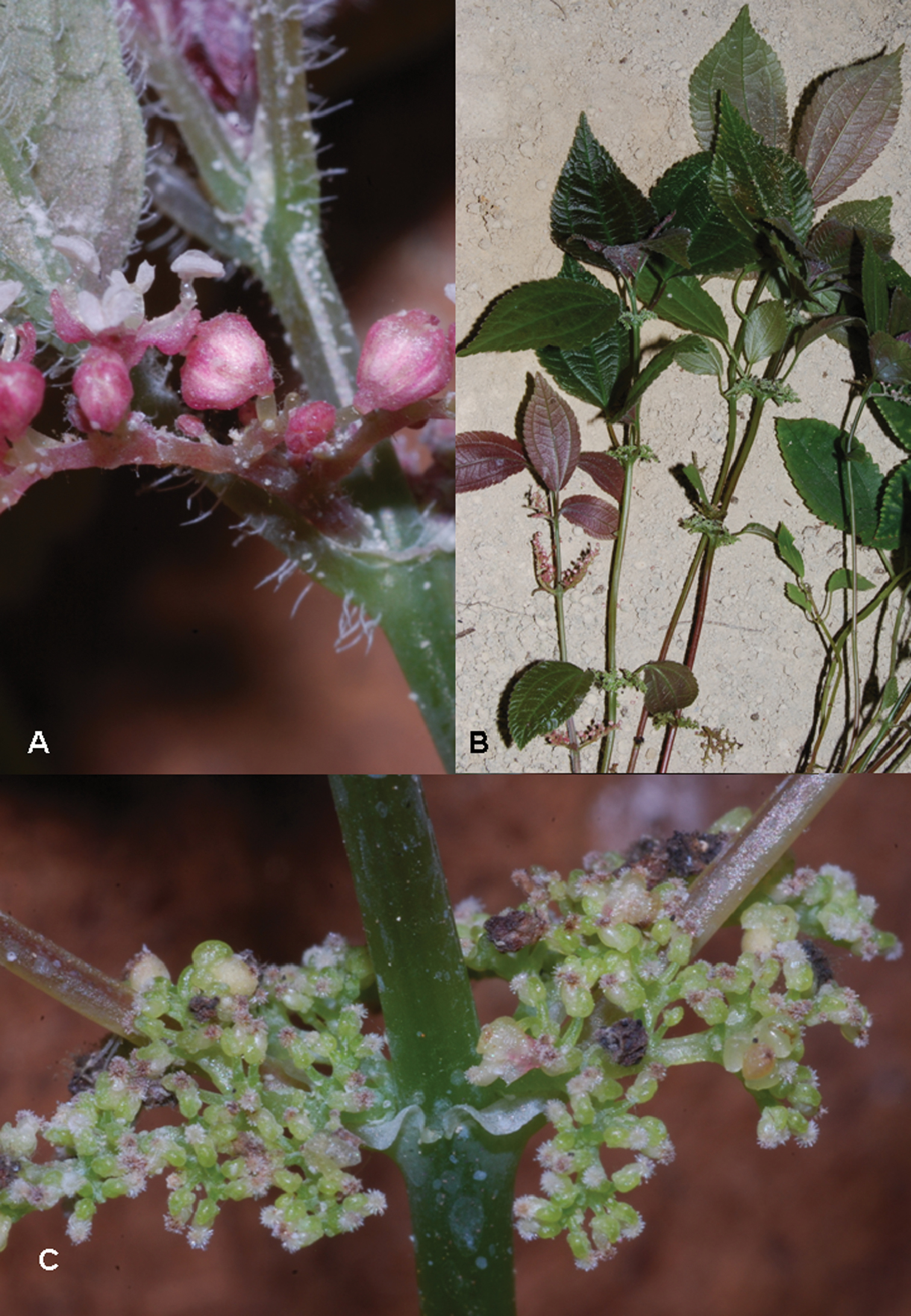
Pilea cavernicola. A Staminate flowers B Habit showing cave floor substrate in the background C pistillate inflorescence and flowers. Images of Monro & Wei 6669.
