(C) 2012 Meghan McKeown. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Revwattsia fragilis (Watts) D.L. Jones (Dryopteridaceae), originally described as a Polystichum Roth by the pioneer Australian botanist Reverend W.W. Watts in 1914, is a rare epiphytic fern endemic to northeastern Queensland, Australia. Known from only a few populations, it is restricted to tropical rainforests in the Atherton Tablelands. We used the cpDNA markers psbA-trnH, rbcL, rbcL-accD, rps4-trnS, trnG-trnR, trnL-trnF, and trnP-petG to infer the relationships of Revwattsia fragilis within Dryopteridaceae. Based on our molecular analysis, we were able to reject Watts’s 1914 hypothesis of a close relationship to Polystichum. Its closest allies are a suite of Asian Dryopteris Adans. species including Dryopteris labordei, Dryopteris gymnosora, Dryopteris erythrosora and Dryopteris cystolepidota; maintaining Revwattsia renders Dryopteris paraphyletic. The epiphytic habit and distinctive long-creeping rhizome of Revwattsia appear to be autapomorphies and do not warrant its generic status. In the course of our investigation we confirmed that polyphyly of Dryopteris is also sustained by the inclusion of Acrorumohra (H.Itô) H.Itô, Acrophorus C.Presl, Arachniodes Blume, Diacalpe Blume, Dryopsis Holttum & P.J.Edwards, and Peranema D.Don. The epithet fragilis is occupied in Dryopteris, therefore we provide the name Dryopteris wattsii nom. nov. to accommodate Revwattsia fragilis in Dryopteris.
Biogeography, Australia, Morphology, Polystichum, Rumohra
The fern genera Polystichum Roth and Dryopteris Adans. are now understood to be closely allied members of the Dryopteridaceae. Polystichum and its allies Cyrtomium C.Presl and Phanerophlebia C.Presl are sister to Arachniodes Blume and Dryopteris (Schuettpelz and Pryer 2007). The breadth of morphological diversity exhibited by Polystichum and Dryopteris has, in hindsight, had at least three impacts on the taxonomic history of these genera and their family. First, a large number of segregate genera have been removed from these two genera based on dramatic morphological transformations. Some of these segregates render Dryopteris and Polystichum paraphyletic; examples include Sorolepidium Christ, which belongs in Polystichum (
The rare Australian monotypic genus Revwattsia D.L.Jones presents a similarly intricate history (Fig. 1A). A high-canopy epiphyte, Revwattsia fragilis (Watts) D.L.Jones (Dryopteridaceae) is endemic to northeastern Queensland, where it is known from only a few small populations (
Revwattsia fragilis. A habit B Rhizome in cross section C Abaxial rachis and costa D Adaxial rachis and costa (M Kessler, M Sundue and M Lehnert 14293).
Indeed, inclusion of Revwattsia in Polystichum is untenable morphologically. Long-creeping rhizomes, reniform indusia, and the epiphytic habit are not characteristic of Polystichum. The herbaceous dark-brown petiole scales of Revwattsia are unknown in Polystichum, which has pale scales or dark indurated petiole scales. The extensive glandular indument characteristic of Revwattsia (
Revwattsia presents a taxonomist’s classic dilemma; taxonomic placement requires a considered set of decisions about which morphological characters are synapomorphies and which are not. To address this dilemma, we assembled a set of chloroplast DNA nucleotide data from seven markers to infer the phylogenetic relationships of Revwattsia and provide insight into its morphological evolution. Included in our inquiry was a test of Jones’ 1998 assertion that Revwattsia fragilis requires a separate genus within the Dryopteridaceae. In order to understand implications of the taxonomic placement of Revwattsia fragilis, we also studied its critical morphological characters, namely those of the rhizome, indument, rachis and costa architecture, lamina segment shape, and indusium shape.
