(C) 2012 Norbert Kilian. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
It is shown that the concept of the Iranian endemic Lactuca polyclada in the sense of both its original author Boissier and its current use actually admixes two entirely different species, as was first noted by Beauverd a hundred years ago but has been neglected by later workers. One is a putative relative of Lactuca rosularis, the other was recognised by Beauverd as a member of the genus Cicerbita. The name Lactuca polyclada Boiss. is lectotypified here, maintaining its use as established by Beauverd for the Cicerbita species. Both species are morphologically delimited and mature achenes of Cicerbita polyclada are illustrated for the first time. The putative relative of Lactuca rosularis, a rare local endemic of the summit area of Kuh e-Dena, which has remained without a valid name by now, is described as a new species, Lactuca denaensis N. Kilian & Djavadi, and illustrated. A third member of the Lactuca rosularis group, Lactuca hazaranensis Djavadi & N. Kilian, discovered among a recent collection and apparently being a rare chasmophyte of the Hazaran mountain massif in the province of Kerman, Iran, is described as a species new to science, illustrated and delimited from the other two species. This new species has peculiar achenes representing a hitherto unknown variant: the body of the beaked achenes is divided into two segments by a transversal constriction in the distal third. The proximal segment contains the embryo, the distal segment is solid with a lipid-containing yellow tissue. The easily detachable pappus and the equally easily detachable beak potentially obstruct dispersal by wind. Since detachment of the beak also exposes the lipid-containing tissue of the distal segment, its potential as an elaiosome and myrmecochory as a possible mode of dispersal are discussed.
Compositae, Asteraceae, Cichorieae, Lactuca rosularis group , Lactuca denaensis, Lactuca hazaranensis, taxonomy, Iran, Kerman, Hazaran Mts, Zagros Mts, carpology, dissemination, elaiosome, myrmecochory
Identification of a collection of a Cichorieae species, made by the third author together with A. Torabi in August 2010, in Kerman Province in the vicinity of the famous waterfalls near the city of Rayen on the eastern foot of Mt Hezar, revealed that it apparently represents a hitherto unknown lactucoid species. The species has strikingly unusual achenes, which show a transversal constriction in the distal third. Morphological comparison and evaluation of its affinities led to the discovery of another still unnamed species among the putatively related species.
The lactucoid genera, which form the subtribe Lactucinae, have a worldwide distribution in the northern hemisphere but extend also into the southern hemisphere in Africa and comprise about 230 species (
This paper (a) gives the description and delimitation of hitherto unknown or neglected plants, respectively, as two species new to science, (b) clarifies their morphological affinities among the lactucoid species, and (c) considers the peculiar achene morphology found in one of the two species with respect to its possible function for fruit dispersal.
Material and methodsThe study is based on herbarium material of the Herbarium of the Iranian Research Institute of Plant Protection (IRAN) and of the Herbarium of the Botanic Garden and Botanical Museum Berlin-Dahlem (B) as well as on digitised type material from the herbaria of G, M, MO, P, WAG (herbarium abbreviations according to
The authors observed all morphological data presented and used in the description and comparison of the new species on the herbarium material cited in the text under the new species or in the Appendix, respectively. Micromorphological features were examined under a WILD M5 optical reflected-light microscope. Documentation of morphological features was done with an Olympus DP72 digital camera mounted on an Olympus SZX16 stereo zoom optical reflected-light microscope equipped with the Olympus analySIS docu software.
Light microscopic histochemical analysis of the achene tissue to test the presence of lipids was performed using Sudan III staining following
urn:lsid:ipni.org:names:77118676-1
http://species-id.net/wiki/Lactuca_denaensis
Fig. 1, 3C ;Habitually similar to Lactuca rosularis but clearly distinguished by the rosette leaves being undivided (instead of lyrately to irregularly pinnatifid to pinnatisect), the involucre being 10–12 mm (instead of 6–9(–10) mm) long, the achenes having a 4–5 mm (instead of 2.2–3 mm) long corpus, a 0.4–0.9(–2) mm (instead of (1.1–)3–5.3 mm) long beak and a 5–6 mm (instead of 2.5–3 mm) long pappus.
