(C) 2012 Marc S.M. Sosef. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Garcinia has some 260 species and is often regarded as a genus with a difficult taxonomy. No recent treatment is available for the botanically rich Lower Guinea phytogeographical region. This study aims at partly filling this gap. First, several taxonomic problems are solved. Garcinia chromocarpa is reduced to a variety of Garcinia quadrifaria. Garcinia gnetoides and Garcinia granulata are both synonyms of Garcinia quadrifaria. Garcinia zenkeri is a synonym of Garcinia densivenia and lectotypes are being designated for both names. Garcinia brevipedicellata is a synonym of Garcinia afzelii, as is Garcinia antidysenterica for which a lectotype is designated. Second, two new species endemic to Gabon are described: Garcinia gabonensis Sosef & Dauby and Garcinia obliqua Sosef & Dauby. Finally, an identification key to all species present in the Lower Guinea region is provided. A few remaining West African species names could not be placed with certainty, because the type material was lost or not traced yet. One is a Rutaceae while the remaining three are provisionally to be regarded as synonyms of Garcinia smeathmannii.
Garcinia, Clusiaceae, Africa, Lower Guinea, Gabon, taxonomy
The genus Garcinia L. is part of the family Clusiaceae which, in its present circumscription (
Most authors now agree upon a broad concept of Garcinia, including the former genera Rheedia L. and Ochrocarpos Thouars, as well as the small genera Pentaphalangium Warb. and Tripetalum Schumann. One might also merge the genus Allanblackia with Garcinia (
Amongst the African genera, Garcinia is characterized by the dioecism of its species and hence its unisexual flowers (or at least functionally so, see also
Garcinia contains approximately 260 species which are mainly confined to the tropics (
In Lower Guinea (roughly comprising the forested regions in Cameroon, Equatorial Guinea, Gabon, the south-west of the Republic of the Congo, Cabinda (Angola) and the southwest of the Democratic Republic of the Congo;
The presence of high numbers of sympatric Garcinia species in almost every tropical region (
In tropical Africa, the Clusiaceae (often in their old concept of Guttiferae) have been treated for West Africa (
The authors studied the available Garcinia material in BM, BR, BRLU, K, L, LBV, MO, P and WAG (herbarium abbreviations follow
The conservation status of the two new species was assessed using the
The species now known as Garcinia quadrifaria was first described by
The species Garcinia quadrifaria belongs to the section Xanthochymus, characterized mainly by having staminal bundles with filaments only partly fused and globose anthers (
After careful examination of the characters of Garcinia quadrifaria and Garcinia chromocarpa, we have come to the conclusion that the only distinction between the two is the minute puberulence on the bracts, pedicels and fruits of Garcinia chromocarpa, where those of Garcinia quadrifaria are glabrous. Although at first glance there might be a geographical distinction, Garcinia chromocarpa in the Congo Basin, west to Gabon and Cameroun (
We therefore conclude that since the differences between the two taxa are minimal, they cannot be upheld as different species. We do, however, want to distinguish them, and the level of variety seems most appropriate since there is no geographical separation. Because the name Garcinia quadrifaria has priority over Garcinia chromocarpa, this leads to the following new combination:
urn:lsid:ipni.org:names:77122646-1
Subsequently, we have studied the West-African species Garcinia gnetoides Hutch. & Dalziel. The type material at K, Chevalier 15157, consists of a plant carrying terminal inflorescences with a dense mass of many racemes composed of a short rachis with closely set bracts which are glabrous. In Garcinia quadrifaria each raceme normally appears solitary. An old note attached to the type already states it might well be a galled inflorescence, because a larva was observed inside. Hutchinson and Dalziel are well aware of its potentially diseased nature which shows from their remark in the Flora of West tropical Africa (
After careful examination of all material at hand, we cannot conclude otherwise than that both Garcinia gnetoides and Garcinia granulata represent the same species known to us as Garcinia quadrifaria and thus are synonyms of the latter. The fact that the first two are in fact synonymous was already concluded by
Some sources cite the publication of the names Garcinia gnetoides and Garcinia granulata in Kew Bulletin (
So, in conclusion, the new situation is as follows:
Finally, Garcinia le-testui, a rare species endemic to southern Cameroon and Gabon, seems sufficiently distinct from Garcinia quadrifaria being larger in most parts (notably wider wings on the twigs, larger leaves, longer pedicels, etc.). Most differences being related to size, it would not be surprising if Garcinia le-testui turns out to be a polyploid of Garcinia quadrifaria.
Two more species of the section Xanthochymus were described by
Garcinia zenkeri was also based on two Zenkercollections from Cameroon: Zenker 1120 (with flowers) and Zenker 3247(with fruits). According to the
Garcinia densivenia Engl., Bot. Jahrb. Syst. 40: 563 (1908). ‒ LECTOTYPE (designated here): CAMEROON. Bipinde, Urwaltgebiet, 1903. Zenker 2547(G!, barcode G00018874; isotype BR!, GOET!, K!, M!, P!, WAG!).
