(C) 2012 M.S. Vorontsova. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new andromonoecious species related to the eggplant and belonging to Solanum subgenus Leptostemonum from southern Africa is described. Solanum umtuma Voronts. & S.Knapp, sp. nov. is found in the eastern part of South Africa, and is sympatric with its close relative Solanum linnaeanum Hepper & P.M-L.Jaeger. It is morphologically very similar to Solanum cerasiferum Dunal of northern tropical Africa. A comparison table with similar and closely related species is provided, as are a distribution map and illustration of Solanum umtuma.
Africa, andromonoecy, eggplant, endemic, South Africa, “spiny solanum”
The Solanaceae is an economically important, cosmopolitan family with approximately 3000 species in some 90 genera. The Solanaceae include globally important food crops such as the cultivated potato (Solanum tuberosum L.), tomato (Solanum lycopersicum L.), aubergine (Solanum melongena L.), and chilli pepper (Capsicum spp.) as well as a number of widely used drug plants such as tobacco (Nicotiana tabacum L.), Datura, and Atropa belladonna L., the source of atropine. The giant genus Solanum L. with ca. 1500 species has become a model system for collaborative online taxonomy in challenging tropical plant groups (see
The “spiny” (or more accurately prickly) solanums (Solanum subgenus Leptostemonum Dunal) are the largest clade in the genus, with some 750 species (
Although the eggplant is generally considered to be a vegetable of Asian origin and distribution (see
Morphological differences between Solanum umtuma and other eggplant relatives in Africa. Calyx characters refer to the long-styled flowers at the base of the inflorescence and not to the smaller, functionally male, short-styled flowers in the distal parts of the inflorescence. This comparison focuses on the characters relevant to the identification of Solanum umtuma and does not include all the characters useful for separating the other members of this group (these will be presented in an up-coming monographic treatment of the prickly solanums of Africa and Madagascar, Vorontsova and Knapp in prep.).