[Iran, Kogiluyeh & Boyer Ahmad], in glareosis alpis Kuh-Daëna, fl. lutescens, 14 Jul 1842, Kotschy Pl. Pers. autral. 662 (G-BOIS G00330211, specimen annotated by
Perennial rosulate herb, with a woody subterranean caudex, rosette shoots often on vertical subterranean axes vested with cataphylls below the sometimes somewhat elongate rosette, acaulescent to usually very shortly caulescent and less than 5 cm tall (Fig. 1A), rarely to c. 15 cm. Stem one per leaf rosette, usually not or little projecting above the leaf rosette. Rosette leaves (Fig. 1A) obovate to spatulate, tapering towards base, 2–7 × (1–)1.5–3.2 cm, somewhat glaucous; base semiamplexicaul, margin ± densely dentate and denticulate, apex rounded to, more rarely, subacute. Synflorescence corymbosely paniculiform, condensed through conspicuously short axes, of c. 6–20 capitula (Fig. 1A, G); peduncles 0.4–0.7 cm long. Capitula with c. 10–14 flowers. Involucre (Fig. 1B–F) narrowly cylindric at anthesis, 10–12 mm long, not elongating during maturation; outer phyllaries imbricate, outermost ± narrowly ovate, c. 2 mm long, following ones gradually longer, lanceolate, the longest up to c. 1/2 as long as inner ones; inner phyllaries linear-lanceolate, 7–8, ± in one row, subequal in length, with ± narrow scarious margin. Receptacle flat to slightly convex, naked. Flowers with corolla yellow, ligule 6.7–7.5 mm, tube 5–5.5 mm long; anther tube without appendages 3.2–3.5 mm, basal appendages c. 0.4–0.6 mm, apical appendages 0.3–0.4 mm long; style arms 2.8–3.2 mm long. Achenes (Fig. 1G, 3C) homomorphic, including beak 4.8–5.7 mm long; corpus 4–5 mm long, up to 1.1–1.3 mm in diam., slender-obovoid, compressed, apex contracted into a stout, easily detachable beak c. 0.4–0.9(–2) mm long; achene body apart from the two lateral ribs with 1 similarly strong median rib on either side, rarely dorsally with 2 equally strong ribs, secondary ribs missing; achene surface ± smooth, brown. Pappus simple, without an outer series of minute hairs, setae thin, white, 5–6 mm long, persistent. – Flowering and fruiting: July to September.