Heterotypic synonym:
Garcinia zenkeri Engl., Bot. Jahrb. Syst. 40: 566 (1908), syn. nov. ‒ LECTOTYPE (designated here): CAMEROON: Bipinde, Urwaltgebiet, 1896, Zenker 1120(G!, barcode G00018871; isotype BM!, GOET!, K!, M!, P!, S!, WAG!).
The material now identified as Garcinia densivenia, shows a remarkable variation in the distinctiveness of the tertiary venation. In the type as well as the paratype collection this venation is indeed, as the name indicates, strikingly dense and prominent. However, in most of the remaining material we observed a large continuous variation towards leaves where the tertiary venation was even hardly visible. Because otherwise, the material is quite uniform, we assume this character to be highly variable within the species, and possibly also depending on the way in which the material was treated and dried after collecting.
A second remarkable variation was observed in the shape of the fruits. These can be subglobose to distinctly 5-lobed and ‘pumpkin-like’. The lobed feature was even already mentioned by Engler in his protologue: “Baccae ….. leviter 5-lobae…..” and “schwach 5 lappigen Früchte…..”. However, again, we found no other characters to correlate with this feature. Moreover, the label of Bos 3639 specifically mentions that the shape of the fruits he collected are “globose to shallowly 5-lobed and resembling a pumpkin”. We thus assume that these observations also illustrate within-species variation, and might be related to the number of seeds that develop within a single fruit.
The Garcinia afzelii complexThe section Tragmanthera is characterized by 4-merous flowers and staminal bundles that are completely fused carrying a row of ellipsoid anthers at their tip (
However, we could not find any clear difference between the remaining two species, Garcinia afzelii and Garcinia brevipedicellata.
Finally, the status of the name Garcinia antidysenterica A.Chev. is unclear. At present, it is regarded as a synonym of Garcinia afzelii (
The above now leads to the following situation:
Garcinia afzelii Engl., Bot. Jahrb. Syst. 40: 570 (1908).
Heterotypic synonyms:
Garcinia antidysenterica A.Chev., Vég. ut. Afr. trop. franc. 6: 445, fig. 52 (1911). ‒ LECTOTYPE (designated here): IVORY COAST: vallée du Moyen-Comoé, entre Yabrouakrou et Tingouéla, 13 décembre 1909, Chevalier 22571 (P!; isotype BR!, K!).
Garcinia mannii Oliv. var. brevipedicellata Bak.f., in Rendle et al., Cat. pl. Oban : 8 (1913). – Garcinia brevipedicellata (Bak.f.) Hutch. & Dalziel, Fl. West trop. Afr., ed. 1, 1(1): 237 (1927), syn. nov.