| Leaf shape | Leaf base | Apices of primary leaf lobes | Secondary leaf lobes | Total calyx length | Calyx lobe length | Calyx lobe shape | Calyx lobe apex | Prickle # on calyx at anthesis | Distribution | |
|---|---|---|---|---|---|---|---|---|---|---|
| Solanum aureitomentosum | ovate | obtuse to cordate | rounded to obtuse | absent | 12–19 mm | 7–10 mm | ovate to oblong and foliaceous | obtuse | 30–60 | Southern Africa, from southern DR Congo to Angola, southern Tanzania, Zambia, and Zimbabwe |
| Solanum campylacanthum | ovate to elliptic or lanceolate | rounded to cordate | rounded, sometimes acute | absent | 7–15 mm | 5–10 mm | deltate to narrow-deltate | acute to obtuse or acuminate | 0–20 | Ubiquitous weed of low altitudes in Southern and Eastern Africa |
| Solanum cerasiferum | ovate to elliptic | attenuate | rounded to acute | sometimes present | 7–12 mm | 4–7 mm | deltate to narrow-deltate | acuminate | 0–20 | From Senegal to Cameroon, Sudan and Ethiopia |
| Solanum incanum | ovate | rounded to cordate | rounded | absent | 6–10 mm | 2.5–5 mm | deltate to narrow-deltate | acute to obtuse | 15–60 | Predominantly in Ethiopia, Somalia, Arabia, and India, with some populations in N Kenya, Sudan, and extending to Mali |
| Solanum insanum | ovate | truncate, sometimes obtuse | rounded | absent | 5–10 mm | 4–6 mm | deltate | acute | 0–15 | Madagascar, India to SE Asia |
| Solanum umtuma | elliptic | cuneate to truncate | obtuse to acute | often present | 11–22 mm | 7–10 mm | ovate and foliaceous | bluntly acute | 30–80 | South Africa |
| Solanum lichtensteinii | ovate | cordate, sometimes cuneate | rounded | absent | 7–15 mm | 3.5–6 mm | deltate to narrow-deltate | acute to obtuse | 20–50 | Angola to South Africa, DR Congo, and Tanzania |
| Solanum linnaeanum | elliptic, sometimes ovate or obovate | cuneate or obtuse | rounded | always present and often well-developed | 10–14 mm | 5–6 mm | deltate to ovate | acute to rounded | 30–100 | Native to South Africa and naturalised in disturbed, often coastal, habitats worldwide |
| Solanum melongena | ovate | cordate to obtuse | rounded | absent | 10–40 mm | 5–17 mm | deltate to narrow-deltate | acute to long-acuminate | 0(-30) | Cultivated worldwide (commonly cultivated in West Africa, sometimes in southern Africa, rarely cultivated in tropical Africa) |