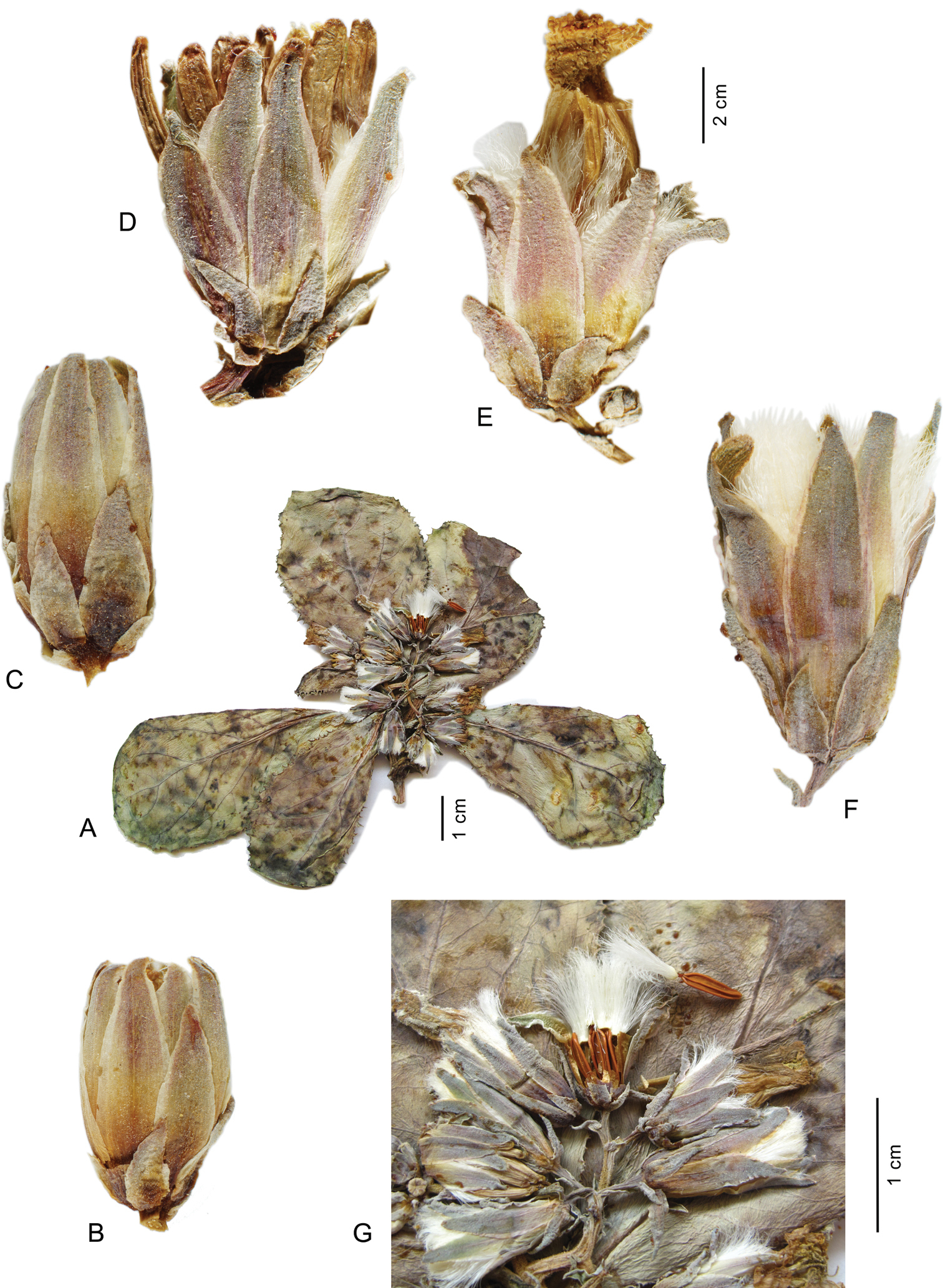
Lactuca denaensis – A habit, acaulescent form B–F capitula, all to the same scale, before flowering (B–D), with faded flowers (E), after flowering (F); G details of synflorescence, one capitulum at fruiting. – All from Remaudière, 5252E (IRAN 10625)
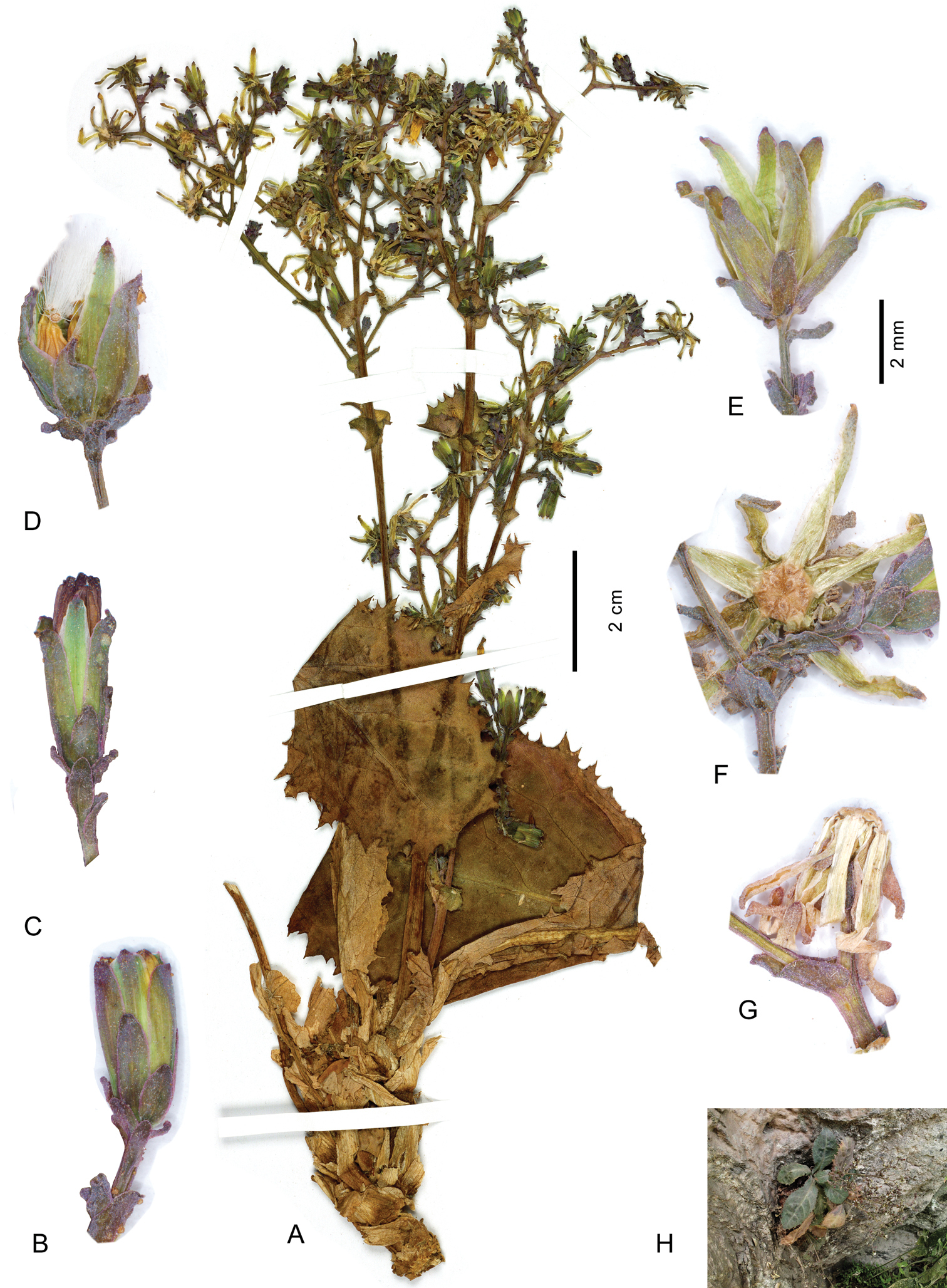
Lactuca hazaranensis – A habit, left plant of holotype sheet B–G capitula, all to the same scale, before flowering (B–C), at fruiting (D–E), after fruiting (F–G); H plant in natural habitat A–G from the holotype H plant in the natural habitat at the type locality; photograph by M. Eskandari, 10 Aug 2010.
According to Art. 7.11 of the Vienna Code, Beauverd’s restriction of the name to Kotschy 603 does not, however, constitute a lectotypification, because he did not use “the term ‘type’ (typus) or an equivalent” (
The second species admixed by Boissier under the name Lactuca polyclada with a usually very short or almost missing flowering stem, yellow flowers, a pappus without an outer row of minute hairs and the achene anatomy as illustrated by
As far as we know, Lactuca denaensis is restricted to the higher elevations, probably above 3000 m, of Kuh e-Dena in the Zagros mountains of SW Iran (Fig. 4). From the rare material with subterranean parts preserved (e.g. on the sheets MO 6264530 and P 00750252 with isotypes), showing a subterranean caudex producing several cm long shoots vested with cataphylls below the leaf rosettes, it can be concluded that the species is a scree plant.
Additional specimen seen: Iran. Kogiluyeh & Boyer Ahmad: Dena [c. 30°56'N, 51°28'E], 3300 m, Sep 1955, Remaudière 5252E (IRAN 10625).
Lactuca denaensis is named after the Kuh e-Dena massif of the Zagros mountains, where the only two collections known to us come from.
