(C) 2011 Norbert Holstein. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Nuclear and plastid sequences from two individuals of a suspected new species of Coccinia from West Africa were added to an available molecular phylogeny for the remaining 27 species of the genus. Phylogenetic analyses of these data indicate the new species' monophyletic status and closest relatives. Based on four fertile collections, we here describe and illustrate Coccinia intermedia Holstein. We also provide a key to the Coccinia species of West Africa and map their distributions.
Benin, Ivory Coast, Ghana, leaky dioecy, molecular phylogenetics, species monophyly, Togo
The genus Coccinia Wight et Arn. so far consisted of 27 species distributed mainly in Sub-Saharan Africa, with centers of diversity in East Africa and southern Africa (Holstein, ongoing monograph). Only four species were known from West Africa, including Coccinia longicarpa Jongkind, Coccinia keayana R. Fern., and Coccinia barteri (Hook. f.) Keay, which apparently evolved during Pliocene-Pleistocene climatic oscillations (
We produced new sequences of the plastid rpl20–rps12 intergenic spacer (JN653687), trnSGCU–trnGUCC intergenic spacer (JN653686) and the nuclear LEAFY-like second intron (JN653688) from the female specimen A. Akoègninou et al. 2625 (WAG0278370) of the new species, following standard procedures (
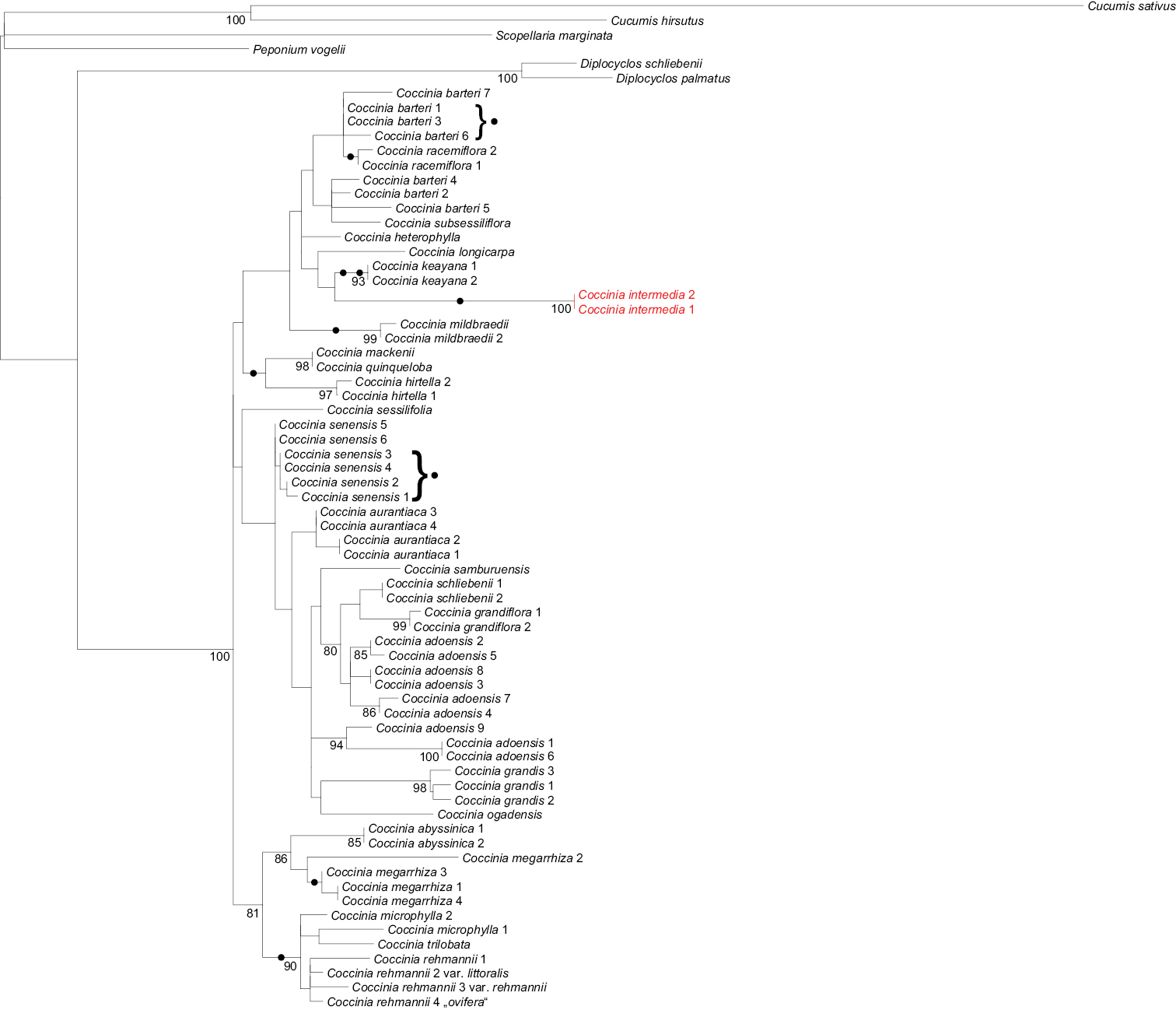
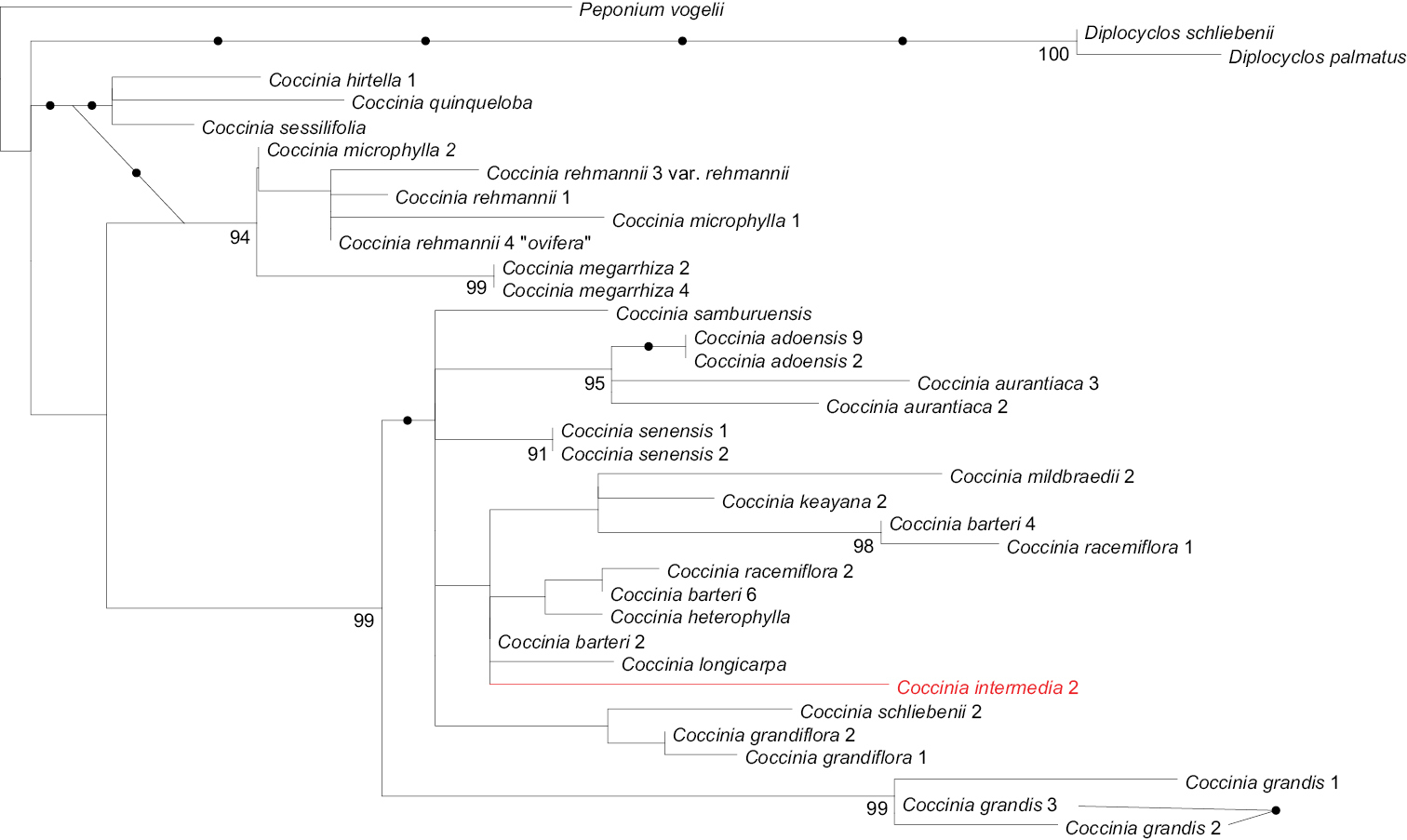
The two Coccinia intermedia accessions in the plastid tree form a clade (Fig. 1) within the barteri clade. In the nuclear LEAFY phylogeny, Coccinia intermedia groups with Coccinia barteri, Coccinia heterophylla (Hook.f.) Holstein, Coccinia keayana, Coccinia longicarpa, Coccinia mildbraedii Gilg, and Coccinia racemiflora Keraudren (Fig. 2), albeit without bootstrap support.
Maximum likelihood phylogeny for Coccinia based on plastid DNA sequences analyzed under GTR+Γ model of substitution. The tree is based on 4, 551 nucleotides (140 parsimony-informative sites) from the trnSGCU–trnGUCC intergenic spacer (IS), the rpl20–rps12 IS, the ndhF–rpl32 IS, trnLUAA intron, trnLUAA–trnFGAA IS, and the matK gene (expanded matrix from
Maximum likelihood phylogeny for Coccinia based on nuclear DNA sequences from the LEAFY-like 2nd intron analyzed under the GTR+Γ model of substitution. The tree is based on 505 nucleotides (56 parsimony-informative sites). Numbers below branches represent bootstrap support ≥ 80% from 100 replicates. Dots on branches and behind brackets refer to uniquely shared insertions or deletions. Species names follow
A Coccinia longicarpa differt calycis dentibus angustis, corolla campanulata et fructu elliptico ad oblongo. A Coccinia keayana et Coccinia grandis differt calycis dentibus ad corollam adpressis vel apicem versus leviter recurvatis et lamina foliorum subtus glandibus fuscis provisa. A Coccinia barteri differt floribus femineis 1–3 fasciculatis non racemosis, corolla campanulata.
BENIN. Atakora: Natitingou, Kouaténa (Perma), 10°12.00'N; 1°30.18'E, river bed, female, fl, fr, 3 Oct 2000, A.Akoègninou et al. 3625 (Holotype: WAG0278370!; isotype: WAG0278369!).