Within the section Tragmanthera some taxonomic questions remain to be solved, for example the distinction between the non-locellate species such as Garcinia epunctata Stapf and Garcinia preussii Engl., and the status of several other names now regarded as their synonyms. For now, we maintain the present status quo, just signalizing the need for a more in-depth study.
Two new endemic Garcinia species from GabonDuring the preparation of the Clusiaceae treatment for Flore du Gabon, material belonging to two new species turned up. Both are endemic to this country that has a plant endemism rate of ca. 11% (Sosef et al. 2006). Gabon is notoriously rich in species (see above), especially its lowland rain forest is reputedly the most species-rich in tropical Africa (
urn:lsid:ipni.org:names:77122647-1
http://species-id.net/wiki/Garcinia_obliqua
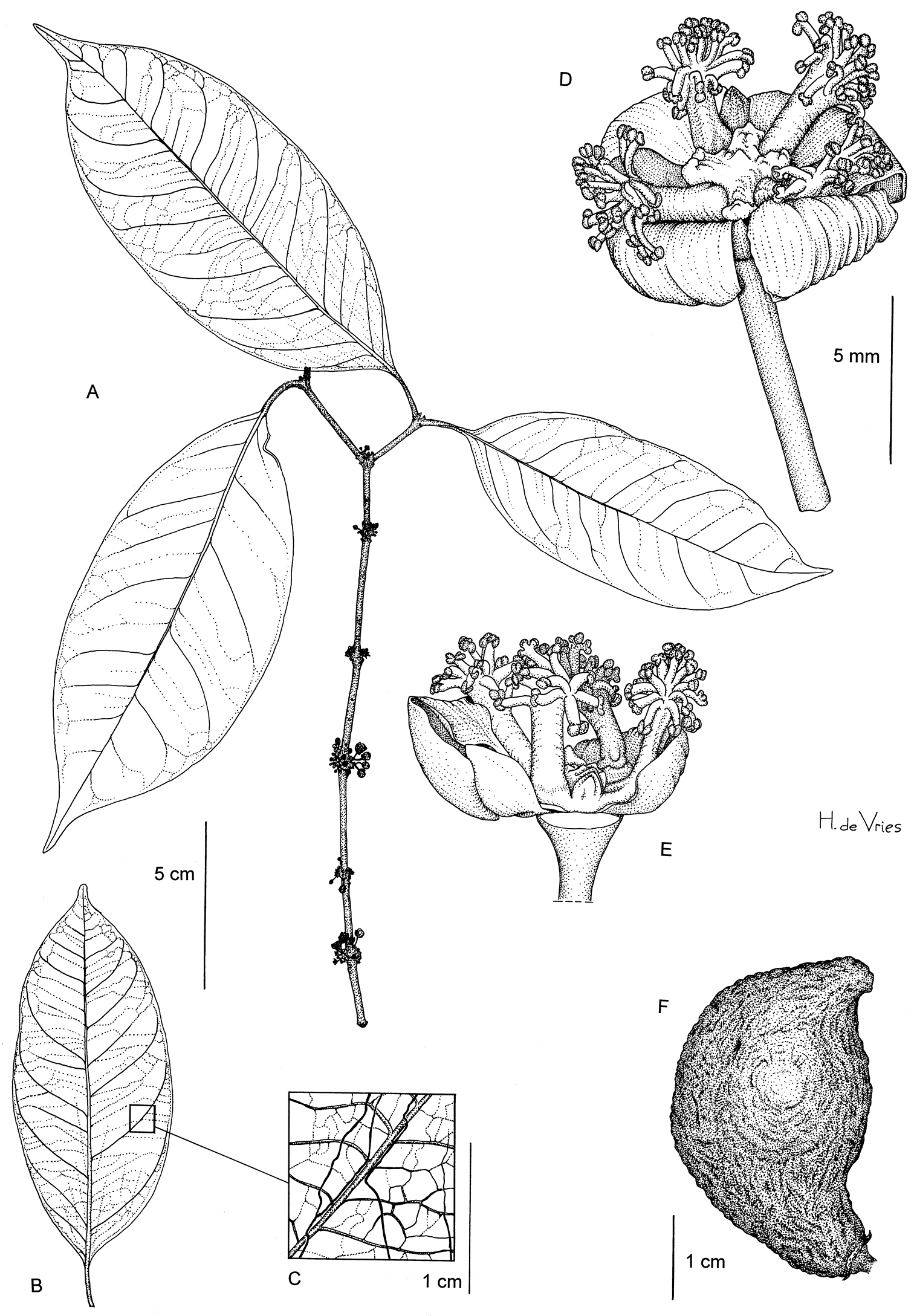
Fig. 1Similar to Garcinia smeathmannii but leaves with 6‒9 pairs of lateral veins, tertiary venation only slightly distinct above, petiole almost smooth, and fruits asymmetric with a coriaceous and ribbed skin.
GABON: Ogooué-Ivindo, near Djidji, 5‒10 km West of Koumémayong, 0°15'N, 11°50'E (DMS), 25-4-1988, Breteler 8993 (holotype WAG!; isotype BR!, K!, LBV!, MO!, P!).
Dioecious tree; bole up to 35 cm dbh; twigs round to slightly angular in cross section; latex transparent or yellow. Leaves opposite; petiole (0.8‒)1‒1.5(‒2) cm long, smooth or slightly transversely wrinkled, with a distinct foveola of up to 3 mm long ; blade elliptic to lanceolate, (8‒)9‒17(‒18) × 2.5‒6 cm, attenuate at base, acuminate at apex, glabrous; lateral veins 6‒9 pairs, distinct below, slightly distinct above, disappearing towards the margin; tertiary venation visible to distinct below, slightly distinct above; resin canals visible on the lower surface, black and subparallel to the midrib. Inflorescence axillary, with fascicles of flowers on swellings in the axils of twigs. Flowers 4-merous, unisexual; pedicel 3‒6 mm; external sepals about 2.5 mm long, internal ones about 3 mm, greenish; petals suborbicular, about 4 mm long. Male flower: staminal bundles tree-like, with 10‒19 irregularly positioned stamens per bundle, filaments free at the apex, anthers small, subglobose; pistillode present. Female flower not observed. Fruit on a 12 mm long pedicel (only 1 fruit with pedicel observed), asymmetric, oblique, probably because of aborted ovules, circular to broadly ovate in cross section, 3‒3.5 × 1.8‒2 cm; exocarp strongly furrowed and coriaceous and green with blue reflection in dry condition. Seed 1, obliquely ellipsoid, 2‒3 × 1.5‒2 cm, black.
Garcinia obliqua: A Flowering twig B Leaf from below C Idem, detail D Male flower E Idem, 1 sepal and 2 petals removed F Fruit. (A: Breteler et al. 8993 B, C, F: Dauby et al. 1570; D, E: Breteler et al. 8738). Drawing by Hans de Vries, NCB Naturalis (section NHN) ©.
