(C) 2011 Alex V. Popovkin. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of Spigelia L. (Loganiaceae), Spigelia genuflexa Popovkin & Struwe, sp. n., from the Atlantic forest of northeastern Bahia, Brazil, is described, being the first reported geocarpic species in the family. During fruit maturation, the basal infructescences bend down towards the ground, depositing the fruit on the surface (and burying it in soft kinds of ground cover, e.g., moss), whereas the upper ones do so slightly but noticeably. The species is a short-lived annual apparently restricted to sandy-soil habitat of the Atlantic forest of northeastern Bahia, with variable and heterogeneous microenvironment and is known from only two restricted localities. A short review of amphi- and geocarpic species is provided. A discussion of comparative morphology within Spigelia with regards to dwarfism, indumentum, and annual habit is included. A phylogenetic parsimony and Bayesian analysis of ITS sequences from 15 Spigelia species plus 17 outgroups in Loganiaceae confirms its independent taxonomic status: on the basis of sequence similarity and phylogenetic topology it is phylogenetically distinct from all Spigelia species sequenced so far.
Dwarfism, evolution, geocarpy, ITS, Loganiaceae, Neotropics, phylogeny, Spigelieae
Spigelia L. is a genus of approximately 60 species of Neotropical herbs to shrubs (
Morphologically, Spigelia species can be recognized by their opposite or whorled leaves, one-sided cymose inflorescences, often brightly colored pentamerous flowers with usually funnelform or tubular corollas, articulated styles, and strongly bilobed capsules with persistent style and fruit bases.
The new species was discovered by José Carlos Mendes Santos (a.k.a. Louro), the house help and fellow plant collector of the first author, when squatting near the latter's house. The tiny plant of no more than 3 cm in height would have been otherwise easily missed. A colony of half-dozen plants, within 5 square meters, was initially discovered. Two more colonies in the same restricted area were eventually uncovered. The habitat is an open-soil roadside, partially covered by leaf litter, at the border of a tabuleiro forest in the Atlantic forest biome of northeastern Bahia, Brazil. The species has been observed for a period of over two years, during weekly visits. This is an ephemeral rainy-season species, with plants almost completely disappearing in the dry season. While collecting at a patch of the well preserved tabuleiro forest some 10 km east of the first find, additional, larger specimens (10–25 cm high) were discovered in forest border leaf litter by the same collectors (Popovkin & Mendes 913).
Relationships among the species of Spigelia
are still poorly understood. Early phylogenetic results focusing on the
north-temperate species showed that there are two distinct
north-temperate lineages of Spigelia and that both of them have close relatives in the tropics (
urn:lsid:ipni.org:names:77114017-1
http://species-id.net/wiki/Spigelia_genuflexa
Figs 1–2Additional photos at Popovkin: http://calphotos.berkeley.edu/cgi/img_query?where-taxon=Spigelia+sp.+nov.&where-lifeform=specimen_tag&rel-lifeform=ne&rel-taxon=begins+with&title_tag=Spigelia+sp.+nov.; http://bit.ly/io7bpT--2009-2011
Haec species Spigelia flemmingiana Cham. & Schltdl. similis, sed plantis brevioribus (1.5–25.0 vs. 17–50 cm), foliis parvis (0.6–2 × 0.2–0.5 cm vs. 2–9 × 1.4–2 cm) ellipticis vel ovatis (vs. lanceolatis), corollis brevioribus (0.4–0.8 vs. ca. 1 cm), inflorescentiis paucifloribus, et infrutescentiis nutantibus in maturitatem (vs. semper erectis) differt.
Similar to Spigelia flemmingiana Cham. & Schltdl. but shorter (1.5–25 cm vs. 17–50 cm tall), with smaller leaves (0.6–2 × 0.2–0.5 cm vs. 2–9 × 1.4–2 cm) that are elliptic to ovate (vs. lanceolate), shorter corollas (0.4–0.8 cm vs. ca. 1 cm), fewer-flowered inflorescences (up to 7 flowers vs. up to 38 flowers), and infructescences bending downward at maturity (vs. staying erect).
Brazil: Bahia: Entre Rios, Fazenda Rio do Negro, Residual stands of the Atlantic Forest. Restinga-type forest of the Rio do Negro valley, ca. 15 km southeast of Entre Rios, Atlantic forest, 12°01'S, 38°02'W, 150 m, 31 July 2009, A.V. Popovkin & J.C. Mendes 617 (holotype: HUEFS).
Annual herb, 1.5–25 cm tall. Roots fibrous,
not very extensive. Stem branched at base, with reddish tint, with 4–6
prominent ribs decurrent from the leaf bases; interpetiolar stipules
triangular, with abundant papillae on outside. Leaves opposite as well
as 4 together higher up on the main branch under the inflorescence,
6–20 mm long, 2–5 mm wide, elliptic to ovate; secondary veins 4–6
pairs, arcuate, inconspicuous below and above, midrib raised below;
base acute, with decurrent lamina; margin flat or slightly revolute,
entire; apex obtuse; upper side with many short, transparent papilloid
hairs, 0.1–0.3 mm long; lower side glabrous; petiole 1–2 mm long.
Inflorescence variable, solitary (occasionally multiple), typically a
one-sided cyme (rarely a simple cyme/dichasium or a single flower),
unbranched, (1-)4–7-flowered, up to 28 mm long, without bracts or
with 1–2 tiny bracts subtending flowers; peduncle 7–15 mm. Flowers
actinomorphic, perfect, 5- (rarely 6-) merous. Calyx divided almost to
base, green, persistent in fruit; lobes triangular, acuminate,
0.8–1.4 mm long, c. 0.3 mm wide, with slightly papillose margins.