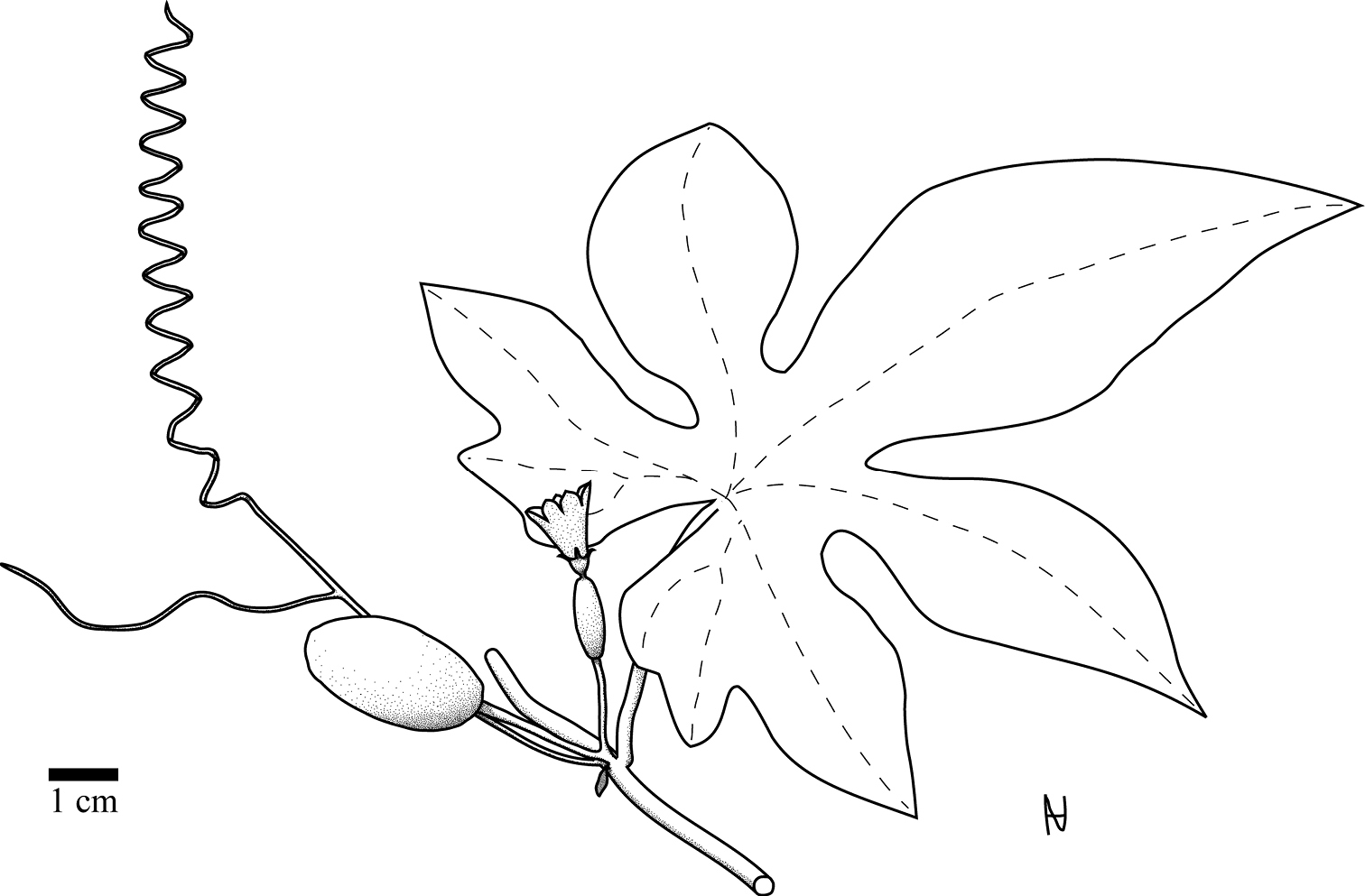
Perennial, diclinous climber. Shoot length unknown, but likely several meters. Shoots lignify with whitish bark and up to 1 cm diam. Fresh shoots green, glabrous, older shoots with clear to white pustules. Petioles 2.8–10.8 cm, glabrous, when older with clear to white pustules (Fig. 3a). Leaves 6–15 × 7–18 cm, shallowly to profoundly 5-lobate, more or less auriculate (Fig. 4). Upper lamina glabrous with clear to whitish pustules. Lower lamina paler than upper lamina, glabrous, often with small dark glands near the leaf base (Fig. 3a). Tendrils simple or bifid. Probracts up to 2.5 mm long, glabrous, apex rounded (Fig. 3a). Male flowers in few-flowered racemes (Fig. 5), likely sometimes accompanied by a single flower. Common peduncle up to 1 cm, pedicels in racemose flowers 2–4 mm, glabrous. Bracts up to 1.5 mm long, round to obovoid. Receptacle pale green, glabrous. Calyx teeth 1.5 mm long, lineal to narrow triangulate, erect with slightly recurved tips (Figs 3–5). Corolla campanulate, 1.6 cm long, pale reddish-yellow to yellow, lobes 0.7 cm long (Fig. 5). Anthers sinuate, in a globose head (Fig. 3c). Pollen unknown. Female