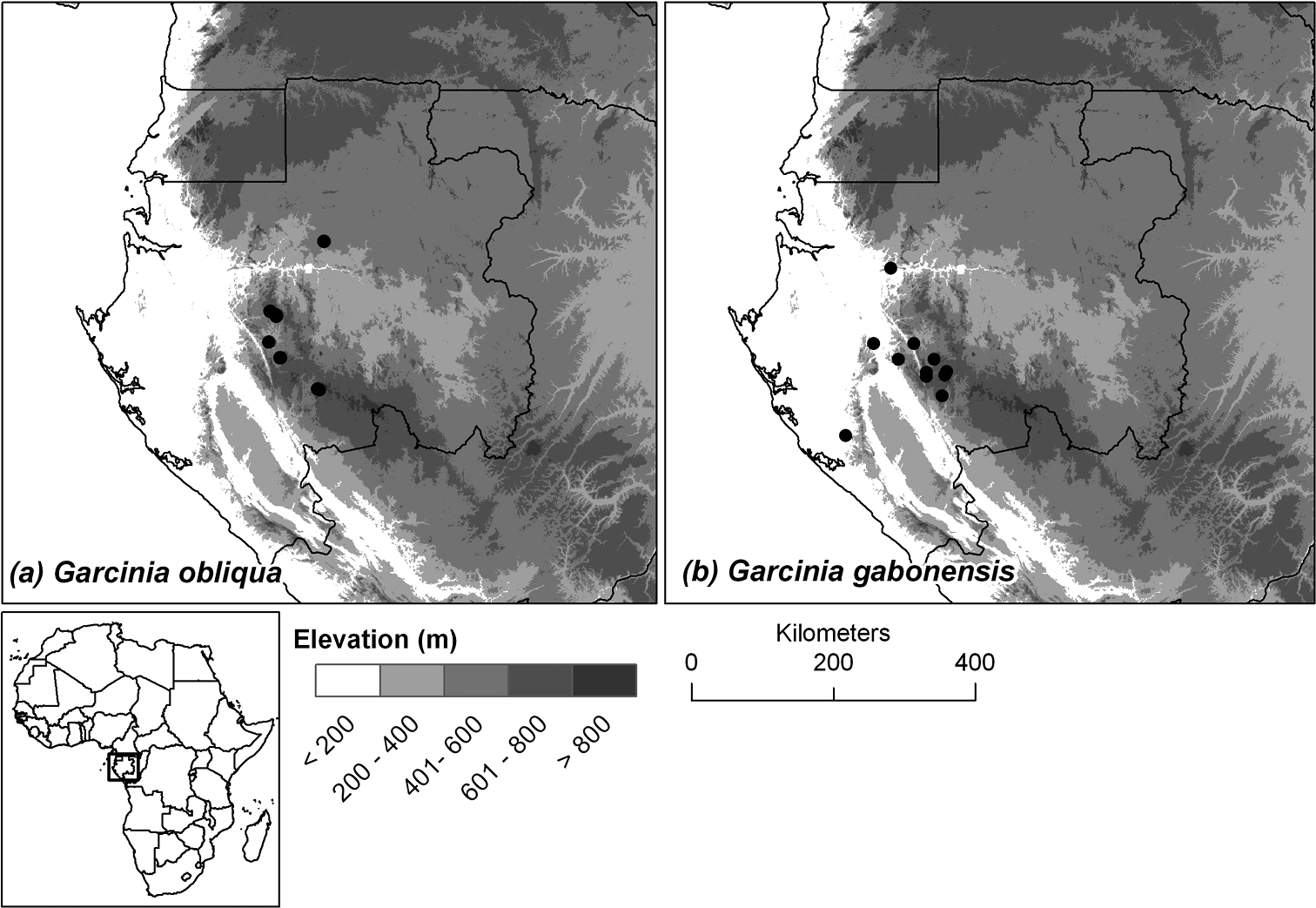
Endemic to central Gabon, known only from the Ogooué-Ivindo and Ngounié provinces (see Figure 3a).
Primary terra firme rainforest; at ca. 450‒800 m altitude. Flowering in April, fruits observed in February.
Garcinia obliqua is currently known from nine collections and six locations. Estimates of its extent of occurrence and area of occupancy are respectively ca. 9488 km2 and 80 km2. One collection (Dauby et al. 1650) corresponded to a dead individual along a forestry road and all locations are currently found within logging concessions. Hence, we assume the extent of occurrence, area of occupancy, quality of habitat and number of sub-populations will decrease in the near future. We therefore assign a preliminary status of Vulnerable (Vu B1ab(i, ii, iii, iv, v)+B2ab(i, ii, iii, iv, v)).
The shape of the staminal bundles and the anthers, as well as the distinct foveola point to a relationship with Garcinia smeathmannii and Garcinia ovalifolia, both belonging to the section Rheediopsis Pierre (Jones, 1980). On the other hand, preliminary molecular data obtained by the second author suggest that Garcinia obliqua is not related to these species. Therefore, the results of an upcoming molecular study are awaited before a firm statement about the sectional position can be made.
(all from Gabon). CFAD de Rimbunan Hijau, au Sud-Ouest du Parc National de la Lopé, 0.64°S, 11.16°E (DD), 2/2/2009 (fr.), Dauby et al. 1570 (LBV, MO, BRLU). CFAD de Rimbunan Hijau, au Sud-Ouest du Parc National de la Lopé, 0.7°S, 11.23°E (DD), 27/2/2009 (fr.), Dauby et al. 1643 (BRLU). CFAD de Rimbunan Hijau, au Sud-Ouest du Parc National de la Lopé, 0.7°S, 11.23°E (DD), 28/2/2009 (ster.), Dauby et al. 1650 (BRLU). near Djidji, 5‒10 km W. of Koumémayong, 0°15'N, 11°50'E (DMS), 15/4/1988 (fl.), Breteler et al. 8738 (WAG). Est du Parc National de Waka, à plus ou moins 5 km au Sud de la rivière Mayi, 1.23°S, 11.28°E (DD), 4/6/2008 (ster.), Dauby et al. 677 (BRLU). Est du Parc National de Waka, à plus ou moins 5 km au Sud de la rivière Mayi, 1.23°S, 11.28°E (DD), 4/6/2008 (ster.), Dauby et al. 666 (BRLU). Bouvala hills, 1.62°S, 11.75°E (DD), 8/10/2007 (ster.), MBG transect (Leal et al.) 1105 (BRLU). Bouvala hills, 1.63°S, 11.78°E (DD), 12/10/2007 (ster.), MBG transect (Leal et al.) 1106 (BRLU). Village Eghuba, nord-ouest du Parc National de Waka, 1.03°S, 11.14°E (DD), 12/5/2008 (ster.), Ngombou Mamadou & Ndjombe 226 (LBV, MO).
