(C) 2012 Hanno Schaefer. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Neotropical genus Melothria (Benincaseae, Cucurbitaceae) is a small group of yellow- or white-flowered climbers with small to medium-sized fruits. In 1899, Alfred Cogniaux described a species from montane rainforest in Haiti as Melothria domingensis, presumably based on the overall similarity in habit, leaf shape, and fruit morphology of his incomplete herbarium material to other Central American Melothria species. Melothria domingensis is still rare in European and American herbaria and the species has never been studied in detail. We here present molecular and morphological analyses, which show that the species is misplaced in Melothria and instead belongs in the distantly related tribe Cucurbiteae in the genus Cayaponia. We illustrate the species, provide the formal transfer and an extended description, and discuss the phylogenetic, biogeographic and ecological implications, including the finding that most likely bee- and not bat-pollination is ancestral in Cayaponia.
Cogniaux, Flora of Hispaniola, Melothria domingensis, pollinator shifts
The genus Melothria L. (including Melancium Naudin, Cucumeropsis Naudin, and Posadaea Cogn.) includes 12–15 species, confined to arid plains, clearings and forest margins, grass- or woodlands from the southern United States through Central and South America down to northern Argentina (
In 1899, Alfred Cogniaux described a new species of Melothria based on herbarium material from the Caribbean island of Hispaniola (Haiti and the Dominican Republic), which he had received from the Krug and Urban collection (
Molecular analyses.Total genomic DNA was isolated from leaf samples of four herbarium specimens of Melothria domingensis (all collected in the Dominican Republic, Table 1) using a commercial plant DNA extraction kit (NucleoSpin, MACHEREY-NAGEL, Düren, Germany), and following the manufacturer’s manual. Polymerase chain reactions (PCR) following standard procedures were used to amplify the entire nuclear ribosomal ITS region plus the following five chloroplast regions: rbcL gene, trnL intron, and the three intergenic spacer regions trnL-trnF, rpl20-rps12, and trnH-psbA. PCR protocols and primers are given in
Taxa, Genbank accession numbers, and voucher information.
| Taxon | Geographic origin | Specimen voucher | rbcL | trnL | trnL-trnF | rpl20-rps12 | trnH-psbA | ITS1-5.8S-ITS2 |
|---|---|---|---|---|---|---|---|---|
| Abobra tenuifolia | Argentina, Entre Rios | T.M.Pedersen 10287 (GH) | -- | -- | -- | -- | -- | JX505456 |
| Calycophysum weberbaueri | Peru, Cuzco | collector unknown (GH) | -- | -- | -- | -- | -- | JX505457 |
| Cayaponia domingensis | Hispaniola, Dominican Republic | G.J.Gastony et al. 640 (GH) | -- | JX505449 | JX505445 | JX505453 | JX505473 | JX505465 |
| Cayaponia domingensis | Hispaniola, Dominican Republic | P.Acevedo-Rodríguez et al. 13274 (GH) | JX505455 | JX505451 | JX505447 | JX505452 | JX505471 | JX505464 |
| Cayaponia domingensis | Hispaniola, Dominican Republic | A.H.Liogier 12563 (GH) | -- | -- | -- | JX505454 | JX505472 | JX505466 |
| Cayaponia domingensis | Hispaniola, Dominican Republic | A.H.Liogier 12908 (GH) | -- | JX505450 | JX505446 | -- | -- | JX505467 |
| Cayaponia (Selysia) prunifera | Suriname | Lindemann et al. 398 (NY) | -- | -- | -- | -- | -- | JX505458 |
| Cionosicys excisus | Mexico | E.Cabrera 15257 (GH) | -- | JX505448 | JX505444 | -- | -- | JX505459 |
| Cionosicys excisus | Mexico | G.F.Gauner 888 (GH) | -- | -- | -- | -- | -- | JX505460 |
| Cionosicys macranthus | Honduras | A.Molina 3838 (GH) | -- | -- | -- | -- | -- | JX505461 |
| Cionosicys macranthus | Mexico | D.M.Kearns 321 (GH) | -- | -- | -- | -- | -- | JX505462 |
| Cionosicys pomiformis | Jamaica | R.A.Howard & G.R.Proctor 14941 (GH) | -- | -- | -- | -- | -- | JX505463 |
| Tecunumania quetzalteca | Costa Rica | R.W.Lent 2311 (GH) | -- | -- | -- | -- | -- | JX505470 |
| Schizocarpum palmeri | Mexico, Rio Mayo | H.S.Gentry 1032 (GH) | -- | -- | -- | -- | -- | JX505468 |
| Schizocarpum reflexum | Mexico, Michoacán | M.Porter 1377 (GH) | -- | -- | -- | -- | -- | JX505469 |
Maximum likelihood (ML) analyses and non-parametric bootstrap searches (BS) with the fast-bootstrap algorithm were performed using RAxML-VI-HPC v. XX (
Morphological analyses. We studied Melothria domingensis specimens from the following herbaria: BR, GH, NY, U, and US. For comparison, both authors also studied a large number of Cayaponia and other Neotropical Cucurbitaceae specimens from all major European and American herbaria over the past decade. All measurements given in the text are from dry herbarium specimens.