flowers 1–3 clustered (strongly reduced raceme; Fig. 4). Pedicels 0.6–1.2 cm, glabrous. Perianth like in males. Ovary fusiform, glabrous. Stigma and staminodes unknown. Fruit 4.5 × 2.5 cm, elliptical to oblong, smooth. Unripe green with pale green longitudinal mottling. Ripe orange?, more likely becoming red via orange ripening stage. Fruit with waxy cover. Size of mature seeds unknown (≥ 5.5 × 3.5 × 1.3 mm), symmetrical (to slightly asymmetrical), face flat (Fig. 3b).
a Coccinia intermedia leaf basis and node with flowers b seeds from late, but immature fruit c node with young fruit and male flower bud with sinuate anthers; all from J.B.Hall & J.M.Lock GC46016 (K).
Habitus of Coccinia intermedia as reconstructed from J.B.Hall & J.M.Lock GC46016 (K).
Male inflorescence of Coccinia intermedia from C.Geerling & J.Bokdam 662 (WAG).
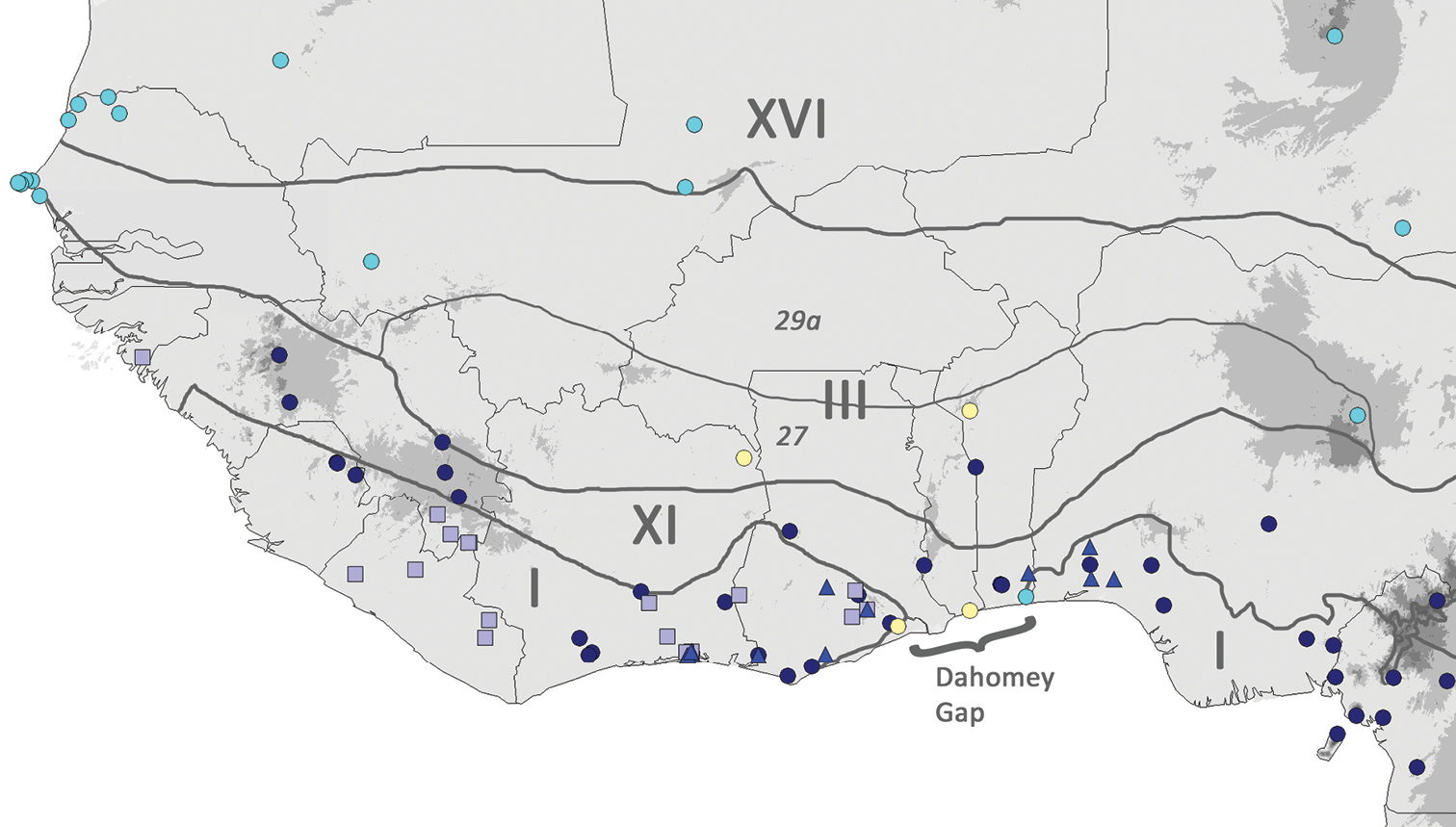
(Fig. 6). NE Ivory Coast, SE Ghana (likely also in the north), S Togo (likely also in the north), NW Benin. Based on the current collections, Coccinia intermedia is likely to occur in the Dahomey Gap region and the Isoberlinia woodlands of West Africa.