Corolla sympetalous, tubular, slightly widening towards mouth, 4–8 mm
long, 2.5–3.0 mm wide at mouth, white with pink lobes, aestivation
valvate with individual corolla lobes plicate in bud, lobes unfolded
when open, closing after a short (8-hour) anthesis, later withering
and deciduous; lobes triangular, 1.0–1.5 mm long, ca. 1 mm wide,
erect, acute, with smooth margin. Stamens epipetalous and adnate to
corolla up to middle of the tube, of equal length, included in
corolla; filaments flattened; anthers 0.7–0.8 mm long, shallowly
sagittate at base, truncate at apex. Ovary bicarpellate, bilocular,
ovoid, ca. 0.4 mm tall, with truncate apex; style 3–6 mm long
(including stigma), simple, articulated at 0.5–1.00 mm above the
ovary, mostly dehiscent in fruit (except the persistent base); stigma
simple, papillose, ‘brush-like' at the height of the anthers. Fruit a
bilobed capsule, 1.5–2 mm tall, 2–3 mm wide; dehiscing septicidally,
loculicidally and circumscissilly, leaving behind on the rachis a
persistent, boat-shaped base with pointed tips (‘carpoatlas' in
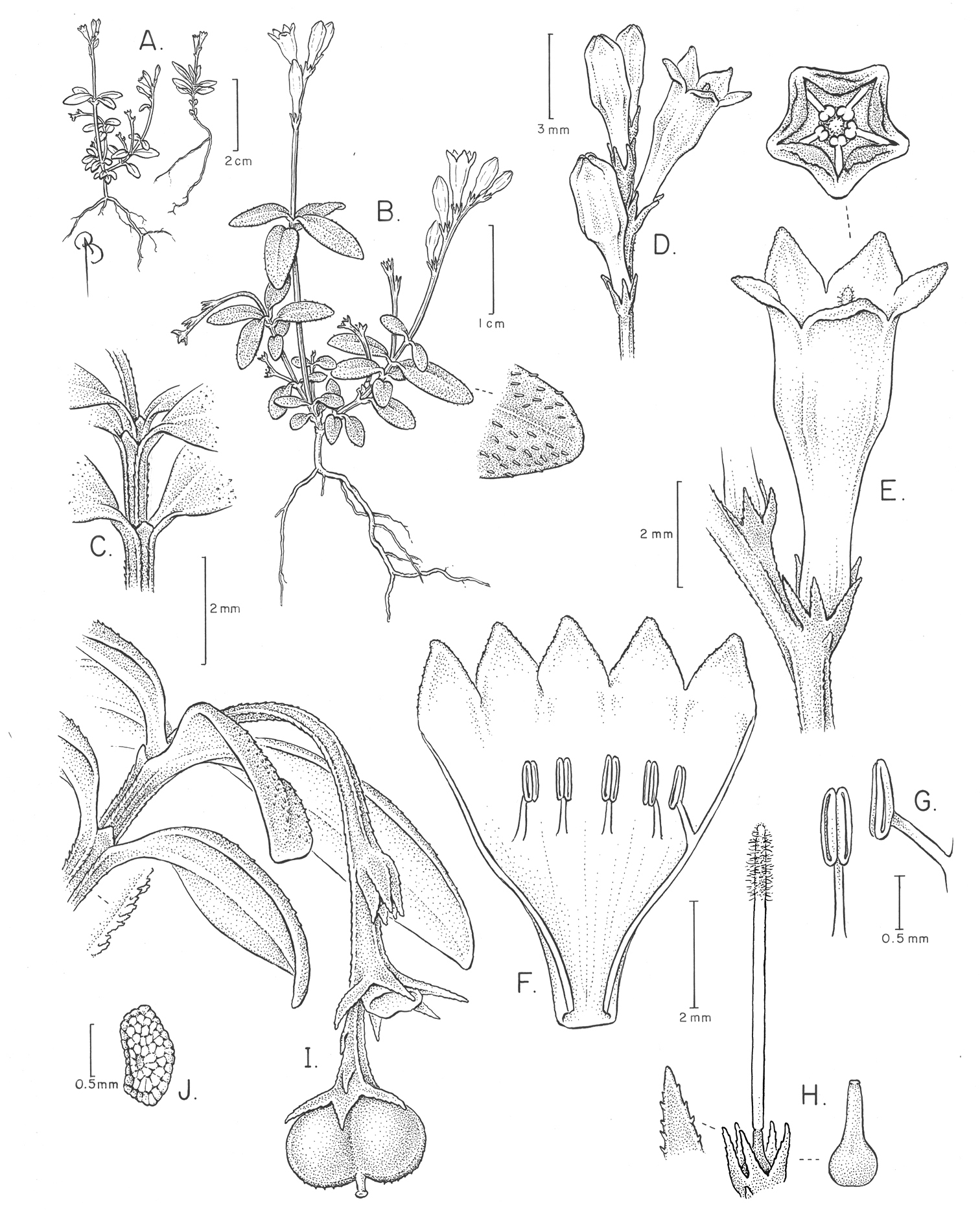
Spigelia genuflexa A–B Habit, showing inflorescences and geocarpic infructescenses, and close-up of apical part of leaf with apressed papilloid hairs C Close-up of node and internode, showing small triangular interpetiolar stipules D Flowers before and at anthesis E Close-up of flower at anthesis; note diminutive bract F Opened corolla with epipetalous stamens G Stamen inserted into corolla and introrse anther H Gynoecium inside papillose calyx, with hairy style (brush-type); older gynoecium, after style has dried and fallen off to the right I Geocarpic infructescence branch with one whole capsule (mitra-shaped, with small style remnant in center), and capsular base of the fruit that has dehisced, above it J Seed. Drawing by Bobbi Angell, based on A.V. Popovkin 602 and 602A.