Habitats ofspecies described in this manuscript. A, B Pilea cavernicola, Yangzi cave, Fengshan County.Locality for Monro & Wei 6669 A Interior of cave B Exterior view of the cave C Habitat ofPilea shizongensis, Feng Huang Gu gorge, Shizong County. Locality for Monro & Wei 6727 D, E Habitat of Pilea guizhouensis, petaloid travertine wall of Malinghe Gorge E close-up of petaloid travertine formation, Locality for Monro & Wei 6715.
North West Guangxi Province, ca 500–1000 m, caves in limestone karst, growing at any point from the back to the entrance of the cave, PAR 0.02-1.39 mmol/m2/sec (ca 0.04-2.78 % full daylight).
The species name refers to the cave-dwelling habit of this species.
CHINA: Guangxi Province: Fengshan County, Paoli Town, Sidui Village, Xibi cave, 740 m, 024°24'38.5"N, 107°04'53.5"E (DMS), 8 May 2010, A. K. Monro & Y.G. Wei 6654 (IBK, BM001001215, PE, MO).
Comparison of the holotype and paratype material with type specimens from the herbaria listed in the methods section recovered Pilea scripta (Buch.-Ham. ex D.Don) Wedd. and Pilea gracilis Handel-Mazzetti as most similar to Pilea cavernicola. Pilea scripta can be distinguished from Pilea cavernicola based on stem height, leaf shape, stipule shape, staminate tepal morphology and achene morphology as summarised in Table 1. Pilea gracilis can be distinguished from Pilea cavernicola based on leaf shape, stipule morphology, staminate and pistillate inflorescence morphology and achene morphology as summarised in Table 2.
Pilea cavernicola falls within
Conservation status. Using IUCN criteria (
| Characters | Pilea cavernicola | Pilea scripta |
|---|---|---|
| Stem height | to 50 cm | to 1.5 m |
| Leaf shape | ovate | elliptic or oblong-lanceolate |
| Stipule morphology | ovate with conspicuous auriculate base | triangular with deltate base |
| Staminate tepal morphology | not conspicuously ribbed | conspicuously ribbed |
| Achene morphology | sub compressed asymmetrical ellipsoid, the surface smooth | compressed asymmetrical ovoid, the surface verrucose |
| Characters | Pilea cavernicola | Pilea gracilis |
|---|---|---|
| Leaf shape | ovate, apex cuspidate | elliptic or elliptic-lanceolate, apex acuminate or caudate-acuminate |
| Stipule morphology | ovate with conspicuous auriculate base, 2.5–4.0 mm | triangular with deltate base, ca 1 mm |
| Staminate inflorescence | 17–22 mm | 20–50 mm |
| Pistillate inflorescence | 8–13 mm | 20–50 mm |
| Achene morphology | the surface smooth | the surface verrucose or verrucose-spinulose |
urn:lsid:ipni.org:names:77123889-1
http://species-id.net/wiki/Pilea_shizongensis
Figs 3 C, 4 A–C, 5 A–DMost similar to Pilea aquarum from which it can be distinguished by the shorter stem, serrate rather than dentate leaves, shorter stipules and glabrous pistillate tepals.
China. Yunnan: Shizong County, Feng Huang Gu gorge, 1200 m, 024°37'54.0"N, 104°14'43.9"E (DMS), 14 May 2010, A. K. Monro & Y. G. Wei 6727 (holotype: IBK; isotypes: BM001001216, MO, PE).