Map of West African Coccinia species. Pale yellow circles = Coccinia intermedia, cyan circles = Coccinia grandis, dark blue circles = Coccinia barteri, pale blue squares = Coccinia keayana, bright blue triangles = Coccinia longicarpa. Thick dark grey lines are phytochoria drawn after
Wooded grasslands (semi-humid savanna), woodlands, dry forests, and along rivers. Flowering specimens have been collected during May, August, and October, which in each site was during or shortly after the rainy season.
The epithet refers to the species' status as the only Coccinia from West Africa that occurs in habitats intermediate between semi-arid and humid conditions. Morphologically, Coccinia intermedia combines characters also found in the other four West African species although not in this combination.
Benin: Atakora, Natitingou, Kouaténa (Perma), 10°12.00'N; 1°30.18'E, river bed, female, fl, fr, 3 Oct 2000, A.Akoègninou et al. 3625 (WAG 2 sheets). Ghana: Shai Hills Game Reserve, monoecious, fl, fr, 25 May 1976, J.B.Hall & J.M.Lock GC 46016 (K 4 sheets, MO). Ivory Coast: Bouna, male, fl, 10 Aug 1967, C.Geerling & J.Bokdam 662 (MO, WAG). Togo: between Lomé and Aného, female, fr, 25 Jun 1994, L.Aké Assi 18982 (MO).