urn:lsid:ipni.org:names:77122648-1
http://species-id.net/wiki/Garcinia_gabonensis
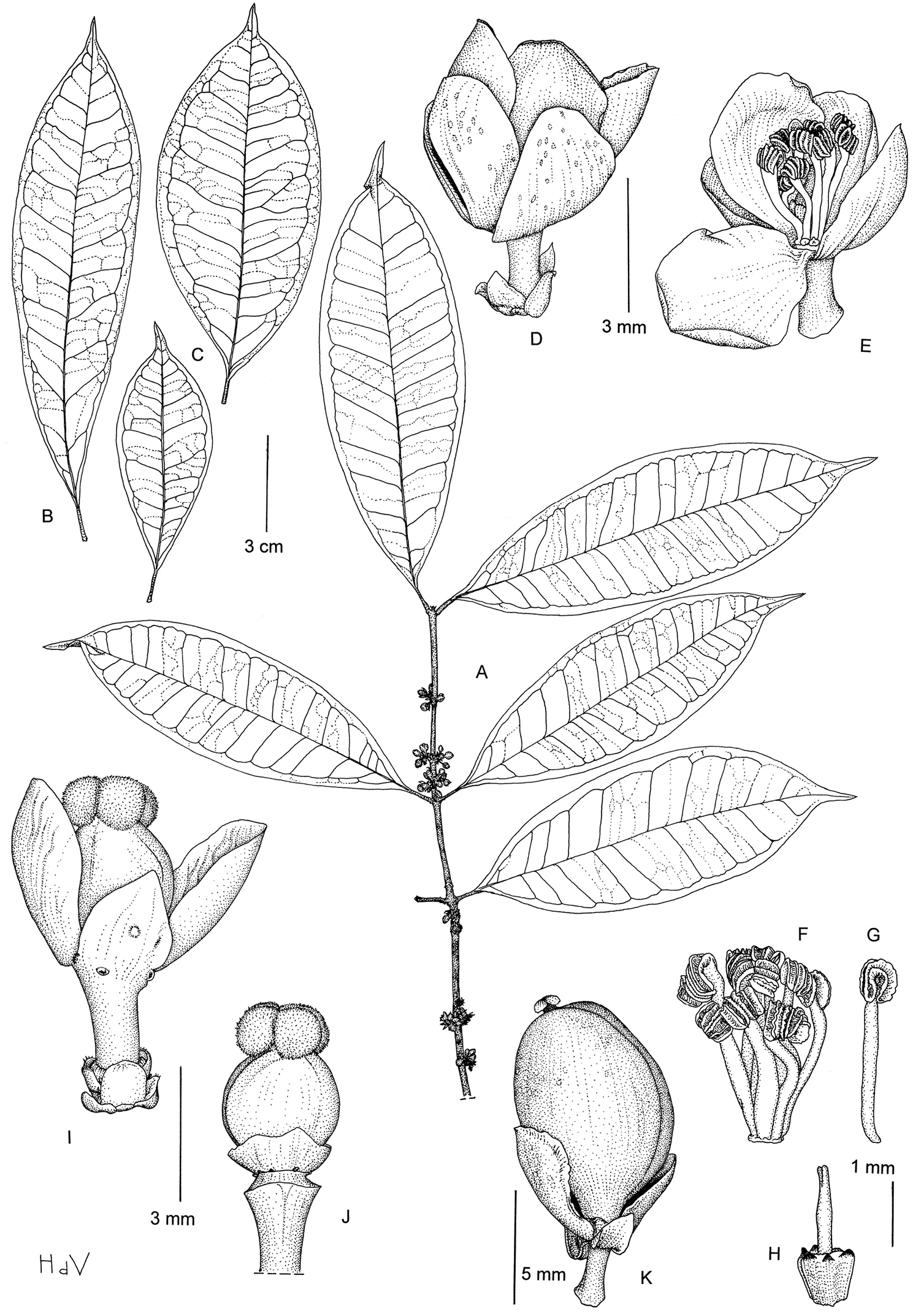
Fig. 2Similar to Garcinia kola, but leaves with lateral veins towards the margin clearly connected in distinct loops and united into an intramarginal vein that runs at (1‒)2‒3 mm from the margin, free stamens and a well-developed, longitudinally ribbed pistillode.
GABON: Ngounié, c. 36 km Mouila to Yeno, 1°45'S, 11°20'E, 19-9-1986, Breteler 7782 (holotype WAG!; isotype BR!, K!, LBV!, MO!, P!, PRE).