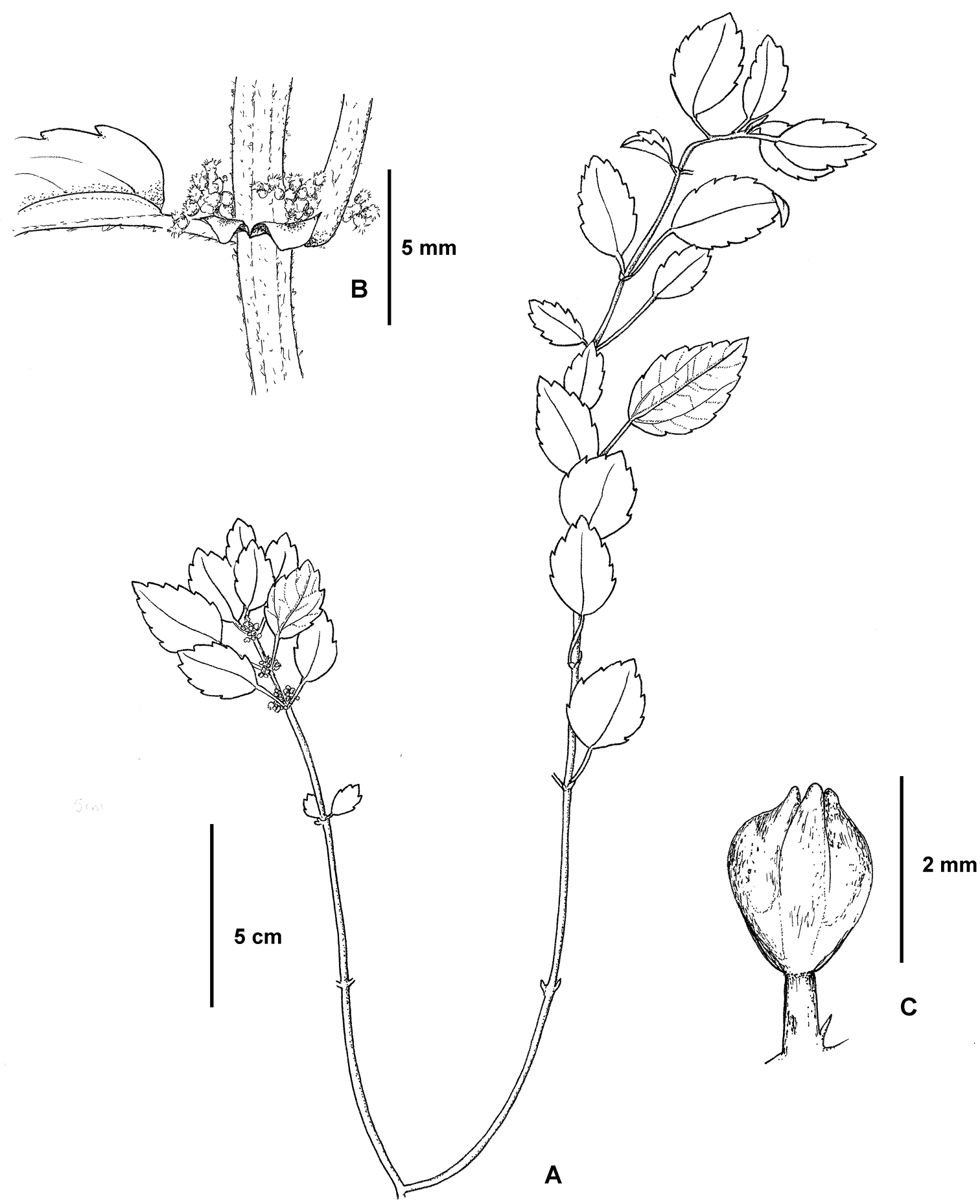
Herb to 20 cm, epipetric and terrestrial. Stems procumbent and erect, drying dark brown, maroon to dark green when fresh, pubescent, more densely so towards the shoot tips, the hairs 0.5 mm, erect or weakly appressed, curved or crooked, orange-brown peltate glandular, cystoliths absent, the internodes 4–38 × 1.5–2.0 mm, angulate in cross-section, striate. Stipules 2.5–3.0 mm, auriculate-cordiform, drying brown. Leaves petiolate, distichous; petioles at each node unequal by ratio 1:3–4.4, 3–20 mm, pubescent, the hairs 0.25-0.375 mm, erect, strongly curved to curved; laminae at each node equal or subequal, 11–35 × 7–17 mm, ovate to broad ovate, chartaceous; 3-nerved, the lateral nerves visible for less than 2/3 of the lamina length, secondary nerves 4–6 pairs, borne 45–60° to the midrib, straight or weakly curved; upper surface drying brown or dark brown, dark green with maroon nerves and green flushed maroon when fresh, sparsely pubescent, the hairs 0.50–0.675 mm, appressed, straight or weakly curved, cystoliths absent, midrib raised; lower surface drying grey-brown when fresh, nerves densely pubescent, the hairs 0.375 mm, weakly appressed, curved, orange-brown peltate glandular, midrib and lateral nerves raised, cystoliths fusiform, randomly scattered; base symmetrical, cuneate or obtuse; margin serrate, the basal 1/4 entire; apex symmetrical, subcuspidate or cuneate. Inflorescences 4–10 per stem, unisexual, staminate and pistillate inflorescences synchronous, born on separate stems; bracts 0.375 mm; bracteoles 0.3–0.5 mm. Staminate inflorescences solitary, 17.5–25 mm, bearing 7–16 flowers in a loose cyme; peduncle 1/4 or less inflorescence length, 0.5 mm in diameter, pubescent, cystoliths absent; pedicels 0.8–1.0 mm, glabrous. Staminate flowers 1.5–2.0 × 1.5–1.8 mm immediately prior to anthesis, deep pink; tepals 4, imbricate, 1.75 mm, fused for their basal 1/4, ovate or elliptic, glabrous, the subapical appendage 0.375 mm, corniculate, glabrous; stamens 4. Pistillate inflorescences solitary, 2.0–2.5 mm, bearing 17–30 flowers in a compact cyme; peduncle 1/2 to 2/3 inflorescence length, 0.375 mm in diameter, glabrous, cystoliths absent; pedicels 0.25–0.375 mm, sparsely pubescent. Pistillate flowers 0.375–0.50 mm, tepals 3, unequal, glabrous, adaxial tepal 0.5 mm, oblong, the dorsal tepal appendage 0.375 mm, oblong, markedly thickened; the lateral tepals 0.25–0.375 mm, asymmetrically ovate. Infructescences not seen.