Methods MaterialRevwattsia fragilis was collected in the Cook District, Queensland, Australia, along the Mt. Lewis road, ca. 12 km before the shelter at the end of the rd. 16°36'S, 145°17'E, 900 m, M Kessler, M Sundue and M Lehnert 14293 (BRI, VT), 10 Aug 2011. Material for genetic analysis was stored in silica gel until DNA could be extracted. The permit used to collect this material was issued by Dept. of Environment and Resource Management Queensland (Michael Sundue, permit number WISP09438311).
MorphologyCharacters for Revwattsia fragilis were scored from M Kessler, M Sundue and M Lehnert 14293at The Pringle Herbarium (VT), and from previously published literature (
One-hundred and ninety-eight taxa from 36 genera were used in the phylogenetic analyses including 32 from the Dryopteridaceae. Taxonomic sampling was informed by an initial blast search of the Revwattsia fragilis rbcL sequence against the NCBI database (
Total DNA extraction from silica-dried specimens was accomplished following the CTAB protocol of
Sequences were edited and aligned using Geneious v5.4.2 (
Characteristics of the cpDNA markers used in the phylogenetic analyses.
| Marker | Model (AIC) | Aligned Length of Marker | % Parsimony Informative | Taxa sampled |
|---|---|---|---|---|
| rbcL | SYM+I+G (26503) | 1506 | 19% | 194 |