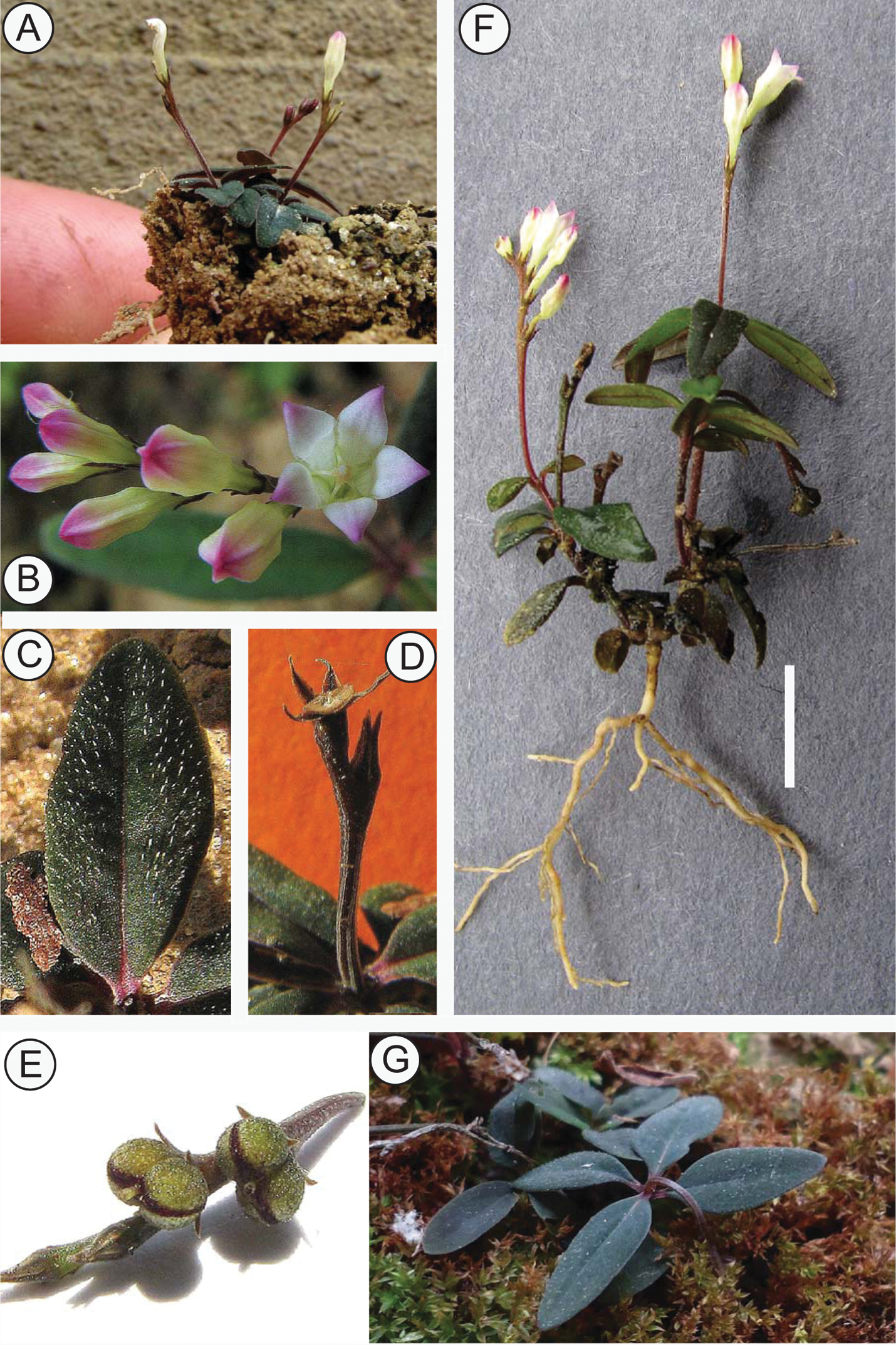
Spigelia genuflexa A Habit of mature plant B Flowers at anthesis and before opening. Note valvate and vertically folded petal lobes C Close-up of leaf with apressed papilloid hairs D Base of fruit after dehiscence (‘carpoatlas') E Fruit before dehiscence F Whole plant with roots. Scale bar = 1 cm G Infructescence showing geocarpy. Photos by Alex Popovkin.
This species is known from only two localities in northeastern Bahia (Brazil), about 30 km from the Atlantic coastline.
The species has been found on sandy, leaf litter- or moss-covered soil areas along the border of a tabuleiro forest. The diminutive flowers appear to be able to self, based on observations of cultivated material, with one to two flowers opening at one time. The anthesis begins early in the morning and ends in the afternoon of the same day. The arrangement and morphology of stamens and pistil, with anthers located closely to the central pistil with hairy upper part (Figure 1), suggests that spatial closeness of flower parts may promote selfing, thus ensuring fruit set. Occasional tiny ant visitors have been observed entering the open flowers, though it is not entirely clear if they might be the pollinators.
The geocarpy, i.e. weak geocarpy (depositors, in Hylander's [1929] terminology), of this species was initially observed on plants transplanted to a pot kept on a windowsill, allowing for daily/hourly observations. Two growth forms have been observed: one with inflorescences forming after the first three pairs of leaves are formed (usually, with a long internode between the first pair of leaves and subsequent two pairs), with the plant height at that stage of about 1 cm, and the other with inflorescences forming after four or five pairs of leaves and the plant reaching the height from 10 to 25 cm. The lower-forming inflorescences at the start of the fruit set would bend down to the soil, depositing the ripe fruit on the ground, while the higher-forming inflorescences would bend down noticeably but, because of the main stem height, would be unable to touch the soil surface. Inflorescences with the fruit not set (a rare phenomenon) stay upright. Later observations of plants growing on moss-covered ground showed that the capsules are actually buried in the soft substrate (Fig. 2G).