Pilea shizongensis. A Habit B Close up of pistillate inflorescence C Close up of a staminate flower. Based on Monro & Wei 6727.
Pilea shizongensis. A Habit B Shoot tip C Close up of a staminate flowers D Close up of pistillate inflorescence and flowers. Based on Monro & Wei 6727.
Yunnan Province, Feng Huang Gu gorge, ca 1200 m, in limestone karst, growing on the floor of the gorge in deep shade.
The species name refers to county of the locality of the only known collection of this species, Shizong.
Comparison of the holotype material with type specimens from the herbaria listed in the methods section recovered Pilea aquarum Dunn as most similar to Pilea shizongensis. It can be distinguished from Pilea shizongensis based on pubescence, leaf margin morphology and pistillate tepal and flower morphology as summarised in Table 3.
Pilea shizongensis falls within
There is some confusion over the delimitation of Pilea aquarum and this is relevant to the delimitation of Pilea shizongensis. It would appear that the relatively rare character trait of pubescent pistillate tepals has been overlooked by several authors and that Pilea aquarum sensu strictu encompass a relatively narrow range of morphological variation which would exclude the subspecies Pilea aquarum subsp. brevicornuta and Pilea aquarum subsp. acutidentata.
| Character | Pilea shizongensis | Pilea aquarum subsp. aquarum |
|---|---|---|
| Stem height | 10–20 cm | 30–40 cm |
| Leaf margin | serrate | dentate |
| Pistillate tepal pubescence | glabrous | pubescent |
Using IUCN criteria (
urn:lsid:ipni.org:names:77123890-1
http://species-id.net/wiki/Pilea_guizhouensis
Figs 3 D, E, 6 A–C, 7 A–CMost similar to Pilea boniana from which it can be distinguished by the shorter inflorescence length, staminate flowers composed of four rather than five and valvate rather than imbricate tepals, and smaller achene size.
Guizhou: Xingyi County, Xingyi City, Malinghe Gorge, 950 m, 025°08'32.9"N, 104°57'11.7"E (DMS), 12 May 2010, A. K. Monro & Y.G. Wei 6715 (holotype: IBK; isotypes: BM001001220, MO, PE).