| trnG-trnR | TIM+I+G (16303) | 1290 | 40% | 100 |
| pbsA-trnH | TVM+G (4308) | 584 | 30% | 101 |
| rbcL-accD | GTR+I+G (9942) | 961 | 37% | 99 |
| trnL-trnF | GTR+G (4594) | 297 | 57% | 102 |
| rps4-trnS | TVM+G (7704) | 576 | 51% | 101 |
| trnP-petG | TIM+G (9370) | 623 | 50% | 99 |
Bayesian inference was conducted on the concatenated data set (psbA-trnH, rbcL, rbcL-accD, rps4-trnS, trnG-trnR, trnL-trnF, and trnP-petG) using MrBayes v3.2.0 (Ronquist et al. 2011) using the appropriate evolutionary models determined for each. Sampling of all seven loci was primarily within Dryopteris; the remaining taxa, including Revwattsia fragilis, had subsets of the seven loci. The Markov chain Monte Carlo permutation of tree parameters was conducted for 2 runs of 5, 000, 000 generations, sampling every 100th generation. A plot of generations versus log-likelihood was examined using Tracer v1.5 (
Parsimony analyses using the same data set were conducted using TNT (Willi Hennig Society,
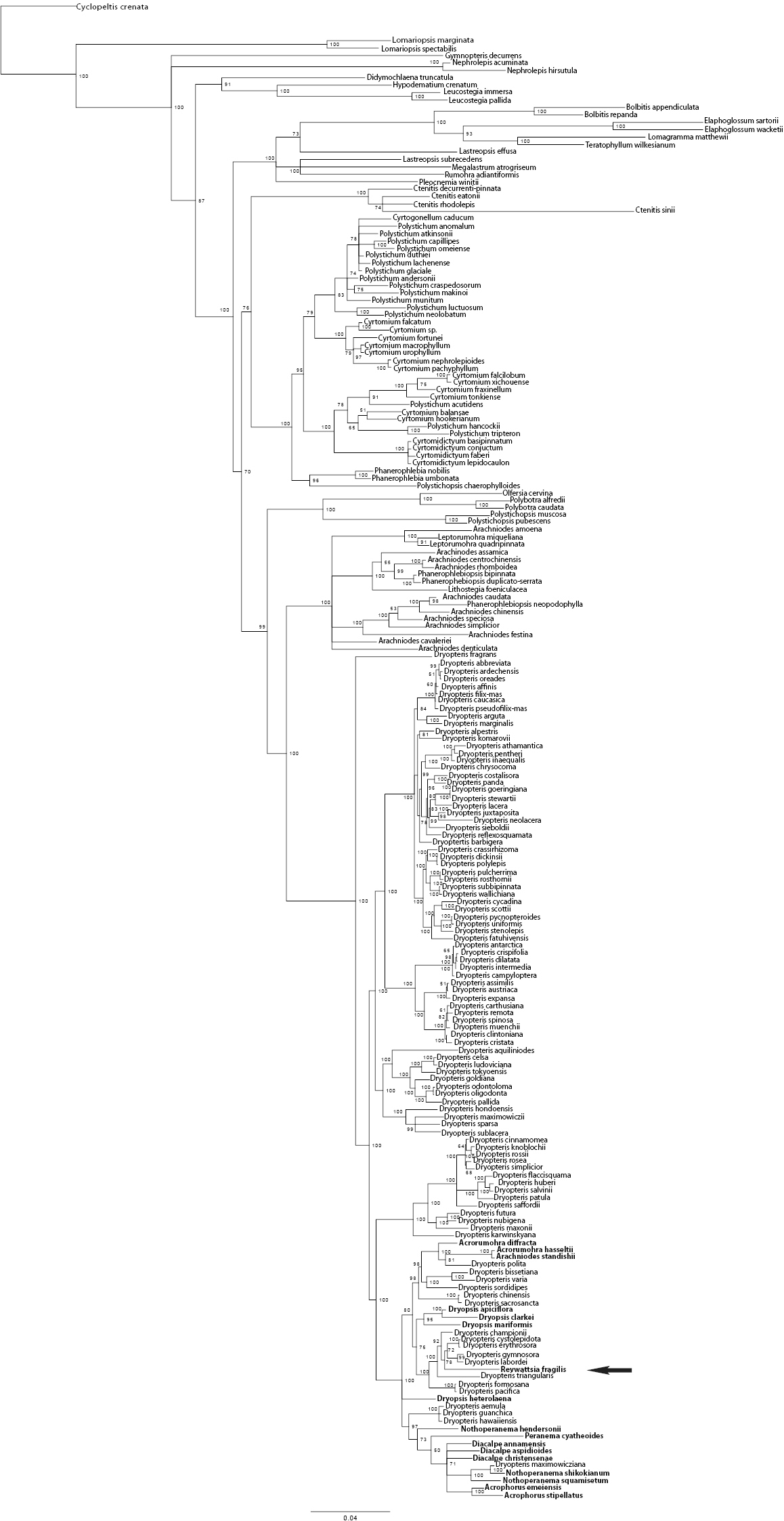
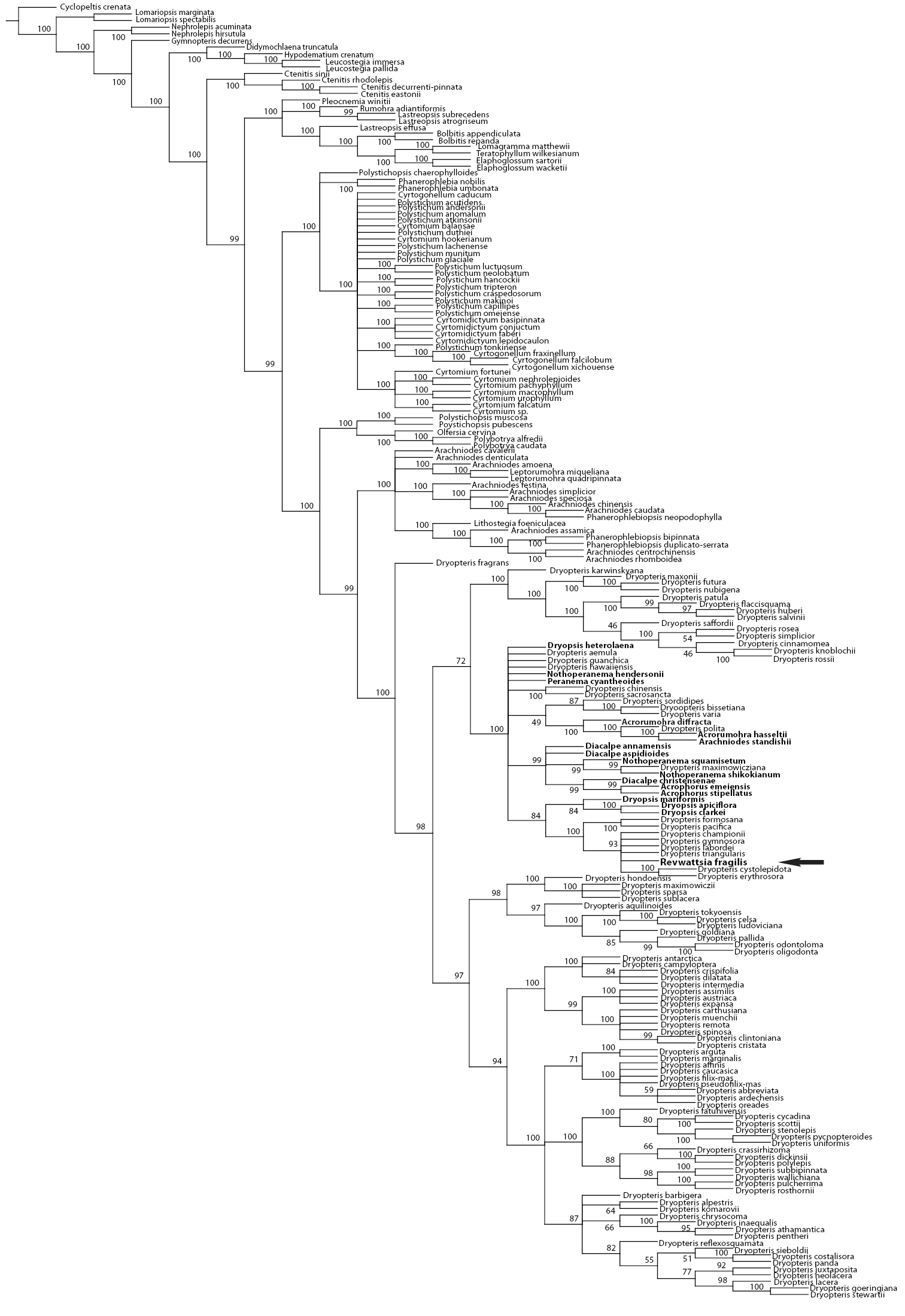
Of the 5425 total characters, 1717 characters (31.6%) were parsimony informative. In the maximum parsimony analysis (MP) 10, 000 most parsimonious trees were retained before maximum storage capacity was reached. The shortest trees had a length of 5531 steps, a consistency index (CI) of 0.40, and retention index (RI) of 0.79. The topology of the Bayesian inference (BI) 50% majority rule tree was largely congruent with the topology of the MP tree but allowed greater resolution of the taxa allied to Revwattsia fragilis. Results of the BI and MP analyses place Revwattsia fragilis in a recently diverged clade within the genus Dryopteris (Figures 2 and 3).
The 50% majority rule tree resulting from Bayesian analysis. Values indicate posterior probabilities, scale bar indicates 0.04 substitutions per site. Arrow indicates position of Revwattsia fragilis.
In both analyses, there is strong support for placement of Revwattsia fragilis within a clade of Dryopteris comprising species from southern and eastern Asia. In the Bayesian analysis, Revwattsia fragilis is sister to the clade comprising Dryopteris cystolepidota, Dryopteris erythrosora, Dryopteris gymnosora (Makino) C.Chr., and Dryopteris labordei (Christ) C.Chr. (78% posterior probability). This clade in turn is sister to Dryopteris championii (92% posterior probability), followed by Dryopteris triangularis Herter(100% posterior probability). These same taxa form a clade in the MP analyses (93% bootstrap support), but relationships between these taxa collapse in the strict consensus of all most parsimonious trees.