The specific name refers to the sometimes repeated bending of its infructescence branches to the ground, figuratively evoking an image of the etiquette of genuflexion.
The species is known from only a handful of
collections from two restricted populations in a non-protected area
(private land), and should therefore be assessed as Data Deficient for
EOO and AOO, following
The species has been found flowering and fruiting from March to November during the local rainy season. It takes about 3–4 weeks from anthesis to fruit maturity. Living plants have not been observed from December to early March.
Brazil: Bahia: Entre Rios: Fazenda Rio do Negro, Residual stands of the Atlantic Forest. Restinga-type forest of the Rio do Negro valley, ca. 15 km southeast of Entre Rios, Atlantic forest, 12°01'S, 38°02'W, 150 m (topotypes), 3 June 2009, A.V. Popovkin 598 (HUEFS); ibid., 10 June 2009, A.V. Popovkin 602 (CHRB, NY); ibid., 15 July 2009, A.V. Popovkin 602A (CHRB, NY); ibid., 31 July 2009, A.V. Popovkin 617 (HUEFS); ibid., 27 May 2010, A.V. Popovkin 703 (HUEFS); ibid., 4 Sep 2010, A.V. Popovkin 744 (HUEFS); ibid., 18 January 2011, A.V. Popovkin 825 (HUEFS); ibid., 8 June 2011, A.V. Popovkin & J.C. Mendes 885 (HUEFS). Bahia: Entre Rios: Imbé, Atlantic forest, 12°05'S, 38°W, 135 m: 1 October 2010, A.V. Popovkin & J.C. Mendes 758 (HUEFS); 1 June 2011, A.V. Popovkin & J.C. Mendes 878 (HUEFS); 8 June 2011, A.V. Popovkin & J.C. Mendes 885 (HUEFS); 17 August 2011, A.V. Popovkin & J.C. Mendes 913 (HUEFS).
Photographs in the field and of cultivated material were made using a Panasonic DMC-ZS3 camera. Pressed and dried herbarium material of Spigelia genuflexa were observed, measured and photographed using a Stemi-2000 Zeiss dissecting microscope with a mounted digital Canon camera.Measurements were made using a caliper or using a graded and calibrated eye piece in a dissecting scope.
Several species concepts were utilized to identify