Dioecious shrub or small tree, up to 4 m high; latex transparent to greenish; branches circular in cross section, fissured, often reddish when dry; twigs flattened on cross section, smooth. Leaves opposite; petiole (4‒)5‒10(‒12) mm, smooth, slightly canaliculated above, with indistinct foveola of about 1 mm long; blade generally oblanceolate, sometimes elliptic or rarely ovate, (7‒)8‒15(–16) × (2–)2.5–5(‒6) cm, pointed at base, caudate-acuminate at apex, coriaceous to papery, glabrous; midrib prominent below, canaliculate above, lateral veins 7‒13 pairs, visible on both surfaces, towards the margin clearly connected in distinct loops and united into an intramarginal vein that runs at (1‒)2‒3 mm from the margin, tertiary veins laxly reticulate, indistinct; resin ducts normally indistinct except in young leaves, subparallel to the midrib. Inflorescence axillary, of few-flowered fascicles; bracts many, small (<1 mm long). Flower 4-merous, unisexual; pedicel slender, 2(‒3) mm; sepals obovate, two external ones about 2 mm long, two internal ones about 4 mm long, greenish to yellowish; petals obovate, about 4 mm long, yellowish or greenish to white. Male flower: stamens 8‒14, free, inserted in a ring around the pistillode, filament broadened and flat, white, anthers ellipsoid, strongly curved; pistillode broadly triangular-obovoid, longitudinally ribbed, stylode simple and slender, 1‒2 mm long. Female flower:disc annular, flattened and pressed against the ovary; ovary globose, about 3 mm in diameter; stigma peltate, lobed, 2 mm wide. Fruit ovoid to subglobose, 5‒11 mm in diameter, greenish, smooth, with persistent sepals at base.
Garcinia gabonensis: A Flowering twig B, C Leaves, showing the variation D Male flower E Idem, open and three petals removed F Androecium G Stamen H Pistillode I Female flower J Gynoecium and disk K Fruit. (A, D–H: Leeuwenberg & Persoon 13683; B, K: Arends et al. 510; C, I, J: Wieringa et al. 4546). Drawing by Hans de Vries, NCB Naturalis (section NHN) ©.
Endemic to southern and central Gabon, in the provinces of Moyen-Ogooué, Ngounié and Ogooué-Maritime (see Figure 3b).
Distribution of the two new species endemic to Gabon: (a) Garcinia obliqua, (b) Garcinia gabonensis.
Primary or late secondary terra firme rain forest, along rivers or on ridges; at ca. 150‒850 m altitude. Flowering in September to November, fruiting in September, November, December and February.
Currently, Garcinia gabonensis is known from eleven collections and nine locations. Estimates of the extent of occurrence and the area of occupancy are respectively ca. 16 000 km2 and 109 km2. Since nine of the eleven collections are within logging concessions or along main roads, we consider that continuing decline in the extent of occurrence, area of occupancy, quality of habitat and number of sub-populations has occurred or will occur in the near future. We therefore assign a preliminary status of Vulnerable (VU B2ab(i, ii, iii, iv)).
For now, it remains unclear as to which section this species belongs. Most striking feature are the free stamens. According to the elaborate work of
(all from Gabon). SE of Sindara, km 12 from Camp Chantier Waka to Ngounié River, 1°14'S, 10°51'E (DMS), 26/9/1985 (fl.), Leeuwenberg & Persoon 13683(BR, K, WAG). Moukabo, about 37 km E of Mouila, on the road to Yeno, 1°40'S, 11°20'E (DMS), 27/11/1984 (fl.), Arends et al. 484 (WAG). about 40 km E of Mouila, on the road to Yeno, 1°40'S, 11°20'E (DMS), 28/11/1984 (fr.), Arends et al. 510(BR, LBV, WAG). Massif du Chaillu, old secondary forest partly primary, near Mouyanama, about 27 km. E. of Mimongo, 1°39'S, 11°46'E (DMS), 25/11/1983 (fl.), Louis et al. 854 (K, WAG). Fougamou, 7 km on forestry road following Bendolo river, 1°12.1'S, 10°32.2'E (DDM), 26/10/1994 (fl.), Wieringa et al. 2916 (LBV, WAG). 10 km on the road Ikobey to Bakongue, Eghaba Mountain, 1°2.0'S, 10°2.6'E (DDM), 28/11/2001 (fl., fr.), Wieringa et al. 4473 (WAG). 5‒15 km NNW of Ndjolé, 0°5'S, 10°45'N (DMS), 13/11/1991 (fl.), Breteler 10445 (LBV, WAG). 13 km on the road Eteké to Ovala, Nyongué, 1°26.1'S, 11°26.1'E (DDM), 8/11/1994 (fl.), Wieringa et al. 3096(WAG). 60 km on the road Mouila to Yeno, 1°41.85'S, 11°23.96'E (DDM), 3/12/2001 (fr.), Wieringa et al. 4546 (LBV, WAG). Doudou mountains, about 60 km along exploitation track in WNW direction from Doussala, 2°12'S, 10°11'E (DMS), 27/11/1986 (fl.), Wilde J.J. de et al. 8984 (K, LBV, WAG). Massif du Chaillu, near Guédévé village about 40 km N of Lébamba, 1°55'S, 11°25'E (DMS), 30/11/1983 (fr.), Louis et al. 1056 (K, WAG). Est du Parc National de Waka, à environ 5 km au Sud de la rivière Mayi, 1°23'S, 11°3'E (DMS), 21/2/ 2008 (fr.), Dauby et al. 735 (LBV, MO, BRLU).