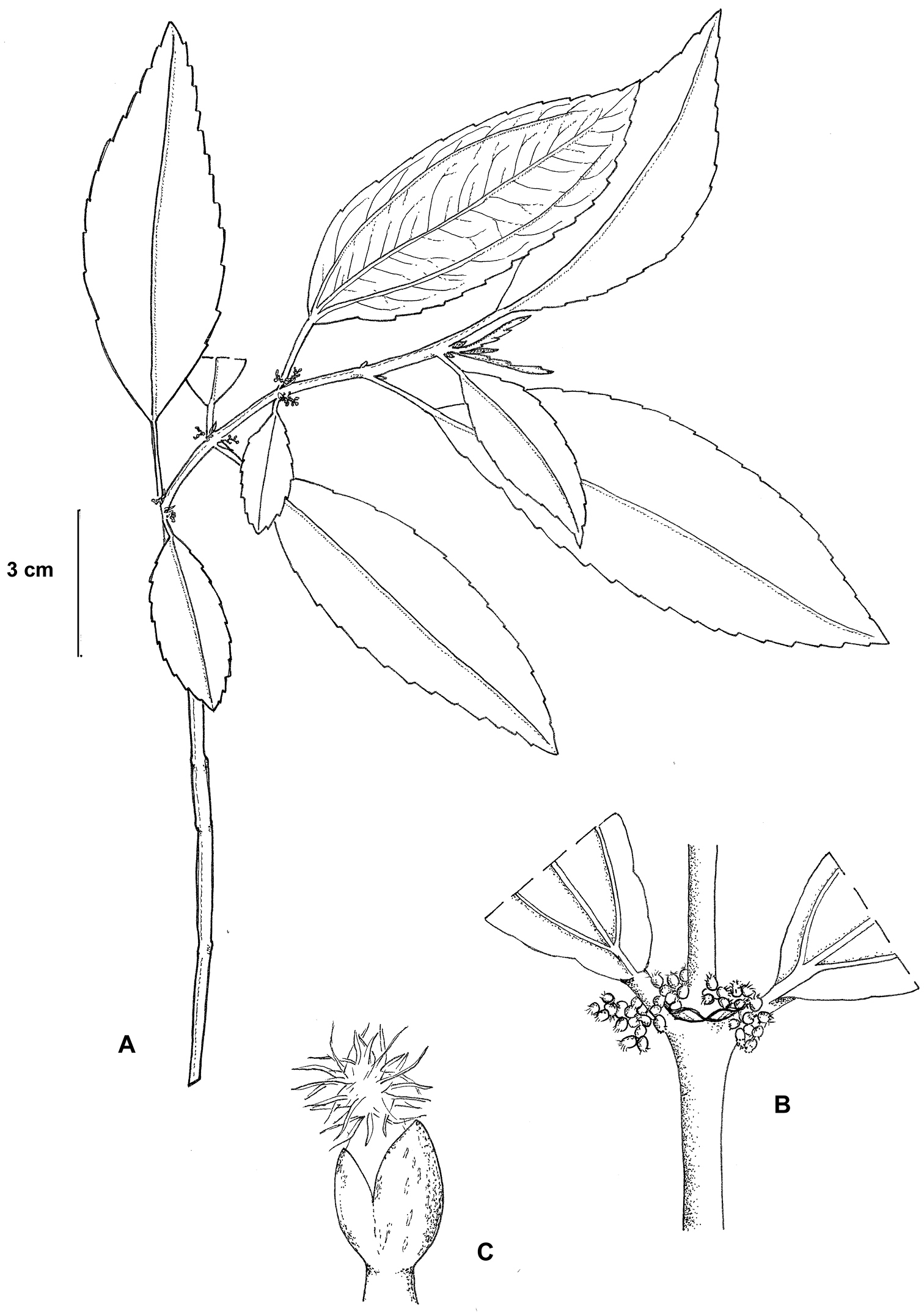
Herb or subshrub to 50 cm, epipetric or terrestrial. Stems erect, drying brown to dark brown, green and maroon at the nodes when fresh, glabrous, brown peltate glandular on young stems and flowering nodes, cystoliths elliptic or absent, the internodes 18–200 × 1.5–2.0 mm, irregularly circular and grooved in cross-section, striate. Stipules 2.0–2.5 mm, deltate, drying red-brown. Leaves petiolate, distichous; petioles at each node unequal by ratio 1:1.8-8.5, 1.5–37 mm, glabrous; laminae at each node subequal or unequal by ratio 1: 1.9–5.4, laminae 12–130 × 5–26 mm, ovate, lanceolate, asymmetrically elliptic, oblanceolate or obovate, chartaceous; 3–nerved, the lateral nerves visible for >7/8 or more of the lamina length, secondary nerves 8-14 pairs, borne 60–75° to the midrib, weakly curved; upper surface drying dark brown, dark green when fresh, glabrous, cystoliths 0.250–0.375 mm, ‘V’ shaped, midrib raised; lower surface drying brown to dark brown, pale green when fresh, glabrous, eglandular; base symmetrical or asymmetrical, cuneate or obtuse; margin discretely serrulate or serrate, the basal 1/8–1/4 entire; apex symmetrical, attenuate to acuminate. Inflorescences ca 3 per stem, unisexual, staminate and pistillate inflorescences synchronous, born on separate stems; bracts 0.75–1.0 mm; bracteoles 0.50–0.675 mm. Staminate inflorescences 15–20 mm, bearing ca 60 flowers in a loose cyme; peduncle ≤1/4 inflorescence length, 0.5 mm in diameter, glabrous; pedicels 1.0–1.5 mm, glabrous. Staminate flowers 1.5 × 1.5 mm immediately prior to anthesis, pale green; tepals 4, 1.75 mm, valvate, broad elliptic or obovate, fused for their basal 1/2, glabrous, the subapical appendage ≤0.25 mm, ridge-like, glabrous; stamens 4. Pistillate inflorescences solitary, 2.0–3.5 mm, bearing 19-42 flowers in a compact cyme; peduncle 1/4 to 1/3 inflorescence length, 0.375 mm in diameter, glabrous, cystoliths absent; pedicels 0.375–0.50 mm, glabrous. Pistillate flowers 0.50–0.75 mm, tepals 3, unequal, adaxial tepal 0.5 mm, oblong to keel-shaped, the tepal appendage 0.375–0.50 mm, appearing inflated; the lateral tepals 0.375 mm, asymmetrically ovate; staminodes not visible. Infructescences 4.0–4.5 mm; achenes 0.675 × 0.375 mm, compressed, asymmetrically ovoid, the margin narrowly thickened.
Pilea guizhouensis. A Habitat B close-up of pistillate inflorescence C close-up of a pistillate flower. Based on Monro & Wei 6715.