and define this particular species, which is in line with previous
species concepts used in this group (
Sequences from the internal transcribed spacer (ITS) of nuclear ribosomal DNA were used to reconstruct a phylogenetic tree of 15 species of Spigelia, including the new species, and several outgroups. The complete methods for the phylogenetic analysis are presented in Appendix I.
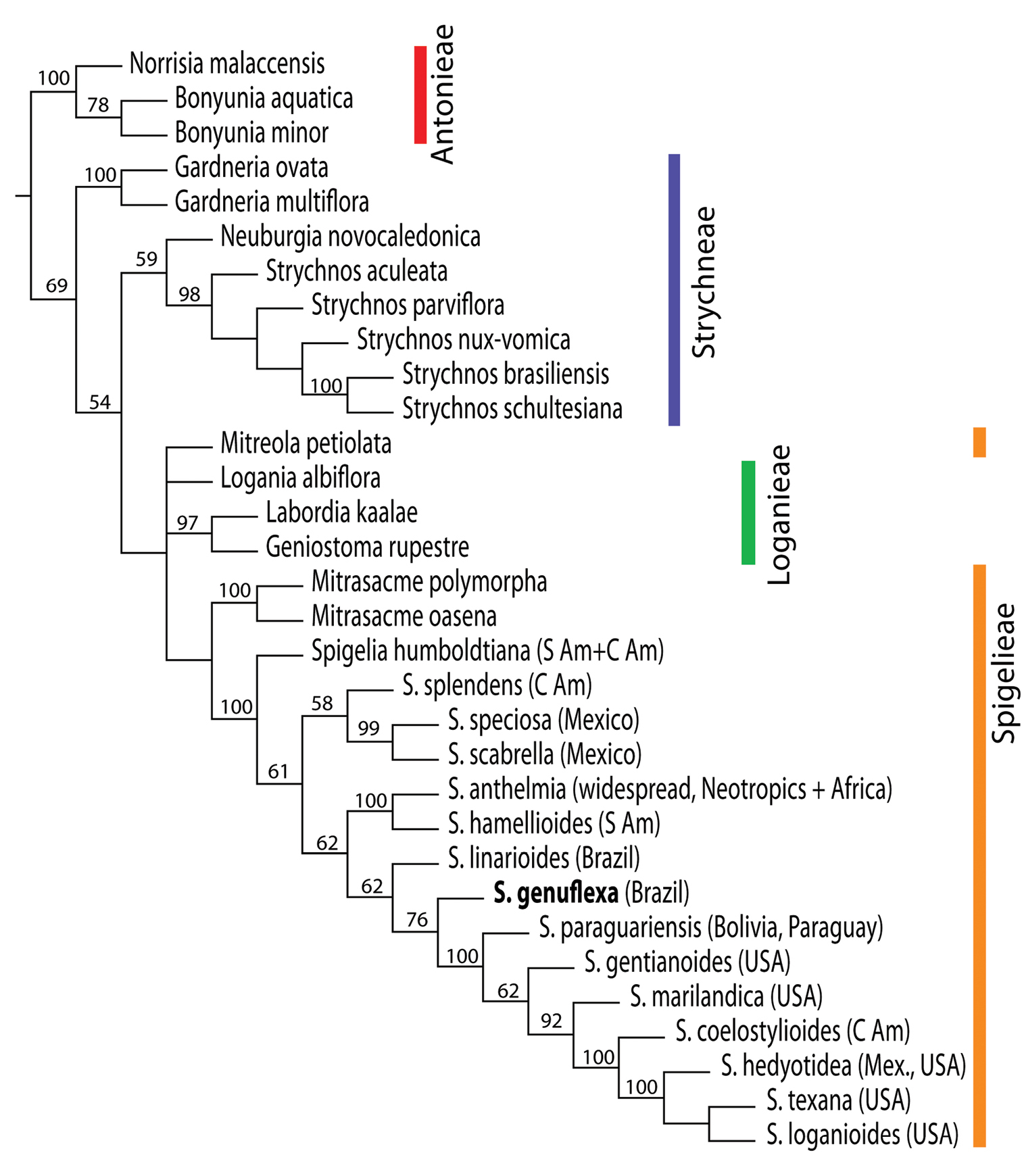
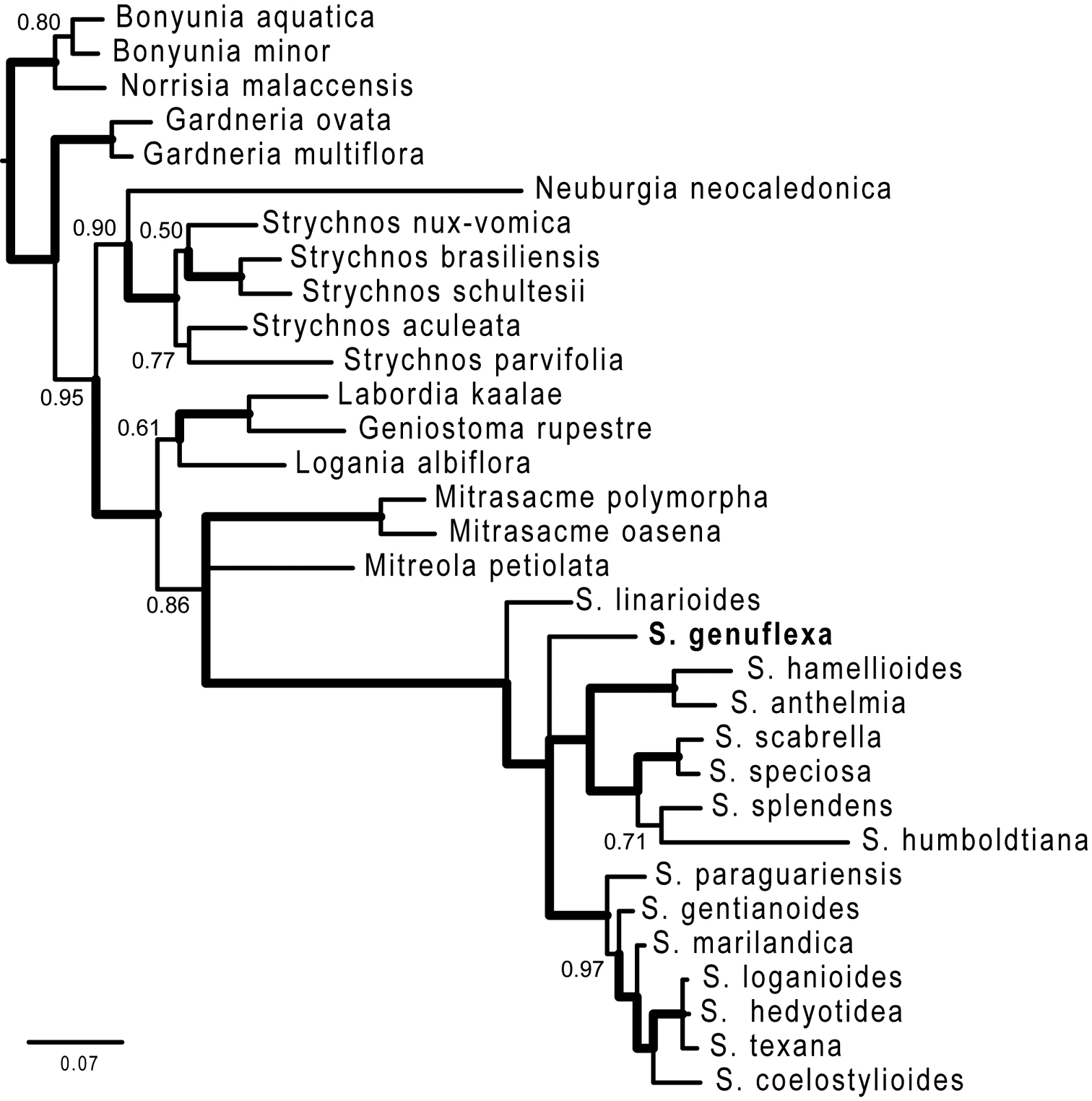
Complete phylogenetic results are presented in Appendix I. Thus far, we have been able to include only one other Brazilian species in the phylogenetic analysis, therefore our results are to be viewed as preliminary but having a bearing on the status of the new species. ITS sequences confirm the position of Spigelia genuflexa within the genus Spigelia relative to multiple outgroups in Loganiaceae. The strict consensus of two most parsimonious trees is shown in Figure 3. In this tree Spigelia genuflexa is placed as sister to a clade containing five northern warm-temperate taxa and two tropical taxa. The only other strictly Brazilian species included in the analysis, Spigelia linarioides DC., is positioned on the node just below Spigelia genuflexa. Below the branch with Spigelia linariodes is a clade formed by the two widespread species, Spigelia anthelmia and Spigelia hamellioides, and below this a clade of three Mexican species. Spigelia humboldtiana, a widespread species from central South America to southern Mexico, is most basal in Spigelia. Figure 4 shows the results of the Bayesian analysis, which differ from the parsimony results primarily in the positions of Spigelia linarioides and Spigelia humboldtiana: Spigelia linariodes from Brazil is on a basal branch outside of all other Spigelia species, and Spigelia humboldtiana is sister to a Mexican species, Spigelia splendens H. Wendl. ex Hook. In the Bayesian analysisthe position of Spigelia genuflexa remains unresolved, but phylogenetically distinct.