For most of the continental tropical African regions, an identification key to the species of Garcinia exists:
| 1 | Inflorescence very large, central axis often over 50 cm and up to 180 cm long, with several very long and unbranched ramifications of similar lengths, carrying distantly spaced clusters of small white sessile flowers; leaf blade (14‒)25‒57 cm long, shiny | Garcinia lucida Vesque |
| – | Inflorescence much smaller; flowers at least shortly pedicellate; leaf blade normally smaller, up to 28(‒35) cm long, shiny or not | 2 |
| 2 | Twigs angular, slightly or sometimes distinctly winged; petioles transversely wrinkled; latex white; flowers 5-merous, in compact racemes with a tetragonous rachis and imbricate bracts; fruit smooth or verrucose | 3 |
| – | Twigs rounded to angular; petioles smooth to transversely wrinkled; latex yellow or transparent; flowers 4-merous, in fascicles, cymes or solitary; fruit smooth | 6 |
| 3 | Twigs narrowly winged; pedicel up to 1.5 cm long (up to 2 cm in fruit); leaf blade 5‒21 × 2‒9.5 cm | 4 |
| – | Twigs strongly winged, wings 3‒5 mm wide; pedicel 3‒5.5 cm long; leaf blade (14‒)18‒41 × (4.5‒)6‒15.5 cm | Garcinia le-testui Pellegr. |
| 4 | Ovary and fruit verrucose; inflorescence almost strictly terminal, 1(‒3) racemes together of 1.5‒3 cm long | 5 |
| – | Ovary and fruit smooth; inflorescence terminal and axillary, often with several racemes together or racemes branched, these 2‒10 mm long | Garcinia densivenia Engl. |
| 5 | Bracts, pedicels and fruit minutely puberulous | Garcinia quadrifaria (Oliv.) Pierre var. chomocarpa (Engl.) Sosef & Dauby |
| – | Garcinia quadrifaria (Oliv.) Pierre var. quadrifaria | |
| 6 | Filaments fused, at least at base; leaf blade without intramarginal vein or with one that runs just inside (at 0.5‒1 mm) of the margin | 7 |
| – | Filaments entirely free; leaf blade with a distinct intramarginal vein running at (1‒)2‒3 mm from the margin | Garcinia gabonensis Sosef & Dauby |
| 7 | Staminal bundles with filaments partly free, at least at the top, and globose or ovoid anthers; leaf blade coriaceous | 8 |
| – | Staminal bundles with filaments completely fused and ellipsoid to oblong, curved anthers; leaf blade papyraceous to coriaceous | 13 |
| 8 | Leaf blade with distinct to very striking reticulations, green to brown in dry condition; sepals smooth to rugose; anthers 3‒20 per staminal bundle | 9 |
| – | Leaf blade with indistinct reticulations, brown-red when dry; sepals finely papillose; anthers very numerous | Garcinia conrauana Engl. |
| 9 | Pedicels and sepals glabrous; sepals smooth or slightly rugose; inflorescence axillary | 10 |
| – | Pedicels and sepals puberulous; sepals distinctly rugose; inflorescence terminal | Garcinia kola Heckel |
| 10 | Stamens 3‒10 per bundle; leaf blade with 15‒20(‒25) pairs of lateral veins; petiole distinctly transversely wrinkled; fruit symmetric | 11 |
| – | Stamens 11‒19 per bundle; leaf blade with 6‒9 pairs of lateral veins; petiole smooth to slightly transversely wrinkled; fruit oblique | Garcinia obliqua Sosef & Dauby |
| 11 | Leaves distinctly petiolate (petiole >4 mm long), with cuneate to rounded or seldom subcordate base | 12 |
| – | Leaves subsessile, with cordate and sometimes amplexicaulous base | Garcinia staudtii Engl. |
| 12 | edicel 1.5‒6(‒10) mm long; staminal bundles in male flower with 3(‒4) stamens; petiole 1‒2 mm thick; leaf blade 3‒15 × 0.5‒6 cm, usually long acuminate but the very top rounded | Garcinia ovalifolia Oliv. |
| – | Pedicel (10‒)15‒45 mm long; staminal bundles in male flower with (5‒)6‒10 stamens; petiole 2‒4 mm thick; leaf blade 8‒28(‒35) × 3, 5‒12(‒17) cm, rounded to tapering or acuminate towards the top, the very top usually acute | Garcinia smeathmannii (Planch. & Triana) Oliv. |
| 13 | Leaf blade with lateral veins making an angle of (45‒)60‒80° with the midrib | 14 |
| – | Leaf blade with lateral veins making an angle of 30‒45° with the midrib | Garcinia buchananii Baker |
| 14 | Leaf blade opaque or with continuous translucent resin canals | 15 |
| – | Leaf blade with translucent resin canals composed of dots and short lines | Garcinia punctata Oliv. |
| 15 | Leaf blade with a distinct acumen to gradually acuminate; with main lateral veins (3‒)4‒11 mm apart, the intermediate ones often clearly not reaching the margin; in dry condition resin canals running parallel to the lateral veins indistinct or invisible; petals white to yellow or yellowish green, not sticky; staminal bundles longer than the pistillode, anthers with septate or non-septate thecae; mature fruit yellow to orange | 16 |