Pilea guizhouensis. A Habitat B close-up of pistillate inflorescence C close-up of a pistillate infructescence. Based on Monro & Wei 6715.
CHINA: Guizhou: Libo County, Yaolu Town, Huangcaoping, 650 m, 025°28'25"N, 108°04'15"E (DMS, both altitude and coordinates taken from Google Earth based on label data), April 24 2005, S. Qing & D. Londong 34 (BM001001221, PE).
Guizhou Province, Xingi County and Libo County, 650–950 m, in limestone Karst. The two localities are separated by ca 310km and represent semi-natural or naturally disturbed sites within an agricultural landscape.
The species name refers to Province from which both collections of this species are known.
Comparison of the holotype and paratype material with type specimens from the herbaria listed in the methods section recovered Pilea boniana Gagnep. and Pilea rubriflora C. Wright as most similar to Pilea guizhouensis. Pilea guizhouensis can be distinguished from Pilea boniana based on stipule, staminate, pistillate inflores and achene length and staminate tepal number as summarised in Table 4. Pilea guizhouensis can be distinguished from Pilea rubriflora based on internode, stipule and, staminate and pistillate flower morphology as summarised in Table 5.
Pilea guizhouensis falls within
| Character | Pilea guizhouensis | Pilea boniana |
|---|---|---|
| Stipules | 2.0–2.5 mm | ca 1.0 mm |
| Staminate inflorescence | 15–20 mm | 60–160 mm |
| Pistillate inflorescence | 2.0–3.5 mm | 60–160 mm |
| Staminate flower tepals | 4, valvate | 5, imbricate |
| Achene | 0.675 mm | ca 2.0 mm |
| Character | Pilea guizhouensis | Pilea rubriflora |
|---|---|---|
| Internodes | 1.5–2.0 mm diameter | 2–3 mm in dianeter |
| Stipules | 2.0–2.5 mm, not ribbed | ca 7 mm, longitudinally ribbed |
| Staminate pedicels | 1.0–1.5 mm | 2–3 mm |
| Pistillate flower tepals | 3 | 4 |
Using IUCN criteria (
We would like to acknowledge the support of the Innovation Fund of the Botany Department of The Natural History Museum, a Travel Grant from the Royal Society, the support of the National Natural Science Foundation of China (Grant No. 3116039), British Airways for the provision of a return flight, Ruth Harker (K) for the illustrations and Sam Pang (HK) for high resolution scans of the holotype collections of Pilea aquarum and the curators of BM, IBK, K and PE. We would also like to thank Melanie Thomas, Dennis Woodland and one anonymous manuscript reviewer for their time and comments.