Work on species limits in the eggplant group was carried out by the late Richard Lester’s students (
urn:lsid:ipni.org:names:77116656-1
http://species-id.net/wiki/Solanum_umtuma
Figs 1–3Differs from Solanum cerasiferum Dunal by its cuneate to truncate leaf bases (versus short-attenuate leaf bases in Solanum cerasiferum), ovate foliaceous calyx lobes 7–10 mm long with between 30–80 prickles at anthesis on long-styled flowers (versus deltate to long-deltate membranous calyx lobes 4–7 mm long with only 0–20 prickles on long-styled flowers of Solanum cerasiferum); also differs from Solanum linnaeanum Hepper & P.-M.L.Jaeger by its shallow, obtuse to acute leaf lobes (versus deep, rounded leaf lobes in Solanum linnaeanum).
South Africa. Eastern Cape: Elliotdale District, The Haven [32°14'S, 28°54'E], forest margin, flower white, 17 Nov 1966, J.L. Gordon-Gray 1017 (holotype: NU [NU-40255]).
Shrub, 0.5–1.5 m. Young stems erect, slender, moderately stellate-pubescent to glabrescent, with porrect sessile or variously stalked trichomes, the stalks to 0.2 mm long, the rays 6–8, ca. 0.2 mm long, the midpoints approximately the same length as the rays, armed with straight prickles 3–4 mm long, 1–2 mm wide at base, deltate, flattened, pale yellow-orange, glabrous, spaced 5–20 mm apart; bark of older stems glabrescent, green-brown to dark brown. Sympodial units plurifoliate. Leaves lobed; blades 8–20 cm long, 5–15 cm wide, 1.5–2 times longer than wide, elliptic, chartaceous, drying concolorous to weakly discolorous, green-brown, moderately stellate-pubescent on both surfaces, with porrect, sessile or stalked trichomes, the stalks to 0.2 mm long, the rays 6–8, 0.2–0.5(-0.8) mm long, the midpoints approximately the same length as the rays, with 5–20 prickles on both surfaces; the primary veins 4–6 pairs, the tertiary venation clearly visible abaxially and not visible adaxially; base cuneate to truncate; margins lobed, the lobes 3–4 on each side, 1–3 cm long, deltate, apically obtuse to acute, extending approximately 1/3 of the distance to the midvein, often with secondary