Morphological comparison shows that Lactuca denaensis is most similar to Lactuca rosularis. Both share the rosulate habit with a woody caudex, glaucous leaves and small yellow-flowered capitula but also the compressed, beaked achenes (compare Fig. 3B and C), characterised by 4 (only exceptionally 5) similar and strongly prominent main ribs (two lateral and one median on either side, exceptionally dorsally 2) and a simple pappus without an outer series of minute hairs (see also Table 1).
Achenes of the Lactuca rosularis group and Cicerbita polyclada – A, F–I Lactuca hazaranensis; complete achene (A); achene after loss of easily detachable beak, dorsal view (F); ventral view (G); longitudinal section of achene showing two segments, proximal large segment with embry, distal small segment solid with yellow tissue (H); embryo (I); B Lactuca rosularis C Lactuca denaensis D–E Cicerbita polyclada, complete achene (D) and longitudinal section of distal portion showing apical cavity (E). – A–D and F–H each to the same scale A, F–I from the holotype at IRAN B from Rechinger 55980 B, C from Kotschy 662 (B 100426936) D–E from Strauss 14091 (B 100312952)
Lactuca denaensis is known only from the higher elevations of the Kuh e-Dena massif, which is part of a Protected Area, possibly also including the populations of the species. The species seems to be rare, since only two collections are known to us, but it has to be taken into account that it is rather inconspicuous. Lactuca denaensis must currently be assessed as Data Deficient (
urn:lsid:ipni.org:names:77118677-1
http://species-id.net/wiki/Lactuca_hazaranensis
Fig. 2, 3A, F–IHabitually similar to Lactuca rosularis but clearly distinguished from it by the rosette leaves being undivided (instead of lyrately to irregularly pinnatifid to pinnatisect), the peduncles being distinct, c. 0.3–0.7(–1) cm long (instead of indistinct, 0–0.2 cm long), the achenes having a 0.3–0.5 mm (instead of (1.1–)3–5.3 mm) long beak and the achene body being transversally constricted and sectioned in the distal quarter into an embryo-containing proximal and a solid distal segment (instead of being unconstricted and unsectioned).