Analysis of pollination syndrome evolution and biogeography.We used the same approach as described in
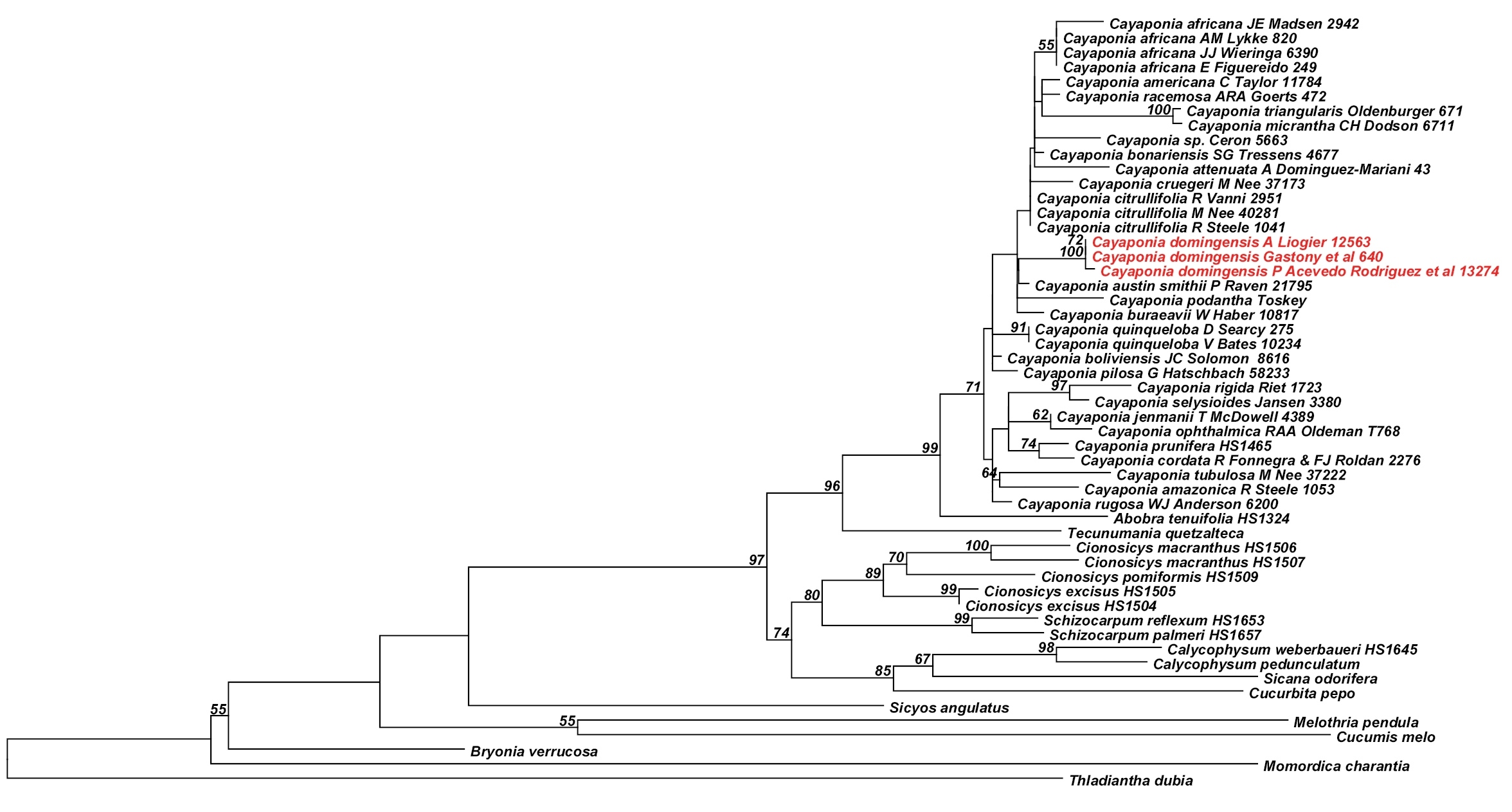
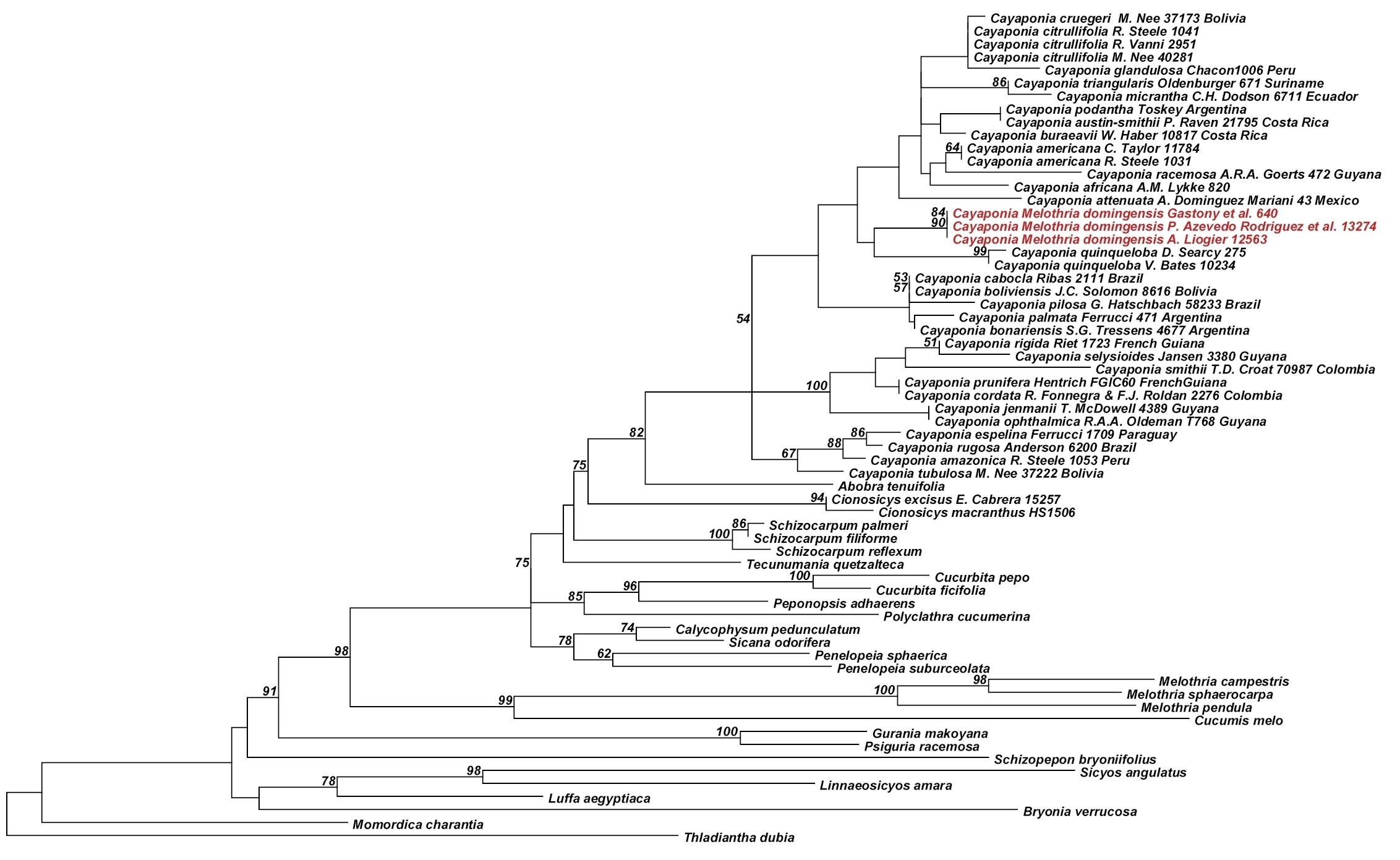
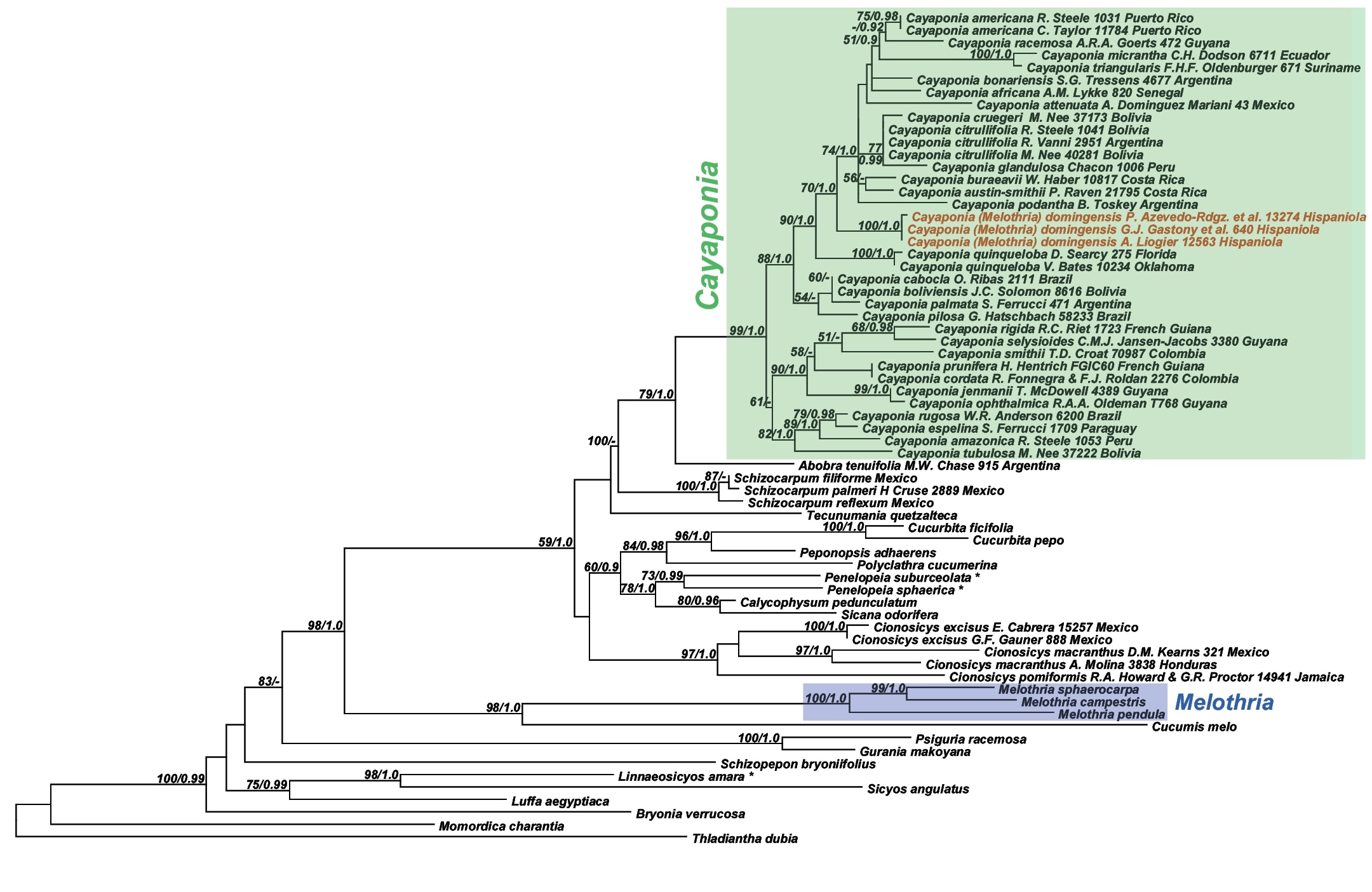
Analyses of the nuclear ITS data matrix (55 accessions, 984 aligned nucleotides) and the individual and combined plastid matrices (67 accessions, 4831 aligned nucleotides) produced congruent phylogeny estimates (Fig. 2, Fig. 3; phylogenies for individual plastid marker not shown), with all areas of discordance being restricted to branches with low support (i.e., BS <60% and Bayesian posterior probability (PP) <0.9). We therefore combined the ITS and plastid matrices into a single matrix (68 accessions, 60 species, 5673 aligned nucleotides) and in the following focus in our