lobing; apex obtuse to acute; petiole 1–3 cm long, approximately 1/6 of the leaf blade length, moderately stellate-pubescent, with 0–5 prickles. Inflorescences apparently lateral, 3.5–9 cm long, rarely branched, with 6–15(-20) flowers, 1–4 flowers open at any one time, weakly stellate-pubescent, with 0(-5) prickles; peduncle 1–3 mm long; pedicels 1–2.3 cm long in long-styled flowers, 0.8–1.2 cm long in short-styled flowers, erect to pendent, articulated at the base, moderately stellate-pubescent to glabrescent, with 0–20 prickles on long-styled flowers, unarmed on short-styled flowers; pedicel scars spaced 2–8 mm apart. Flowers 5-merous, heterostylous and the plants andromonoecious, with the lowermost 1–3 flowers long-styled and hermaphroditic, the distal flowers short-styled and functionally male. Calyx 11–22 mm long in long-styled flowers, 5–9 mm long in short-styled flowers, the lobes 7–10 mm long in long-styled flowers, 3–4 mm long in short-styled flowers, ovate and foliaceous in long-styled flowers, deltate in short-styled flowers, apically bluntly acute in long-styled flowers and acute to obtuse in short-styled flowers, moderately stellate-pubescent, with 30–80 prickles in long-styled flowers and 0–30 prickles in short-styled flowers. Corolla 2.5–3.3 cm in diameter in long-styled flowers, 1.5–2.5 cm in diameter in short-styled flowers, usually white or white with purple midveins, sometimes mauve, stellate, lobed for 1/4–1/2 of its length, the lobes ca. 7 mm long, ca. 10 mm wide in long-styled flowers, 6–10 mm long and 5–8 mm wide in short-styled flowers, broad-deltate, spreading, sparsely stellate-pubescent abaxially, the trichomes porrect, sessile or stalked, the stalks to 0.2 mm, the rays 5–8, 0.2–0.4 mm long, the midpoints approximately the same length as the rays. Stamens equal, with the filament tube 1–3 mm long, the free portion of the filaments ca. 0.5 mm long; anthers 5–6 mm long in long-styled flowers, 4.5–5.8 mm long in short-styled flowers, connivent, tapering, poricidal at the tips. Ovary glabrous, with a few stellate trichomes towards the apex; style 1.1–1.2 cm long in long-styled flowers, stout, straight or gently curved, moderately stellate-pubescent