Strict consensus tree derived from molecular data (ITS and coded gaps) showing phylogenetic relationships of Spigelia and outgroups within Loganiaceae (tribal classification according to
50% majority rule consensus tree from the Bayesian analyses. Numbers are clade posterior probability (pp) values; thickened branches indicate pp = 1.00. The genus name Spigelia is abbreviated to the first letter.
Morphology. Morphologically, Spigelia genuflexa displays a mosaic of traits similar to other species, as well as several unique characteristics, including geocarpy. Its leaves are densely covered with short, simple, slightly hooked papillae-like hairs, a trait that appears to be unique within the genus. Its small, white, funnelform flowers with included stamens (vs. long-tubular or campanulate, brightly colored flowers, with exserted stamens) make it similar to Spigelia flemmingiana (not included in the phylogeny), Spigelia anthelmia, Spigelia hamellioides (syn. Spigelia multispica Steud.), and many others. Other traits shared with the latter three species are the annual lifespan, the warty (vs. smooth) capsule and the persistent fruit base with pointed tips (vs. rounded or emarginate tips). Like Spigelia anthelmia, Spigelia genuflexa has a persistent style segment on the capsule that is short and stout (vs. long and threadlike). Like Spigelia hamellioides, Spigelia genuflexa has pedunculate inflorescences (vs. sessile). Spigelia genuflexa is distinctly different from other species occurring in the Atlantic coastal forest biome of Bahia, including Spigelia anthelmia, Spigelia blanchetiana A.DC., Spigelia flemmingiana Cham. & Schltdl., Spigelia glabrata Mart., Spigelia laurina Cham. & Schltdl., Spigelia linarioides (in the phylogeny), Spigelia schlechtendaliana Mart., Spigelia spartioides Cham., and Spigelia tetraptera Taub. ex L.B. Sm.. Determining the definitive relationships of Spigelia genuflexa within Spigelia will undoubtedly depend on the future inclusion of many additional species in a phylogenetic analysis.
Phylogeny. Very little phylogenetic work has been published in Spigelia,
despite it being a relatively large genus with interesting Neotropical
distribution and variable morphology linked to ecological traits, such
as life span, pollination syndromes, and weediness (but see
The main goal with our phylogenetic analysis, however, was to place Spigelia genuflexa in a taxonomic neighborhood within its genus and the Loganiaceae. It is clearly supported as included in Spigelia, and it is also clearly not very close to any other Spigelia species we have sequenced so far, based on sequence similarity or position in the cladograms. In fact, we cannot say with any certainty where its affinities lie since it is not definitively grouped with any other species. There is low jacknife support in the parsimony analysis for its inclusion in a clade with Spigelia paraguariensis Chodat and more northern species. It does not group with any member of section Anthelmiae Progel (Spigelia anthelmia, Spigelia hamellioides and Spigelia humboldtiana), with which it shares morphological similarities, although this section is not monophyletic in any analyses. It also does not group with the only other Brazilian species, Spigelia linarioides, which is understandable, considering their quite different vegetative morphologies.
Like section Anthelmiae, sections Graciles Progel, Stenophyllae Progel and Speciosae
Progel are not monophyletic in any analyses. This is not surprising,
given the superficial nature of Progel's (1868) classification, based
on a small number of morphological characters. Vegetative characters
used by Progel, which may be highly plastic, include vestiture, stem
shape and leaf venation. Floral characters he used, which may be
related to pollinator selection, include corolla shape and exsertion of
anthers and stigma. In the future, mapping morphological characters
onto a better resolved molecular phylogeny might give some insight into
character evolution in Spigelia. The one infrageneric classification that holds up in all analyses is the North American section Coelostylis(Torr. & A. Gray) Fern. Casas, the members of which (Spigelia texana(Torr. & A. Gray) A. DC., Spigelia loganioides(Torr. & A. Gray) A. DC., Spigelia hedyotidea A. DC.) form a strongly supported clade. According to our trees, the more tropical Spigelia coelostylioides K.R. Gould is closely related to this group, as hypothesized earlier (
The sister group to Spigelia is shown to be other members of tribe Spigelieae sensu Leeuwenberg and Leenhouts (1980), with strong support in the Bayesian analysis (1.00 pp). Mitrasacme Labill. is sister to Spigelia in the parsimony tree, but with less than 50% jacknife support. Mitrasacme and Mitreola L. share a clade with Spigelia in the Bayesian analysis (0.86 pp), though which of the former is closer to Spigelia remains unresolved. In the parsimony tree, Mitreola's position within Loganiaceae is also unresolved.