Iran. Kerman: Rayen, near Rayen falls [c. 29°33'N, 57°18'E], 2850 m, 10 Aug 2010, M. Eskandari & A. Torabi(IRAN 55199; photo at B).
Perennial rosulate herb (Fig. 2A), with a taproot(?) and a woody caudex covered by the marcescent remains of old leaf bases. Stem one or a few per leaf rosette, erect, (2–)10–18 cm tall, branched already in lower half. Rosette leaves obovate to spatulate, (2–)5–11 × (1–)2–5 cm, somewhat glaucous; base semiamplexicaul, margin densely, coarsely and ± irregularly dentate-denticulate, apex subacute to acute. Lower and middle stem leaves spatulate to lanceolate, with auriculately clasping base, smaller, otherwise similar to rosette leaves; upper stem leaves distinctly smaller than lower and middle ones, lanceolate to ovate, with conspicuously auriculately clasping base, margin usually entire, apex acute to acuminate; uppermost stem leaves bractlike. Synflorescence (Fig. 2A) of a stem corymbosely paniculiform, with some to many capitula, axes wiry; peduncles c. 0.3–0.7(–1) cm long, capillaceous. Capitula with 7–14 flowers. Involucre (Fig. 2B–G) narrowly cylindric at anthesis, 6–7 mm long, not elongating during maturation; outer phyllaries imbricate, outermost ovate to narrowly ovate, 1.5–3 mm long, similar to the bracts on the peduncle, following ones gradually longer and ovate-lanceolate to lanceolate, the longest up to c. 1/2 as long as inner ones; inner phyllaries linear-lanceolate, 6–9, ± in one row, somewhat unequal in length, with ± narrow scarious margin. Receptacle flat to slightly convex, naked. Flowers with corolla yellow, ligule 5.5–6.5 mm long, tube shorter than ligule; anther tube without appendages 1.6–2 mm, basal appendages c. 0.4 mm, apical appendages 0.4 mm long; style arms 1.6–1.8 mm long. Achenes (Fig. 3A) homomorphic, including beak 3.2–3.6 mm long, corpus 2.9–3.2 mm long, up to 0.8–1.1 mm in diam., ellipsoidal, compressed, with a transversal constriction of 0.4–0.8 mm diam. in the distal 1/3–1/4, distal segment c. 1.5 × 1.5 mm, contracted into a stout, easily detachable beak c. 0.3–0.5 mm long; achene body (Fig. 3F–I) apart from the two lateral ribs with 1 similarly strong median rib on either side, rarely dorsally with 2 equally strong ribs, secondary ribs missing or rarely 1–2 per side; achene surface faintly transversally wrinkled, proximal segment brown, distal segment and beak yellowish, ribs straw-coloured to yellowish in distal segment; proximal segment containing the whitish embryo (Fig. 3H–I), distal segment containing yellowish tissue (Fig. 3H). Pappus simple, without an outer series of minute hairs, setae thin, white, 3–3.5 mm long, easily detachable. – Flowering and fruiting: June to August.