for most of its length. Fruit a spherical berry, 1(-2) per infructescence, 2.7–3.5 cm in diameter, the pericarp smooth, dark green with pale green and cream markings when young, yellow at maturity; fruiting pedicels 2–3 cm long, 1.2–2.2 mm in diameter at base, woody, pendulous, with 0–20 prickles; fruiting calyx not accrescent, covering 1/4–1/3 of the mature fruit, reflexed, with 10–80 prickles. Seeds ca. 100–200 per berry, 2.7–3.5 mm long, 2–2.5 mm wide, flattened-reniform, orange-brown.
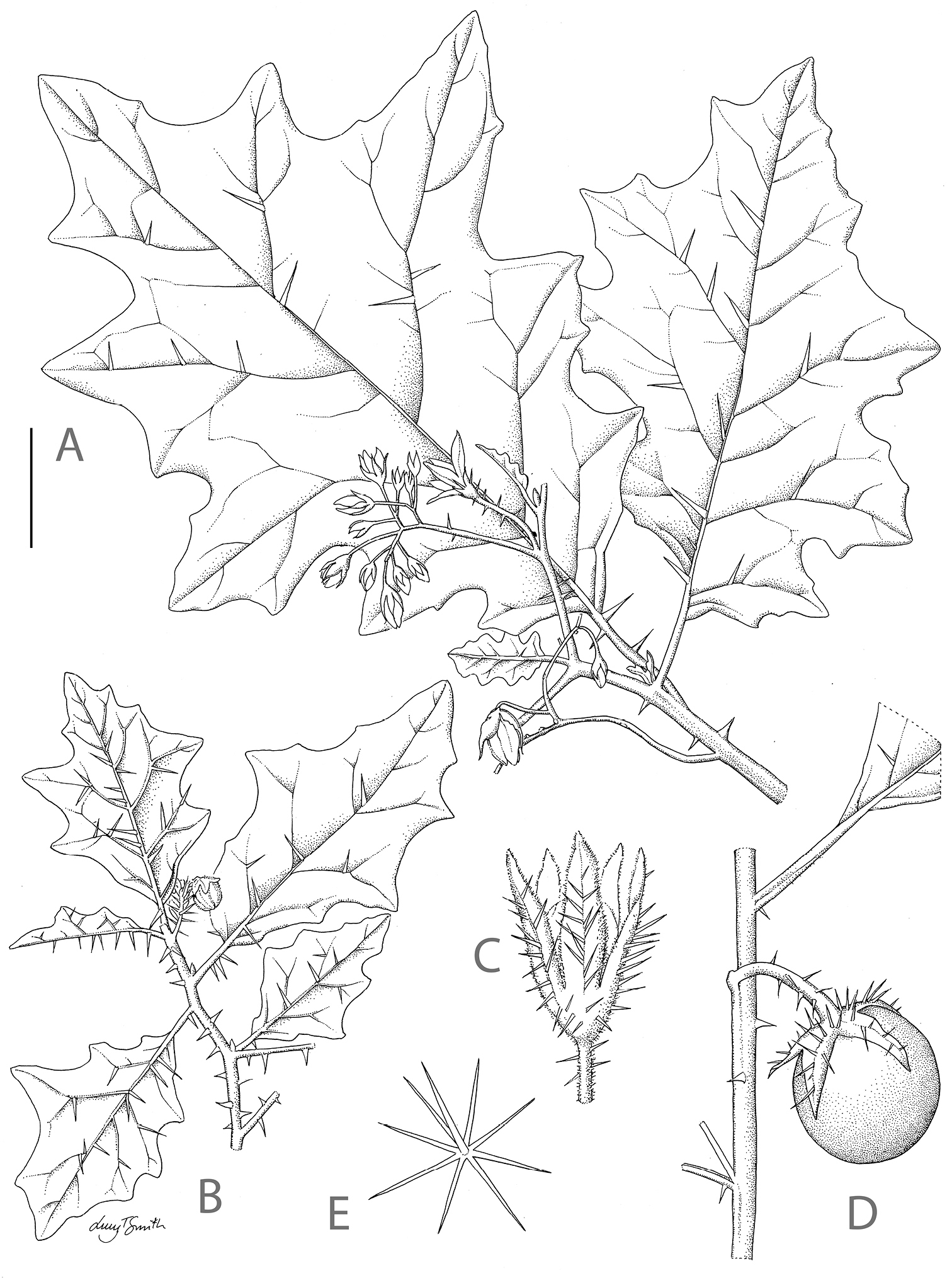
Solanum umtuma. A Habit with pronounced secondary leaf lobes and sparse prickles B Habit with few secondary leaf lobes and dense prickles C Calyx of a long-styled flower at anthesis D Fruiting branch E Porrect stellate trichome from the adaxial surface of a leaf. Scale bar: A, B, C = 3 cm; C = 1.5 cm; E = 0.5 mm. A, E from Gerrard 295; B-D from Arnold 35934. Drawn by Lucy T. Smith.
Photograph of the holotype of Solanum umtuma (J.L. Gordon-Gray 1017, NU-40255).
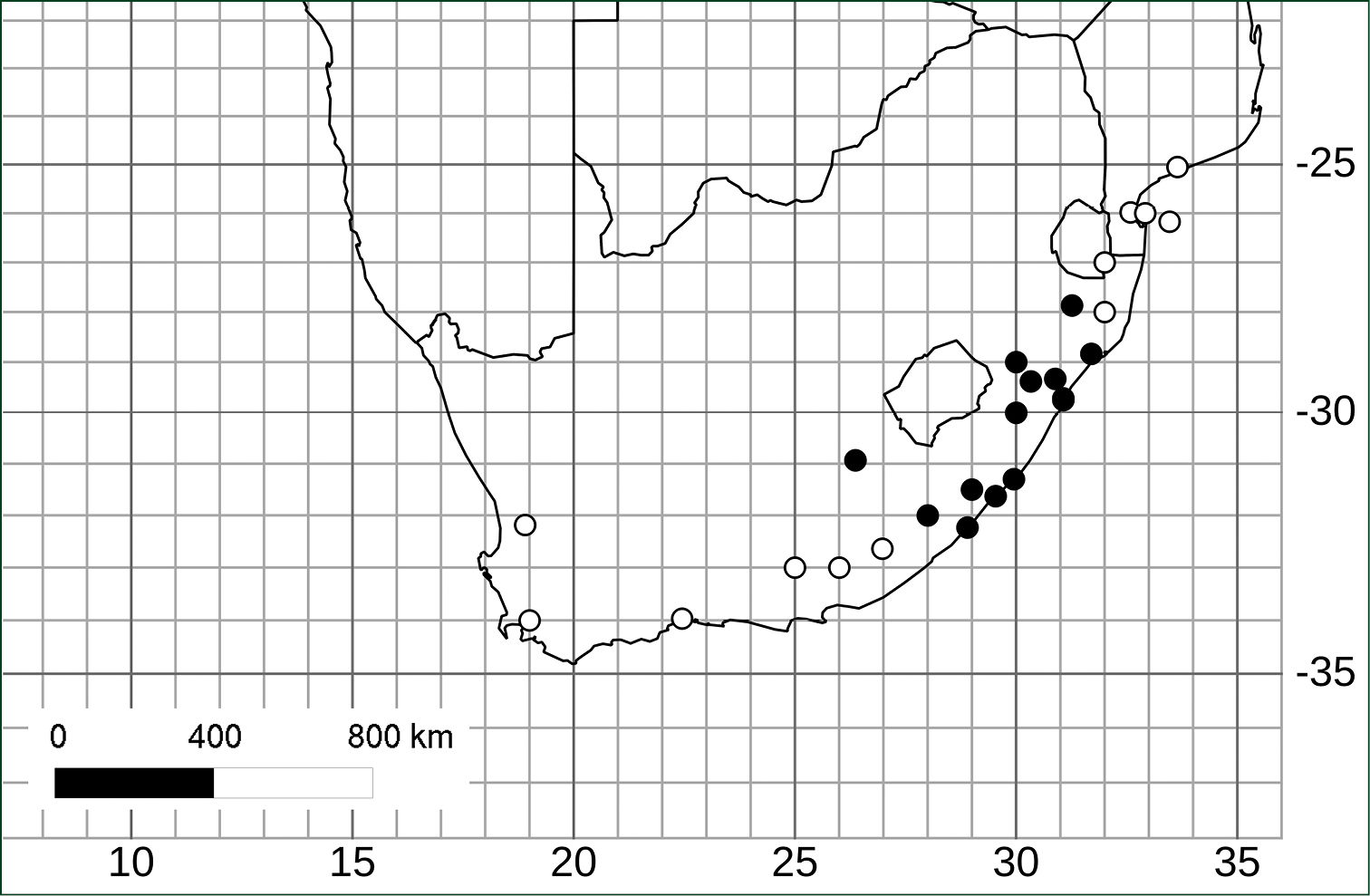
(Fig. 3). Endemic to South Africa in KwaZulu-Natal and Eastern Cape provinces (most specimens from KwaZulu-Natal); 50–1300 m elevation. Solanum umtuma is limited to the Maputaland-Pondoland Floristic Region (
Distribution of Solanum umtuma (black circles) and its putative sister species Solanum linnaeanum (white circles) in southern Africa (specimen details for Solanum linnaeanum can be found on the Solanaceae Source website, http://www.solanaceaesource.org).
Occasional on grassland, scrub, and forest edges, usually growing on sandy soil.
“Umthuma” is an isiXhosa vernacular name for many species of prickly Solanum; in the Xhosa language the “th” is pronounced as “t”, so we have here written the epithet phonetically as “umtuma”. The epithet is used here as a noun is apposition and thus not latinized to agree in gender.
Solanum umtuma is a species of open and somewhat disturbed habitats (as are many prickly solanum species) and occupies an area of approximately 8000 km2 and appears to be relatively evenly distributed within that area (Fig. 3). Although not normally common where it occurs, it is not a species of immediate conservation concern.