Dwarfism in ephemeral annuals. Dwarfism is also seen in two other Spigelia species, Spigelia pygmaea D.N. Gibson and Spigelia polystachya Klotzsch, both of which are annuals and are characteristically shorter than other Spigelia species. We have not yet been able to include either of these in the phylogenetic analysis. Spigelia pygmaea is known only from Chiapas, Mexico, and Guatemala, growing in generally dry habitats, including dry, deciduous forest, pine forest, and savanna. Like Spigelia genuflexa, it has warty-papillose capsules. Spigelia polystachya grows in seasonally flooded fields and mud flats from southern lowland Mexico south to Bahia and Goiás, Brazil, and appears to flower year-round. Since the only collection of Spigelia genuflexa of much taller size (to 25 cm) was made in the multi-layer leaf litter of the tabuleiro forest, the dwarfism of the initially collected plants could be strongly influenced by the differences in their respective micro-habitats, with the dwarfed plants found in a rather inhospitable bare sandy soil environment, with very little leaf litter. Such size plasticity is not uncommon in plants; however, even the larger specimens of Spigelia genuflexa represent plants of a small stature.
Geocarpy. There are several adaptive advantages of
geocarpy for plants growing in variable, heterogeneous, or ephemeral
environments, such as the retention of offspring in advantageous
microhabitats, protection of seeds from environmental extremes, fire,
and predators. The depositor-style geocarpy, in Hylander's terminology
(
Spigelia genuflexa is a new and unique species, with geocarpic fruits, the first known case of geocarpy in the Loganiaceae. It is not surprising it has not been detected earlier, given its diminutive stature and high biodiversity in the area. Northeastern Brazil contains the greatest number of known Spigelia species, most of which have been little studied. To better understand the taxonomic and distributional ranges of Spigelia species in Brazil, the threats to their survival, and their relationships and evolution, and last but not least, to get a better estimate of their actual number, a revision of Brazilian species is greatly needed.
This work was partially funded through a grant provided to LS (USDA/NJAES-NJ17112). M. C. Molina received a grant as a three-month Visiting Scientist from Estancias Breves Investigación, URJC, Spain. We are grateful to the staff at the HUEFS and NY herbaria for their help. We thank Daniela Zappi for her early input in the project, and two anonymous reviewers for constructive criticism on an earlier version of this manuscript. Bobbi Angell provided the line drawings; Cynthia Frasier provided DNA sequences from Loganiaceae; Jason R. Grant translated the diagnosis; and Domingos Benício Oliveira Silva Cardoso helped with the Portuguese abstract.
Molecular studies. Total genomic DNA extraction of Spigelia genuflexa was done by grinding one leaf from a NY herbarium specimen with sterile glass pestle in a mortar, and then processed using the DNAeasy Plant Mini Kit (Qiagen), according to the manufacturer's instructions.
The nuclear ITS rDNA region was amplified using the
primers 5'-AACAAGGTTTCCGTAGGTGA-3' (modified from Baldwin 1992) and
5'-GCTACGTTCTTCATCGATGC-3' (
Additional ITS sequences from other Spigelia species were provided by co-author K. Mathews (
Material for phylogenetic analysis using ITS
sequences, with voucher information, Genbank accession number, and
tribal classification according to
| Species | Tribal classification | Infrageneric classification of Spigelia, if applicable | Genbank accession number | Voucher or publication |
|---|---|---|---|---|
| Bonyunia aquatica | Antonieae | JF937926 | Berry et al. 5771 (NY) | |
| Bonyunia minor | Antonieae | JF937927 | Berry & Brako 5522 (NY) | |
| Gardneria multiflora | Strychneae | JF937929 | Ceming 9611186 (MO) | |
| Gardneria ovata | Strychneae | JF937930 | Klackenberg & Lundin 214 (NY) | |
| Geniostoma rupestre | Loganieae | DQ499095 | Wright et al. (2006) | |
| Labordia kaalae | Loganieae | JF937931 | Motley 1203 (BISH) | |
| Logania albiflora | Loganieae | DQ358879 | Hubbard 4198 (G) | |
| Mitrasacme oasena | Spigelieae | JF937932 | Forste et al. PIF24800 (NY) | |
| Mitrasacme polymorpha | Spigelieae | JF937933 | Anonymous 20495 (NY) | |
| Mitreola petiolata | Spigelieae | AF054635 | Gould 150 (TEX/LL) | |
| Neuburgia novocaledonica | Strychneae | JF937935 | Struwe 1301 (NY) | |
| Norrisia malaccensis | Antonieae | JF937936 | Stone 14107 (HUH) | |
| Spigelia anthelmia | Spigelieae | Anthelmiae | JF937937 | Worthington 21205 (NY) |
| Spigelia coelostylioides | Spigelieae | AF177992 | Gould 139 (TEX/LL) | |
| Spigelia gentianoides | Spigelieae | JN005877 | Bok Tower Gardens Rare Plant Collection, Lake Wales Florida (living collection) | |
| Spigelia genuflexa | Spigelieae | JN005878 | Popovkin 602 (NY) | |
| Spigelia hamellioides | Spigelieae | Anthelmiae | JN005879 | Gould 7 (TEX/LL) |
| Spigelia hedyotidea | Spigelieae | Coelostylis | AF178008 | Gould 103 (TEX/LL) |
| Spigelia humboldtiana | Spigelieae | Anthelmiae | JN005881 | Gould 162 (TEX/LL) |
| Spigelia linarioides | Spigelieae | Graciles | JN005880 | Taylor et al. 1508 (K) |
| Spigelia loganioides | Spigelieae | Coelostylis | AF178000 | Goldman 433 (TEX/LL) |
| Spigelia marilandica | Spigelieae | Graciles | AF177991 | Gould 163 (TEX/LL) |
| Spigelia paraguariensis | Spigelieae | Stenophyllae | JN005882 | Zardini & Velasquez 27462 (G) |
| Spigelia scabrella | Spigelieae | Stenophyllae | JN005885 | Williams 9565 (TEX/LL) |
| Spigelia speciosa | Spigelieae | Speciosae | JN005884 | Gould 136 (TEX/LL) |
| Spigelia splendens | Spigelieae | Speciosae | JN005883 | Panero 5758 (TEX/LL) |
| Spigelia texana | Spigelieae | Coelostylis | AF178006 | Gould 135 (TEX/LL) |
| Strychnos aculeata | Strychneae | JF937940 | Merello 1338 (MO) | |
| Strychnos brasiliensis | Strychneae | JF937956 | Medri etal. 446 (NY) | |
| Strychnos nux-vomica | Strychneae | JF938015 | Maxwell 90–622 (MO) | |
| Strychnos parvifolia | Strychneae | JF938021 | Rodal et al. 502 (MO) | |
| Strychnos schultesiana | Strychneae | JF938036 | Liesner & Gonzalez 9170 (MO) |
The Spigelia genuflexa sequence was edited and assembled using Sequencher ver. 4.10 (GeneCodes). The sequences were aligned using MUSCLE ver. 3.7 (
Phylogenetic analysis. The phylogenetic data matrices were analyzed using maximum parsimony using NONA (
For the second, Bayesian, analyses we chose the best
fitting nucleotide substitution model (excluding the binary indel
partition), using the Akaike information criterion (AIC) in JMODELTEST
ver. 0.1.1 (
Results. The parsimony analysis of ITS yielded only four most parsimonious trees, with 993 steps, consistency index (ci) = 0.59, and retention index (ri) = 0.68. When coded gap characters were included, two most parsimonious trees were found, with a length of 1348 steps, ri = 0.57, and ci = 0.73 (strict consensus tree shown in Figure 3, with jackknife support above branches). Spigelia is supported as monophyletic in both analyses, with a jackknife value of 99% in the ITS-only analysis. Placed as sister to Spigelia is Mitrasacme, but with low support (50%, ITS only; below 50%, ITS plus gaps).