discussion on the phylogeny estimate built using this largest matrix (Fig. 4): the different accessions of Melothria domingensis are almost identical in their sequences and group together with high support (100% BS, 1.0 PP, Fig. 4). They are deeply nested in Cayaponia, which is monophyletic after inclusion of Selysia Cogn. as already suggested by
Comparison of seed, floral, fruit, and vegetative characters of Melothria domingensis with all available Cayaponia, Cionosicys, and Melothria material reveals that seed number, size and shape are most similar to Cayaponia and not to Melothria or Cionosicys. Mottled fruits like those of Melothria domingensis also occur in Cionosicys, but all currently known species of Cionosicys have much larger fruits and are many-seeded. In Melothria, fruits can be small and striped or mottled but all currently known species of that genus have many-seeded fruits with strongly compressed seeds. Only in Cayaponia do we find small fruits with few, tumid seeds.
Within Cayaponia, the relatively small leaves and few-flowered fascicles of Melothria domingensis are most similar to Cayaponia quinqueloba, an endemic from the southeastern United States. The fruits of Cayaponia quinqueloba, however, have the typical coriaceous or chartaceous exocarp of many other Cayaponia species, and not the distinctive spots of Melothria domingensis fruits. Another Cayaponia species with mottled fruits is Cayaponia tibiricae (Naudin) Cogn. (syn. Cayaponia martiana (Cogn.) Cogn.) of eastern Brazil, but its inflorescence is branched like that of Cayaponia racemosa (Mill.) Cogn. and a few others.