| – | Leaf blade with a distinct acumen; main lateral veins 1‒2(‒3) mm apart, because the intermediate ones are almost equally strong and often reach the margin; in dry condition those resin canals running parallel to the lateral veins often distinct and prominent; petals red or orange-red or sometimes yellow, often sticky; staminal bundles as long as the pistillode, anthers with septate thecae; mature fruit orange to purplish red | Garcinia mannii Oliv. |
| 16 | Leaf blade with lateral veins almost straight, curved just before the margin to be united with an intramarginal vein; petals white to pale yellow or yellowish green; thecae not septate | 17 |
| – | Leaf blade with lateral veins gradually and distinctly curved up towards the margin and finally subparallel to it; petals yellow to pale green; thecae septate | Garcinia afzelii Engl. |
| 17 | Flowers and fruits on a 1‒4 mm long pedicel | Garcinia epunctata Stapf |
| – | Flowers and fruits on a 7‒18 mm long pedicel | Garcinia preussii Engl. |
http://species-id.net/wiki/Garcinia_arbuscula
CAMEROON. Mfongu, am Muti-abhang, 1700‒1900 m alt., Ledermann 5863 & 5943. Not located, probably lost at B, no duplicates traced yet.
The protologue states there are 20‒30 stamens in 4 bundles (so some 5‒8(‒9) per bundle), fused until halfway, and leaves similar to Garcinia ovalifolia, but with less pronounced veins and a cuneate base, on 1‒1.5 cm long petioles. Flowers are positioned in glomerules on the twigs, below the leaves. This description fits that of Garcinia smeathmannii and we momentarily place this name under that species.
http://species-id.net/wiki/Garcinia_danckelmanniana
CAMEROON. Genderogebirge, Tschape pass, 1420 m alt., Ledermann 2671 & 2750. Not located, probably lost at B, no duplicates traced yet, but a sketch of Ledermann 2750 is present at BM.
The protologue states there are 30‒40 stamens in 4 bundles, fused almost to the top, a distinct foveola, leaves with 12‒15 lateral veins and flowers in many-flowered bundles on the nodes. The sketch at BM shows large leaves (12–19 × 3.5–8 cm) with an acute apex and flowers on pedicels of 1.5–2 cm. All this fits Garcinia smeathmannii best, and for the moment we regard it as a synonym of that species.
http://species-id.net/wiki/Garcinia_laurifolia
SIERRA LEONE. Scott-Elliot 4806.
The type collection was located through the JSTOR Plant Sciences website (http://plants.jstor.org ) at BM. It was identified as a Rutaceae belonging to the genus Teclea. It shows a twig with alternate leaves and a single young fruit.
http://species-id.net/wiki/Garcinia_tschapensis
CAMEROON. Genderogebirge, Tschape pass, 1430 m alt., Ledermann 2771. Not located, probably lost at B, no duplicates traced yet, but a sketch of Ledermann 2771 is present at BM.
The protologue states the material concerns a fairly large tree (18‒22 m), with twigs soon rounded, rugose petioles of 1.5‒2 cm long with a distinct foveola, leaf blade coriaceous and shiny, male flowers 4‒7 in a fascicle, pedicel 2.5 cm, white petals, stamens 20, in 4 bundles, fused to halfway, alternating with verrucose disc lobes. The sketch at BM shows large elliptic-obovate leaves with slightly acuminate apex and a fasciculate inflorescence with three flowers on 22–28 mm long pedicels.
Again, this description, as well as the sketch, fit Garcinia smeathmannii and for now we regard it as a synonym of that species.
The following herbaria are kindly thanked for giving access and support to both authors: BM, BR, K, L, LBV, P. Herbarium material was kindly send on loan by MO. Dr. P. Bamps (BR) has very generously and freely shared his thoughts and partial manuscripts on West and Central African Garcinia, which was highly appreciated. The PhD project of G.D. was funded by the FRIA. His visit to WAG was funded by the European Commission’s Research Infrastructure action via the SYNTHESYS Project (application NL-TAF-1244). Field surveys carried out by G.D. in Gabon have been financed by the Communauté Française de Belgique, the Belgian Fund for Scientific Research (FRS-FNRS), the Fond Cassel and the United States Agency for International Development (USAID) through the Central Africa Regional Program for the Environment (CARPE). We are very grateful to the CENAREST, in particular the Herbier National du Gabon (IPHAMETRA), for permission to conduct research in Gabon and to the Central Africa program of Missouri Botanical Garden for logistic support. Tariq Stévart, Diosdado Nguema, Etienne Mounoumoulossi and Prince Bissiemou are acknowledged for their help during the field expeditions.