The strict consensus trees from these two analyses are largely congruent, with a few exceptions. The results from ITS only differ from matrix 2 (ITS with coded gaps) by: 1) positioning a species of Mitreola as sister to Logania, and this clade as sister clade to Labordia and Geniostoma; 2) collapsing the node below Strychnos parvifolia within Strychnos; and 3) rearranging relationships among Spigelia species in the clade, including Spigelia paraguariensis. These three differences are nodes that are relatively poorly supported by jackknife analysis (up to 62% support) based on ITS only data.
The new species, Spigelia genuflexa, is placed as sister (76% support) to a clade containing five northern warm-temperate taxa (Spigelia marilandica, Spigelia gentianoides, Spigelia texana, Spigelia hedyotidea, Spigelia loganioides) in addition to two tropical taxa, Spigelia paraguariensis (Paraguay) and Spigelia coelostylioides (Chiapas, Mexico; Figure 3) in the parsimony results. The only other strictly Brazilian species included in the analysis, Spigelia linarioides, is positioned on the node right below Spigelia genuflexa. Below the branch with Spigelia linariodes is a clade formed by the two widespread species, Spigelia anthelmia and Spigelia hamellioides, and below this a clade of three Mexican species (Spigelia scabrella, Spigelia speciosa, Spigelia splendens). The species placed on the most basally positioned branch within Spigelia is Spigelia humboldtiana, a multi-stemmed, basally woody herb widespread from central South America to southern Mexico.
In the Bayesian analysis, Mitreola and Mitrasacme are in an unresolved sister clade to Spigelia (0.86 posterior probability [pp]), and Spigelia is monophyletic (1.00 pp; Figure 4). Within Spigelia, the Bayesian results differ from the parsimony results primarily in the positions of Spigelia linarioides and Spigelia humboldtiana: Spigelia linariodes from Brazil is on a basal branch outside of all other Spigelia species (1.00 pp), and Spigelia humboldtiana is sister to a Mexican species, Spigelia splendens (0.71 pp). Spigelia genuflexa's position remains unresolved, but phylogenetically distinct, in the Bayesian analysis. We experimented with setting priors in the Bayesian inference by fixing the substitution rates and nucleotide frequencies to the values in the Q-matrices output by jModelTest (results not shown). This corresponded to the more parameter-rich TIM3 model. The resulting consensus tree placed Spigelia genuflexaas sister to the Spigelia paraguariensis + North American species clade (the same position as in the parsimony analyses), albeit with a low posterior probability (0.55). Other relationships within Spigelia did not change.
DNA alignment of ITS from Loganiaceae taxa, especially Spigelia, shown in Fasta format. (doi: 10.3897/phytokeys.6.1654.app2)
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Citation: Popovkin AV, Mathews KG, Santos JCM, Molina MC, Struwe L (2011) Spigelia genuflexa (Loganiaceae), a new geocarpic species from the Atlantic forest of northeastern Bahia, Brazil. PhytoKeys 6: 47–65. doi: 10.3897/phytokeys.6.1654.app2
Alignment of ITS from Loganiaceae taxa, especially Spigelia, including coded gaps, Nexus format. (doi: 10.3897/phytokeys.6.1654.app3)
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Citation: Popovkin AV, Mathews KG, Santos JCM, Molina MC, Struwe L (2011) Spigelia genuflexa (Loganiaceae), a new geocarpic species from the Atlantic forest of northeastern Bahia, Brazil. PhytoKeys 6: 47–65. doi: 10.3897/phytokeys.6.1654.app3
Bayesian run-file of ITS plus coded gaps from Loganiaceae taxa, especially Spigelia, Nexus format. (doi: 10.3897/phytokeys.6.1654.app4)
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Citation: Popovkin AV, Mathews KG, Santos JCM, Molina MC, Struwe L (2011) Spigelia genuflexa (Loganiaceae), a new geocarpic species from the Atlantic forest of northeastern Bahia, Brazil. PhytoKeys 6: 47–65. doi: 10.3897/phytokeys.6.1654.app4