The type collection of Lactuca hazaranensis comes from the northeastern foot of Mt Hezar, which rises to 4465 m elevation, and has been collected in the vicinity of the Rayen falls, at an altitude of 2850 m, in rock crevices. A second collection, with mature achenes of the precisely the same variant, was traced in the Berlin herbarium and had been made by J. Bornmüller in 1892 some 50 km further NW on rocks at an altitude of 3700 m on Mt Jupar (c. 29°55.8'N, 57°11.5'E; spelled “Khu-i-Dschupar” by Bornmüller, see also
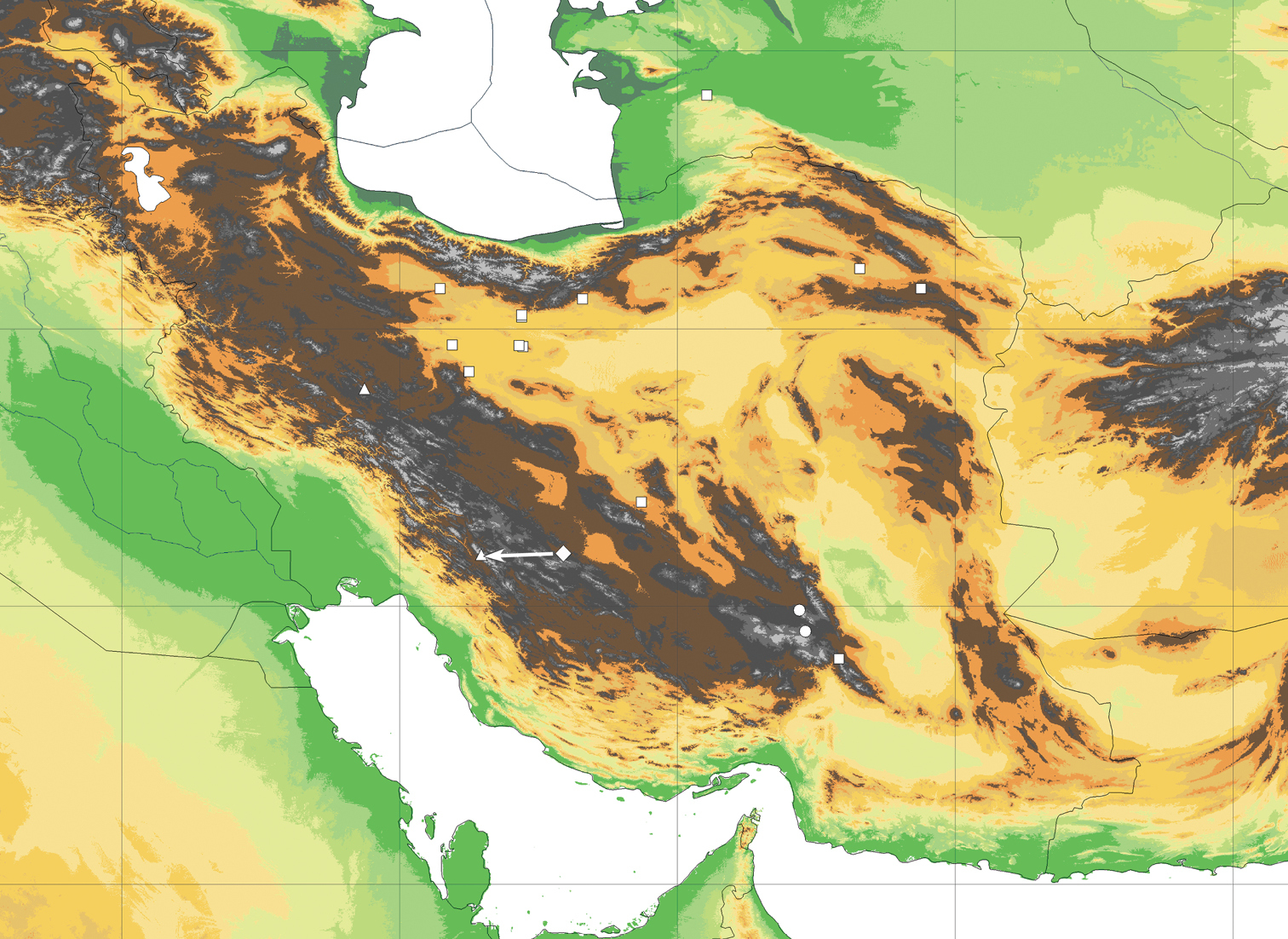
Distribution of Lactuca hazaranensis (circles) and the related species Lactuca rosularis (squares) and Lactuca denaensis (rhomb, actucal position as indicated by arrow), as well as of Cicerbita polyclada (triangles). – Georeferenced map based on the known collections (see Appendix and supplemented by collection cited in
Additional specimen seen: Iran. Kerman: in rupibus summi jugii m. Kuh-i-Dschupar [Kuh-i-Jupar, c. 29°55.8'N, 57°11.5'E], 3700 m, 13 Jun 1892, J. Bornmüller 4119 (B).
Lactuca hazaranensis is named after its provenance, the Hazaran mountain massif in the Iranian province of Kerman, which is a southeastern outlier of the Zagros mountain range and reaches a maximum elevation of about 4500 m in the peak Kuh-e Hazar.
. Morphological comparison revealed that the new species is most similar to both Lactuca rosularis and Lactuca denaensis. The three species are perennial rosette herbs of montane to high-montane environments, which are most likely closely related to each other, and considered here as the Lactuca rosularis group. They are all rare, being known from few collections only, and are endemic or almost so to the Iranian Highlands (Fig. 4). They share the rosulate habit with a woody caudex, the glaucous leaves, small yellow-flowered capitula and the principally same achene morphology. The differences between these three species are summarised in Table 1.