Morphological assessmentRevwattsia fragilis exhibits a massive (3 cm diam.) long-creeping rhizome with dorsal leaves and ventral roots (Figure 1A). A rhizome cross-section revealed an elongate ventral meristele (Figure 1B arrow). The rhizome and basal petiole are densely provided with thin, dark brown attenuate scales. The rachis and costa are rounded abaxially (Figure 1C), and are shallowly grooved adaxially (Figure 1D). The grooves are shallowly continuous with the next-order axis (Figure 1D) and they lack a central ridge. These axes are densely provided with short capitate-glandular hairs (Figure 1D). Frond dissection is 2-pinnate-pinnatifid to 2-pinnate-pinnatisect with symmetrical (neither basiscopically nor acroscopically enlarged) pinnae and pinnules (Figure 1A). Fertile fronds have medial sori and light brown reniform indusia.
Discussion Monophyly of DryopterisResults presented here demonstrate that the monotypic genus Revwattsia is nested within Dryopteris (Figures 2 and 3). Maintaining Revwattsia renders Dryopteris paraphyletic; we therefore recommend placing the monotypic Revwattsia in synonymy under Dryopteris.
Strict consensus of 10, 000 most parsimonious trees. Values indicate bootstrap support of 1000 pseudoreplicates. Arrow indicates position of Revwattsia fragilis.
Paraphyly of Dryopteris is further perpetuated by the inclusion of the sampled Acrophorus (two species), Acrorumohra (two species), Arachniodes standishii (T. Moore) Ohwi, Diacalpe (three species), Dryopsis (three species), Nothoperanema (three species), and Peranema cyatheoides D. Don. These results do not come as a surprise given the results of other recent phylogenetic studies (
Our assessment of morphological characters largely corroborates those of
The long-creeping rhizome and elongate ventral meristele of Revwattsia (the latter first demonstrated here, Fig. 1B) are distinctive autapomorphies. Although a long-creeping rhizome is known to occur in Dryopteris amurensis and Dryopteris angustifrons, neither is closely allied to Revwattsia fragilis. These two characters occur in combination sporadically in Eupolypods I (e.g., in Lomariopsis Fée (
Biogeographic patterns in Dryopteris were recently examined by
The phylogenetic position of species treated as Acrophorus, Acrorumohra, Arachniodes standishii, Dryopsis, Nothoperanema, Peranema, and Revwattsia fragilis demonstrate that the circumscription of Dryopteris needs to be expanded. Several of these genera include unique character states that do not occur in Dryopteris as currently defined. In addition to the morphological redefinition, expansion of Dryopteris to include these segregate genera necessitates numerous nomenclatural innovations. We provide here a name for Revwattsia fragilis in Dryopteris. The name Dryopteris fragilis is previously occupied; therefore a new name is provided.
Taxonomy and nomenclatureDryopteris wattsii, M. McKeown, Sundue, & Barrington nom. nov. ≡ Polystichum fragile Watts, Proc. Linn. Soc. New South Wales 39: 775. 1914 (1915). ≡ Revwattsia fragilis (Watts) D.L.Jones [as “Revwattsia fragile”], Flora of Australia 48:711. 1998. non Dryopteris fragilis C. Chr. TYPE: Australia, Queensland, Majors Homestead, near Ravenshoe, W. W. Watts s.n., Aug 1913 (Syntypes: BRI n.v., MEL n.v., NSW n.v.).
The authors thank Michael Kessler and Marcus Lehnert for assistance with field work, and Darren Crayn and Frank Zich for assistance at CNS. We thank Ashley Field for helping us to locate a population of Dryopteris wattsii. This research was funded in part by NSF DEB-1119695. The authors thank the two anonymous reviewers for their constructive and helpful comments.
GenBank accession numbers for McKeown et al. 2012 (doi: 10.3897/phytokeys.14.3446.app) File format: Adobe PDF file (PDF).
Explanation note: Genbank Accession numbers are listed in the following order: psbA-trnH, rbcL, rbcL-accD, rps4-trnS, trnG-trnR, trnL-trnF, trnP-petG. The “—“ indicates markers that were not available for the taxon.