Since molecular and morphological analyses agree that Melothria domingensis should be placed in the genus Cayaponia, we here provide the necessary new combination followed by an extended description:
urn:lsid:ipni.org:names:77123716-1
http://species-id.net/wiki/Cayaponia_domingensis
Monoecious climber with 3–4 m long, slender, grooved stems (Fig. 1A); leaves triangulate-cordate, often deeply 3(-5)-lobed, shortly tomentose on both sides, glabrescent above, margin finely dentate; petioles 10–20 mm, striate; tendrils simple. Flowers probably diurnal (based on herbarium specimens with open flowers), solitary or in few-flowered fascicles in the leaf axils (Fig. 1B), the staminate and pistillate coaxillary, shortly pedicillate; corolla pale yellow, inner petal surface white; calyx glabrous, broadly campanulate; stamens inserted near the base of the tube; filaments distinct, two anthers 2-thecous, one 1-thecous; ovary globose, glabrous; style erect, linear, inserted on a basal nectary; stigmas 3, dilated, reflexed; staminodes 3, minute; fruit globose, glabrous, 10–20 mm diam., with thin, leathery wall, dark green turning orange with greenish white mottling; fruiting pedicels 10–30 mm long (Fig. 1C); seeds 6, broadly ovoid, 5 × 4 × 2.5 mm; testa light brown, smooth (Fig. 1D).
Cayaponia domingensis (Cogn.) H. Schaef. & M. Nee A habitus with leaves, tendrils, staminate and pistillate flowers, and young fruits B pistillate flowers C fruit D seeds; scale bar: 5 mm. Photographs of A.H.Liogier 12563 (GH) by H. Schaefer.
Flowering and fruiting specimens have been collected in April, June, August and September.
Endemic to the Greater Antilles, island of Hispaniola, and perhaps Puerto Rico (one record from Ponce, Toro Negro Commonwealth Forest, between Cerro Maravillas and Monte Jayuya, along road off highway 143, 1190–1200 m, 26 Feb 1993, Breckon et al. 4427, NY, specimen not seen).
According to specimen label information, the species occurs in rainforest, thickets and disturbed areas of cloud forest 1200–2200 m a.s.l., on limestone rocks with Ardisia Gaertn., Brunellia Ruiz & Pav., Buddleja L., Cestrum L., Coccoloba P.Browne, Cordia L., Daphnopsis Mart., Garrya Douglas ex Lindl., Heterotrichum M.Bieb., Lobelia L., Meliosma Blume, Miconia Ruiz & Pav., Myrsine L., Palicourea Aubl., Persea Mill., Ocotea Aubl., Trema Lour., Turpinia Raf., and Weinmannia L.
Listed as in danger of extinction (“peligro de extinción”) on an informal webpage for the flora and fauna of Hispaniola (