Morphological differences between Lactuca hazaranensis and its relatives Lactuca rosularis and Lactuca denaensis, as well as between the latter and Cicerbita polyclada. Based on the material studied. – The investigated material is listed in the Appendix.
| Features | Lactuca hazaranensis | Lactuca rosularis | Lactuca denaensis | Cicerbita polyclada |
|---|---|---|---|---|
| Rosette leaves: division | undivided | lyrately to irregularly pinnatifid to pinnatisect with large terminal lobe | undivided | undivided, towards base sometimes shallowly pinnately divided |
| Rosette leaves: margin | coarsely and ± irregularly dentate and denticulate | irregularly dentate and denticulate | ± densely dentate and denticulate | subentire to dentate and denticulate |
| Branching of stem | with dominating main axis, corymbosely paniculiform | with dominating main axis, paniculiform to corymbosely paniculiform | subacaulescent, with dominating main stem only few cm long or branched from base, corymbosely paniculiform |
± without dominating main axis, repeatedly divaricately branched from base |
| Branches | slender to capillaceous | ± slender (to capillaceous) | slender to capillaceous | conspicuously inflated |
| Penduncles, length [cm], shape | c. 0.3–0.7(–1), capillaceous | 0–0.2, capillaceous if developed | 0.4–0.7, capillaceous | 1–3.5, inflated if well developed |
| Cauline leaves | usually present, gradually reduced to bracts | basally present, soon reduced to bracts | usually absent, reduced to bracts | absent |
| Involucre, length [mm] | 6–7 | 6–9(–10) | 10–12 | 7–9 |
| Corolla, colour | yellow | yellow | yellow | blue |
| Achene, length [mm] | 3.2–3.6 | (3.8–)6–8 | 4.8–5.7 | 3.4–5.2 |
| Achene: corpus, length [mm] | 2.9–3.2 | 2.2–3 | 4–5 | 3–4.2 |
| Achene: ribs | 4(–5), strongly prominent | 4, strongly prominent | 4(–5), strongly prominent | 5, subprominent to subdistinct |
| Achene: beak, length [mm] | 0.3–0.5 | (1.1–)3–5.3 | 0.4–0.9(–2) | 0.5–1 |
| Achene: transversal constriction | present | absent | absent | absent |
| Pappus, length [mm] | 3–3.5 | 2.5–3 | 5–6 | 3–4 |
| Pappus: outer ring of minute hairs | absent | absent | absent | present |
Lactuca hazaranensis is known only from two localities c. 50 km apart, which are not in protected areas. The species seems to be rare, but it has to be taken into account that it is rather inconspicuous in its rocky environment. Members of the tribe are among the most favoured food of livestock, grazed wherever in reach and are therefore particularly threatened by overgrazing. Lactuca hazaranensis must currently be assessed as Data Deficient (
The shape of the achenes of Lactuca hazaranensis with the transversal constriction in the distal third (Fig. 3A, F–H) is curious. The fact that all achenes of all fruiting heads in two collections from different localities and centuries invariably show the same morphology, rules out the possibility that this achene variant represents a teratogenic manifestation.
Conspicuous transversal constrictions are, as far as we know, a very rare phenomenon in Asteraceae fruits. The present case is parallelled, however, by a few Pulicaria species of the Horn of Africa and southern Yemen (
In contrast to the cases in Pulicaria, where the constrictions chiefly affect the pericarp (
Conspicuous segmentation of the achene, although not precisely by a transversal constriction, is otherwise known from the probably unique case of the bispecific genus Urospermum (Cichorieae, Hypochaeridinae): the achenes of this genus consist of a proximal, compressed segment, which contains the embryo, and a larger, inflated distal segment tapering into the beak (for images, see Urospermum picroides in
A morphological transition towards a segmentation might perhaps be an achene with a cavity below the beak as it can be observed, e.g. in Cicerbita polyclada (Fig. 3D–E). Within subtribe Lactucinae, the achenes of Cephalorrhynchus polycladus (Boiss.) Kirp., not to be confused with the habitually similar Cicerbita polyclada, might represent an even stronger morphological transition (pers. com. A. Sennikov, Feb 2012); the presumably empty apical achene portion below the beak is, as stated by
Being shortly beaked and provided with a pappus, the achenes of Lactuca hazaranensis appear principally suited for wind dispersal (anemochory). The entire beak is, however, detachable at its base from the distal segment of the achene by slightest pressure and also the pappus setae are very easily detachable and are thus not functional for dispersal by wind. Finally, the solid distal segment brings additional weight and thus impedes wind dispersal.