South Africa. Eastern Cape: Transkei, outside Umtata [31°30'S, 29°00'E], 17 May 1975, M.N.M. Arnold s.n. (K [K000441994]); Port St Johns, 1 May 1899, E.E. Galpin 2869 (K [K000545863]); Port St. Johns, 21 Dec 1932, A.O.D. Mogg 1300 (K [K000545864]). —KwaZulu Natal: 50 km from Nongoma, 13 May 1975, M.N.M. Arnold 35934 (K [K000795077]); Berea, 1862, T. Cooper 1272 (BM [BM000887022], K [K000441992, K000441993]); Berea, 1862, T. Cooper 1273 (K [K000441998, K000441999]); Noodsberg, Feb 2002, T. Edwards 2973 (NU); location unknown, “Zululand”, received Jul 1865, W.T. Gerrard 295 (BM [BM000887021], K [K000795076]); Umhlanga Rocks, 2 Sep 1966, R.K. Grosvenor 168 (K [K000441995]); Weza forestry Area - beyond Lorna Doone [31°18'S, 29°57'E], 2 Jul 1986, P.E. Hulley 134 (NU); Mkambati, Mkambati Enviromental Education Centre, 6 Apr 1988, P.E. Hulley 230 (NU); Umgeni Park near Howick; Endulu Camp road, 18 Dec 1988, P.E. Hulley & T. Olckers 279 (NU); 11 km N of Butterworth, 27 Apr 1990, P.E. Hulley & T. Olckers 333 (NU); Vernon Crookes Nature Reserve, 27 Apr 1990, P.E. Hulley & T. Olckers 336 (NU); Umvoti, Umvoti valley S.W. of Mapumulo river bank, 9 Feb 1965, E.J. Moll 1538 (K [K000442000]); Swart Umfolozi, Mpembeni, 1257 m, 27 Jan 2005, L.S. Nevhutalu, LA. Nkuna, & E. van Wyk 921 (K [K000441997]); La Lucia, 14 Aug 1966, R.G. Strey 6750 (K [K000441991]); Umhlanga Rocks, on gentle slopes above Umhlanga Rocks Hotel, 30 Dec 1959, R.H. Watmough 461 (K [K000441996]); Ixopo, 22 Aug 1986, J.O. Wirminghaus s.n.(NU); Ngoye Forest, Zululand [28°50'S, 31°42'E], 17 Sep 1987, J.O. Wirminghaus 628 (NU).
Solanum umtuma is a medium-sized subshrub with straight prickles, acute to obtuse leaf lobes, and large yellow fruits. It is almost certainly a close relative of the sympatric Solanum linnaeanum; the two species share long, leafy, prickly calyx lobes on long-styled flowers and fruits and differ primarily in the shape of their leaf lobes. Solanum linnaeanum is immediately recognisable by its quite deeply incised leaves with rounded lobes; a few intermediate specimens of Solanum umtuma have somewhat rounded lobes, e.g. R.G. Strey 6750 (K000441991). Label data indicate that Solanum umtuma has white or only occasionally violet to mauve flowers, while Solanum linnaeanum always has purple flowers.
Solanum umtuma is morphologically very similar to Solanum cerasiferum and more superficially similar to other species with straight prickles and acute to obtuse leaf lobes, including the African highland Solanum dasyphyllum Schumach. & Thonn. (
Specimens of Solanum umtuma have sometimes been annotated as “Solanum fuscatum L.” or “Solanum ferrugineum Jacq.” These names are both widely misapplied. No original material of Solanum fuscatum L. has been located and the application of this name has been in doubt (
Our work on Solanum is supported by the National Science Foundation's Planetary Biodiversity Inventory programme through the project 'PBI Solanum - a worldwide treatment' (DEB-0316614). We thank Lucy T. Smith for Figure 1; the curators of the herbaria cited in the text for loan of and permission to examine their material; Dirk Bellstedt (University of Stellenbosch), Benny Bytebier (NU), and Brian Schrire (K) for help and advice; Pawel Ficinski for technical and logistical support and for the preparation of the map; Abigail Brady for assistance with analysis of leaf trichomes; Jennifer Potgieter and Kholeka Sylvia Mbomboza of George, Western Cape, South Africa, for helping us find a suitable epithet; and the botanical community for voting in Melbourne in July 2011 to allow electronic publication of new names of plants.