Mirliton blanc, Mirliton marron, Mirliton sauvage (
Dominican Republic. Baoruco: Sierra de Neiba, Sabana del Silencio, 18°39'07"N, 71°33'26"W, 2201 m, 19 Jun 2003 (fl., fr.), P.Acevedo-Rodríguez et al. 13011 (NY); Elías Peña: Sierra de Neiba, near La Doscientos, Hondo Valle, 1750–1850 m, 5–7 Sep 1968, A.Liogier 12501 (NY, US); Sierra de Neiba, near La Doscientos, S of Hondo Valle, 1750–1850 m, 5–7 Sep 1968, A.Liogier 12563 (GH, NY). Independencia: Sierra de Neiba, between Ángel Félix and Aniceto Martínez, 18°41'37"N, 71°46'56"W, 1867 m, 24 Jun 2003 (fl., fr.), P.Acevedo-Rodríguez et al. 13274 (GH, NY); Sierra de Neiba, along the Carretera Internacional near the crest of the range, along the Haitian border, vic. the San Rafael and Independencia border, 18°39'00"N, 071°37'48"W, 1700–2000 m, 9 Aug 1967, G.J.Gastony, G.C.Jones & D.H.Norris 640 (GH, NY, US); near Sapotén, above El Aquacate, Duvergé, 1500–1700 m, 4–5 Jan 1972, A.Liogier 18359 (F, NY); Sapotén, El Aguacate, Duvergé, 1300 m, 25 Jun 1977, A.Liogier & Liogier 27009 (NY); Sierra de Neiba, 30 km above Sabana Real on road paralleling the Haïtian border, 18°40'00"N, 71°46'00"W, 1845 m, 18 Apr 2003 (fl., fr.), M.Nee & J.C.Montero Castro 52304 (MO, NY). La Vega: near La Ciénaga, N of Constanza, 1700 m, 16 May 1959, Jiménez 4010 (US); La Nevera, from Valle Nuevo to San José de Ocoa, 2100 m, 18 Oct 1968, A.Liogier 13134 (NY); cabezadas de Ciénaga de la Culata, Constanza, 1650 m, 16 Oct 1968, A.Liogier 13072 (NY); Loma Redonda, Ciénaga de la Culata, Constanza, 1600–1950 m, 23 Sept 1969, A.Liogier 16015 (NY, US). Pederales: Sierra de Bahoruco, sección Los Arroyos, 18°25'54"N, 71°74'45"W, 1500 m, 10 Jul 2007 (fl., fr.), T.Clase, L.Raz, D.Castillo, L.Reinoso & E.Soto. 4539 (MO); above Los Arroyos, along the International Highway from Pedernales to Duvergé, 1500–1600 m, 8 Nov 1969, A.Liogier 16775 (NY, US). Peravia [now San José de Ocoa]: 33.9 mi. N of the Parque Central de San José de Ocoa on the road to Constanza, 1800 m, 7 Jul 1982, T.A.Zanoni, M.M.Mejía & J.D.Pimentel B. 21376 (MO, NY, U). San José de Ocoa: La Horma Arriba, 1800–2000 m, 1 May 1972, A.Liogier 18595 (F, NY); La Nevera, 2100 m, 22–23 Oct 1976, A.Liogier & Liogier 25690 (NY). Santiago: upper Río Bao valley, at the base of La Pelona, 1700 m, 1–7, A.Liogier 12908 (NY). Haïti. Ouest: Fourcey, Massif de la Selle, ca. 1750 m, 5 Aug. 1924 (fl., fr.), E.L.Ekman 1319 (B, S, US). Massif de la Selle; Parc National Morne la Visite; Morne d›Enfer, eastern slopes and ridge, 1850–1880 m, 14 May 1984, W.S.Judd 4669 (FLAS).