Light microscopic histochemical analysis, using Sudan staining, of the yellow tissue of the distal segment of Lactuca hazaranensis revealed abundant presence of lipid drops, which were similarly found also in the embryo. Lipids are well known in the embryo of Lactuca as a major component of the reserves for the germination, accounting for 33 % dry weight of the achene in lettuce (Lactuca sativa) (
Their spatial separation from the embryo makes it unlikely that the lipid reserves of the distal segment are related to the germination. A potential function for the dispersal of the achene seems more probable, in particular in connection with the easy detachment of the achene beak. The detachment of the beak has not only a potentially atelechorous effect but the rupture also exposes the lipid reserves of the distal segment. The distal segment could thus perhaps be an “elaiosome”, a structure developing from seed or fruit tissue and aiding diaspore dispersal by ants (myrmecochory) in that it both attracts and rewards them (
Usually, elaiosomes develop in Asteraceae at the base of the achenes from tissue separating the achene from the receptacle. In this way, the elaiosome is separated from the embryo by the indurate pericarp, which hinders the ants to get access to the embryo in the interior. The development of an elaiosome at the apex of the achenes and inside the pericarp appears in this context much less favourable. Apart from the presumably higher cost of this solution, the constriction only incompletely locks off the lipid reserves from the embryo, with the risk of its damage. In case the hypothesis of the myrmecochorous property of the distal achene segment of Lactuca hazaranensis is confirmed, e.g. by an experimental approach such as exemplified by
The unparallelled and, so far as we know, transition-free occurrence of the transversally constricted achenes in the Lactuca rosularis group, is, independently of its potential function, a particularly striking evidence for a considerable developmental plasticity in achene features in the Lactucinae.
We are most grateful to Dr Laurent Gautier (Conservatoire et Jardin botaniques de la Ville de Genève) for his instantaneous invaluable help with the material in G-BOIS and G, to Dr Julia Lazar-Schurreit (Dahlem Center of Plant Sciences, Institute of Pharmacy, Pharmaceutical Biology, Freie Universität Berlin) for guiding the histochemical analysis, to Monika Lüchow for her assistance with the photographic documentation, Michael Rodewald for the preparation of the photographic plates, Prof. H. Walter Lack for reading a previous version of the manuscript, Prof. Werner Greuter for nomenclatural advice (all Dahlem Center of Plant Sciences, Botanic Garden and Botanical Museum, Freie Universität Berlin) and to Alexander Sennikov and an anonymous reviewer for their constructive comments on a previous version of the manuscript.
Specimens of related species investigated
Lactuca rosularis
Iran. Khorasan: prope Robat-e-Safid inter Mashad et Torbat-e-Heydariyeh, [c. 35.72°N, 59.38°E], 1800–2000 m, 29 May 1977, Renz & Runemark in Rechinger 55980 (B). Semnan: Momen-abad [c. 35°32.5'N, 53°17.5'E], 1100–1150 m, 26 Jun 1992, Termeh, Moussavi & Tehrani (IRAN 54459). Tehran: 13 km E Eyvanki, [35°20.9'N, 52°12.3'E], 1100 m, 8 Jul 1974, Renz & Iranshahr, Rechinger 16608 (IRAN 10627). Tehran: 13 km W Garmsar [35°15.3'N, 52°11.8'E], 25 May 1984, Moussavi & Karavar (IRAN 10626). Qom: Qom to Qomrud, [c. 34°42.9'N, 50°57.3'E], 16 Oct 1972, Iranshahr & Moussavi (IRAN 10629). Kerman: Bam, Deh Bakri, 29.054°N, 57.913°E, 6 May 1969, Pazouki & Hashemi (IRAN 10628).
Cicerbita polyclada
Iran. Kogiluyeh & Boyer Ahmad: [in glareosis alpis] Kuh-Daena [c. 30°56'N, 51°28'E], [Jul 1842], [Kotschy 603 ed. R. F.] Hohenacker (B 100312954 isolectotype). Markazi: Kuh-Shasinde [nr. Shahsand, c. 33°56'N, 49°22'E], Conglomeratabhänge, 18 Jul 1902, T. Strauss 14091 (B 100312952, 100312953).