| 1 | Plant glabrous. Leaves with few large pale glands between main nerves of lower lamina. Nerves on lower lamina with or without white pustules. Leaf margin dentate, in mature plants often red to brown (black when dry). Tendrils always simple. Male and female flowers 1 solitary (rarely male flowers clustered or in short-peduncled racemes). Calyx teeth spreading to reflexed, tips red to brown. Corolla campanulate, white or buff. Fruit ovoid. Plant of semi-arid habitats. | Coccinia grandis |
| 1* | Plant glabrous or with hairs, especially on adaxial petiole. Leaves with small blackish glands (often many) centered towards the leaf base or without glands on lower lamina. Tendrils simple or bifid. Male and female flowers in racemes or solitary. Corolla in yellowish tones, never white. | 2 |
| 2 | Plant glabrous. Leaves with small blackish glands centered towards the leaf base (Fig. 3). Nerves on lower lamina with or without white pustules. Leaf margin at maturity with colored teeth (color in living plants unknown, black when dry). Tendrils simple or bifid. Male flowers (Fig. 5) bracteate, in few-flowered racemes, female flowers 1–3 solitary/clustered (Figs 3 and 4). Calyx teeth erect with recurved tips (Figs 3–5). Corolla campanulate. Fruit ovoid to short cylindrical. Plant of wooded grasslands (tree savanna), woodlands, or dry forests. | Coccinia intermedia |
| 2* | Plant glabrous or with hairs, esp. on adaxial petiole. Leaves with small blackish glands centered towards the leaf base or without glands. Nerves on lower leaf lamina without white pustules. Tendrils simple or bifid. Male flowers in few to many-flowered racemes, rarely accompanied by a solitary flower. Female flowers in few- to many-flowered racemes or solitary. Flowers bracteate or ebracteate. Corolla urn-, cup- to funnel-shaped. Plant of humid climates (rainforests, gallery forests, etc.) | 3 |
| 3 | Leaf margin with pale (when dry blackening) glandular teeth. Tendrils simple. Flowers without bracts, calyx teeth erect, > 1.5 mm at base. Fruits long cylindrical. | Coccinia longicarpa |
| 3* | Leaf margin without conspicuously colored teeth. Tendrils simple or bifid. Flowers with or without bracts. Calyx teeth erect, spreading, or reflexed, but narrow (< 1.2 mm at base). Fruits ovoid. | 4 |
| 4 | Tendrils simple. Male flowers in lax racemes, female flowers solitary or in few-flowered racemes. Flowers without bracts. Calyx teeth in buds spreading, later reflexed. | Coccinia keayana |
| 4* | Tendrils simple or bifid. Male flowers in dense few- to many-flowered racemes, with or without bracts. Female flowers in racemes, rarely solitary. Flowers with or without bracts. Calyx teeth variable. | Coccinia barteri |
Coccinia intermedia is morphologically similar to the other West African species. From Coccinia grandis, it differs most readily in the glands on the lower lamina and in its calyx teeth (erect with recurved tips in Coccinia intermedia and spreading to reflexed in Coccinia grandis). From Coccinia longicarpa, it differs in its ovoid fruits (instead of long cylindrical fruits in Coccinia longicarpa). Additionally, Coccinia longicarpa has ebracteate racemes and much broader (> 1.5 mm at the base) erect calyx teeth, and an urn-shaped corolla. From Coccinia keayana, it differs in having bracteate male flowers in denser racemes, a campanulate corolla and calyx teeth that are adpressed to the corolla with recurved tips, instead of spreading (in buds) to reflexed calyx teeth. Secure distinction of Coccinia intermedia from Coccinia barteri requires fertile material with flowers (see the key above).
Ecologically, the new species is a member of
Two DNA characters, namely base pairs 310 and 323 in the trnSGCU–trnGUCC intergenic spacer region, suggest the placement of Coccinia intermedia as sister to a clade that we have earlier referred to as the Coccinia barteri clade (
However, true monoecy in Coccinia intermedia would be surprising as none of ca. 1, 500 specimens of other Coccinia species studied is bisexual (Holstein, ongoing monograph).
We thank Dietrich Podlech for help with the diagnosis. We also thank the curators of K, MO, and WAG for sending material on loan, and permission to dissect material (K) and extract DNA (MO, WAG). The work was supported by German Research Council (RE 603/6–1 and 6–2).