Best Maximum likelihood tree for Cayaponia and relatives based on the nuclear ribosomal ITS region (55 accessions, 984 aligned nucleotides). Likelihood bootstrap values ~ 60% is indicated above branches.
Best Maximum likelihood tree for Cayaponia and relatives based on six combined chloroplast loci: rbcL gene, trnL intron, trnL-F spacer, rpl20-rps12 spacer, and trnH-psbA spacer (64 accessions, 4831 aligned nucleotides). Likelihood bootstrap support ~ 60% is indicated above branches.
Best Maximum likelihood tree for Cayaponia and relatives based on 973 nucleotides from ITS plus combined chloroplast loci (67 accessions, 5673 aligned nucleotides). Likelihood bootstrap support ~ 60% and Bayesian posterior probabilities ~ 0.9 are indicated at the nodes.
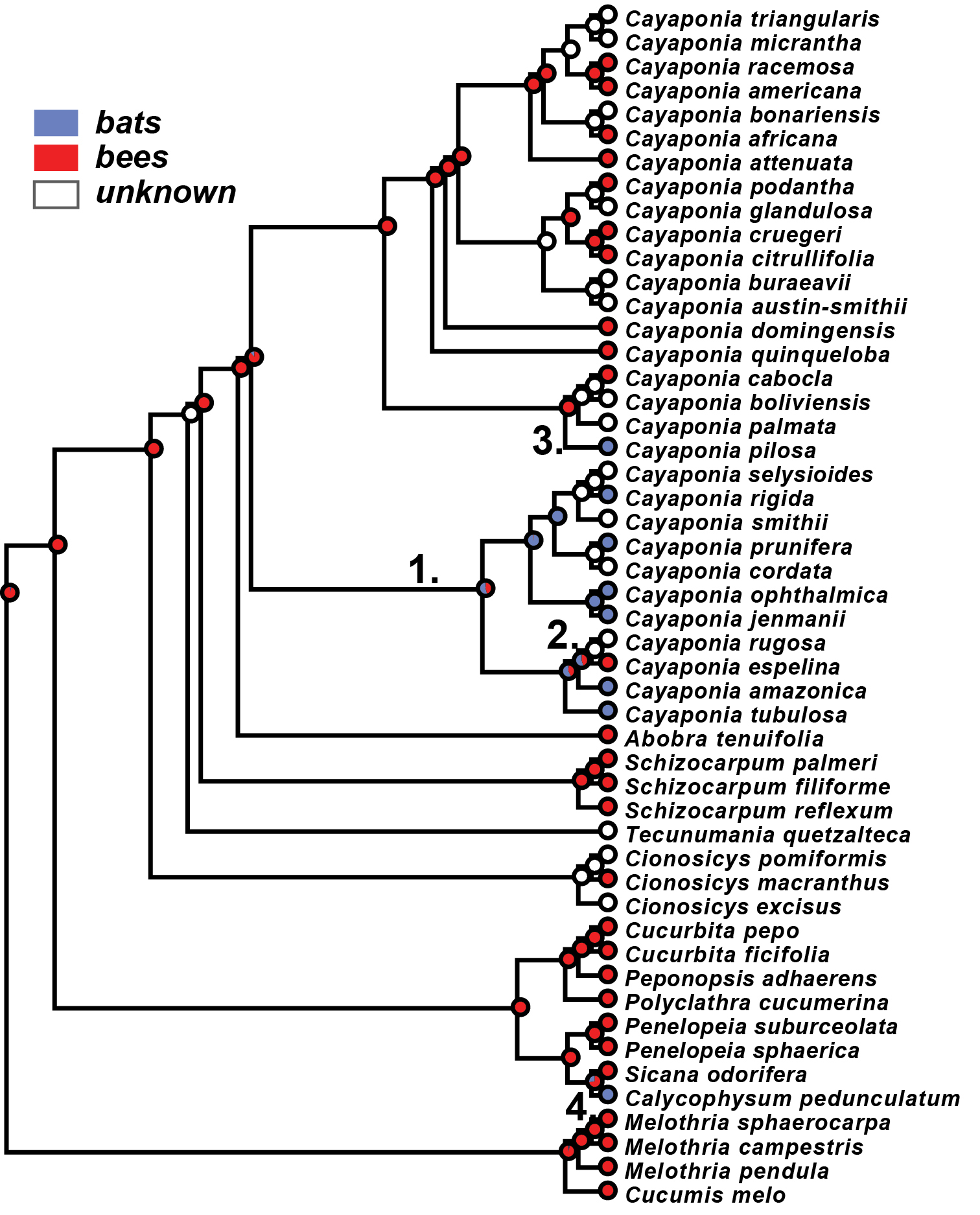
Our re-analysis of the evolution of pollination syndromes in Cayaponia reveals that bee pollination is most likely the ancestral state for the clade (Fig. 5) followed by one shift to bat pollination along the stem of the Cayaponia tubulosa Cogn.-C. prunifera (Poepp. & Endl.) P.Duchén & S.S.Renner clade and one reversal to bee pollination within the same clade (Cayaponia espelina (Silva Manso) Cogn.). A third shift is inferred for Cayaponia pilosa Cogn., which is bat pollinated but nested in a bee pollinated clade (Fig. 5).
Maximum likelihood reconstruction of ancestral pollination syndromes: bat pollination – blue; bee pollination – red; unknown – white; inferred pollinator shifts numbered 1–4.
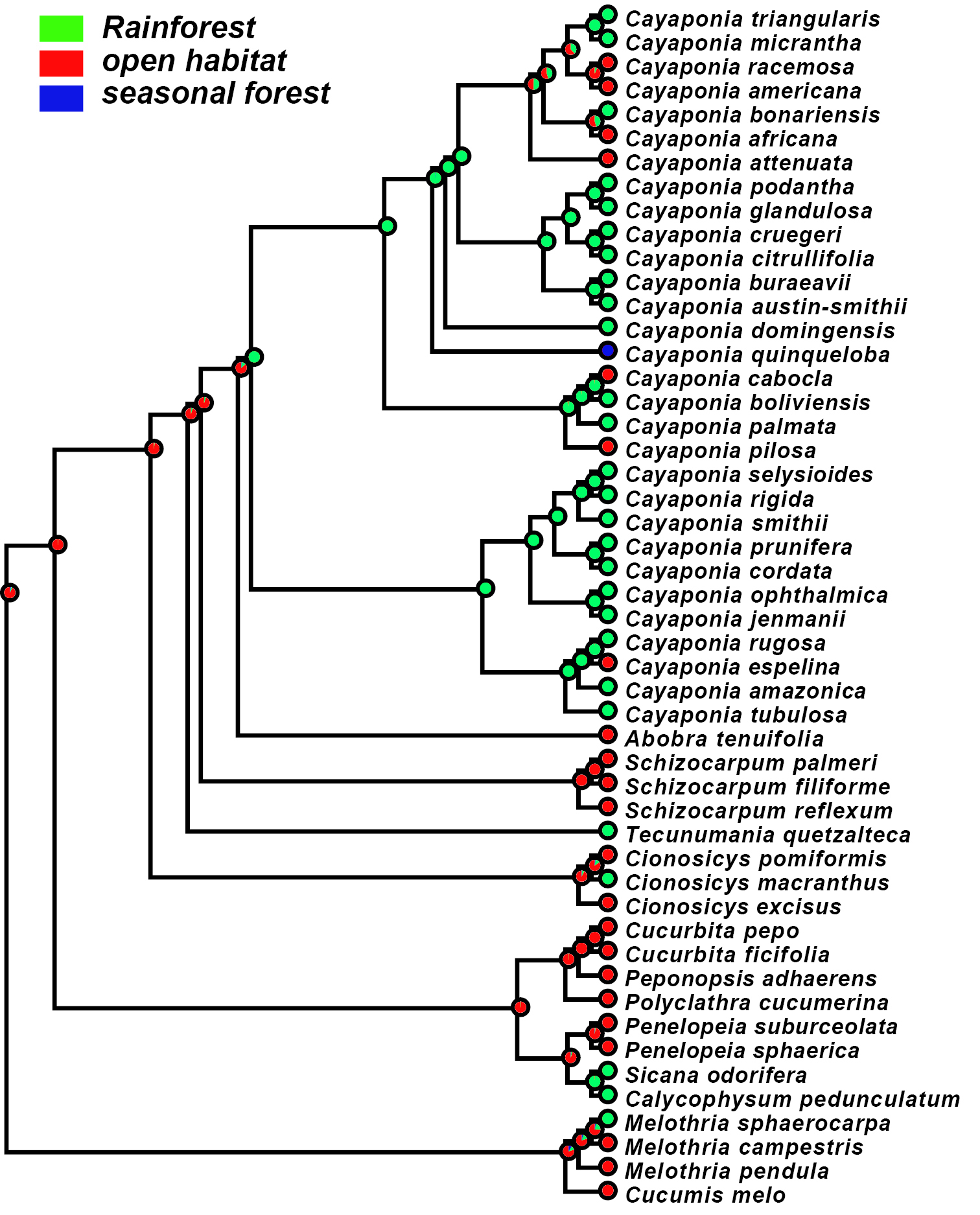
We find that the ancestral habitat of the Cayaponia lineage was most likely rainforest, which is in agreement with most extant species still being confined to some type of rainforest habitat (Fig. 6). Only few species, including Cayaponia espelina, Cayaponia pilosa, and some members of the clade containing Cayaponia attenuata (Hook. & Arn.) Cogn. shifted to more open habitats (Fig. 6).
Maximum likelihood reconstruction of ancestral habitat type: rainforest - green; open habitat (including cerrado, savanna, bushland) - red; seasonal forest - blue.
Molecular and morphological evidence both show that Melothria domingensis is best placed in the Cayaponia clade and probably close to the North American Cayaponia quinqueloba. However, due to the poor resolution in parts of our Cayaponia phylogeny, we are unable to identify exact sister group relationships. Better taxon sampling and additional variable DNA markers are needed to solve this question.
The newly identified Cayaponia species from Hispaniola is a rain- or cloud forest inhabitant with small, probably diurnal flowers on short pedicels that match all characters for bee-pollination in Cayaponia discussed in
Cayaponia domingensis is one of only few species in the genus and in the entire family Cucurbitaceae that occur above 2000 m a.s.l. (the highest collections known are from 2100 m a.s.l., Liogier & Liogier 25690 (NY), A. Liogier 13134 (NY) and 2201 m a.s.l., P. Acevedo-Rodríguez et al. 13011 (NY)). The mechanisms that allow this species to thrive at those altitudes are unknown but might be interesting to study. Most Cucurbitaceae are highly frost-sensitive and survive cold periods only as seeds (e.g. North American Sicyos angulatus L.), rootstocks (e.g. Central European Bryonia dioica Jacq.), or tubers (e.g. Thladiantha dubia Bunge). The only perennial Cucurbitaceae that thrive at even higher altitudes are some Himalayan Thladiantha species that reach up to 3500 m a.s.l.
The mottled fruits characteristic for Cayaponia domingensis are very rare in the genus and might have evolved as an adaptation to a specialised seed dispersal agent on the island. Unfortunately, we lack field observations on dispersal agents and judging from fruit and seed size, a wide range of animals, including birds, lizards, and small mammals might be involved.
Recent transoceanic dispersal has been inferred for the ancestors of Cayaponia africana s.l. (
Our results highlight that even after two decades of Molecular Systematics, we still need more sequencing combined with morphological analyses to sort out taxonomic problems in Cucurbitaceae and other understudied families. We can now be confident about the phylogenetic position of this enigmatic Caribbean endemic. This is, however, only a first step and we now plan to do fieldwork on Hispaniola to obtain accurate distribution data, find out more about the ecology of this species, identify the threats to the remaining populations and ultimately develop a management plant to guarantee the survival of this unique endemic.
We are grateful to R. Wunderlin (F) for information on specimens of Melothria domingensis and to Ian Telford and Thomas Zanoni for comments on earlier versions of the manuscript. H.S. thanks Emily Wood and Walter Kitredge (Harvard) for herbarium assistance and the keepers of NY, U and US for hospitality during visits.