(C) 2011 Mario Martínez-Azorín. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The name Albuca caudata Jacq. has been widely misunderstood or even ignored since its description in 1791. After studying herbarium specimens and living populations in South Africa, plants fitting Jacquin´s concept of that species are found to be widely distributed in the Eastern Cape, mainly in the Albany centre of Endemism. Furthermore, some divergent specimens matching Baker´s concept of Albuca caudata are described as a new related species: Albuca bakeri. Data on typification, morphology, ecology, and distribution are reported for both taxa. Affinities and divergences with other close allies are also discussed.
Albuca bakeri sp. nov., Albuca caudata, distribution, taxonomy, typification
The genus Albuca L. is accepted to include about 60 species in recent treatments (cf.
Taxa of Albuca in its traditional sense (cf.
Herbarium specimens from the following herbaria were
studied: BOL, BNRH, GRA, J, K, KEI, KMG, NBG, NH, NU, PEU,
PUC, UFH, WIND (acronyms according to
Albuca caudata was described by
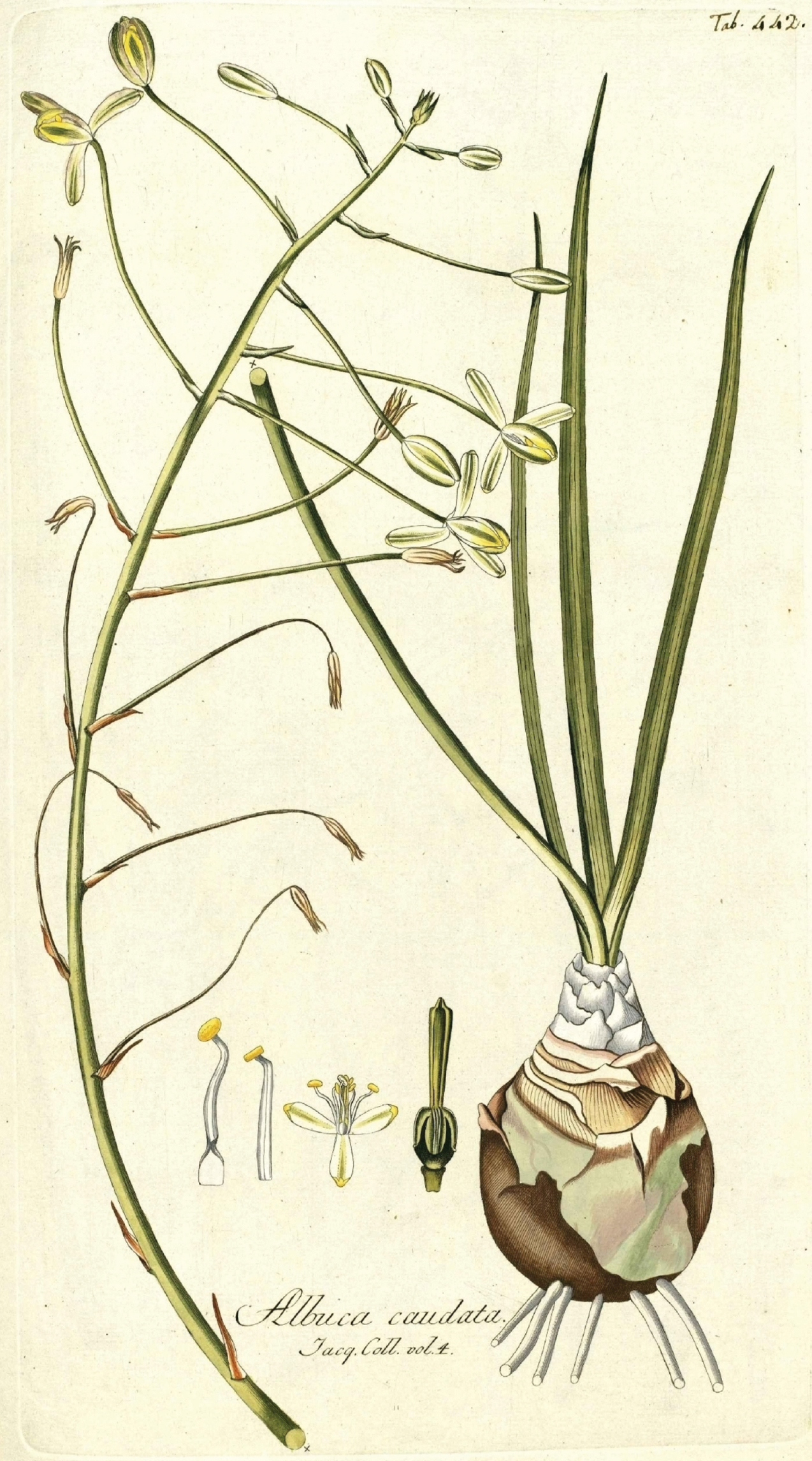
Jacquin, Ic. Pl. Rar. 2(16): 20, t. 442 (1795), ex Promontorio bonae Spei. Apud nos in caldariis floret Decembri & Januario (Fig. 1).
SOUTH AFRICA. Eastern Cape: Alexandria, Addo National Park, 400 feet, 29.X.1954, S.M. Johnson 1077 (GRA).
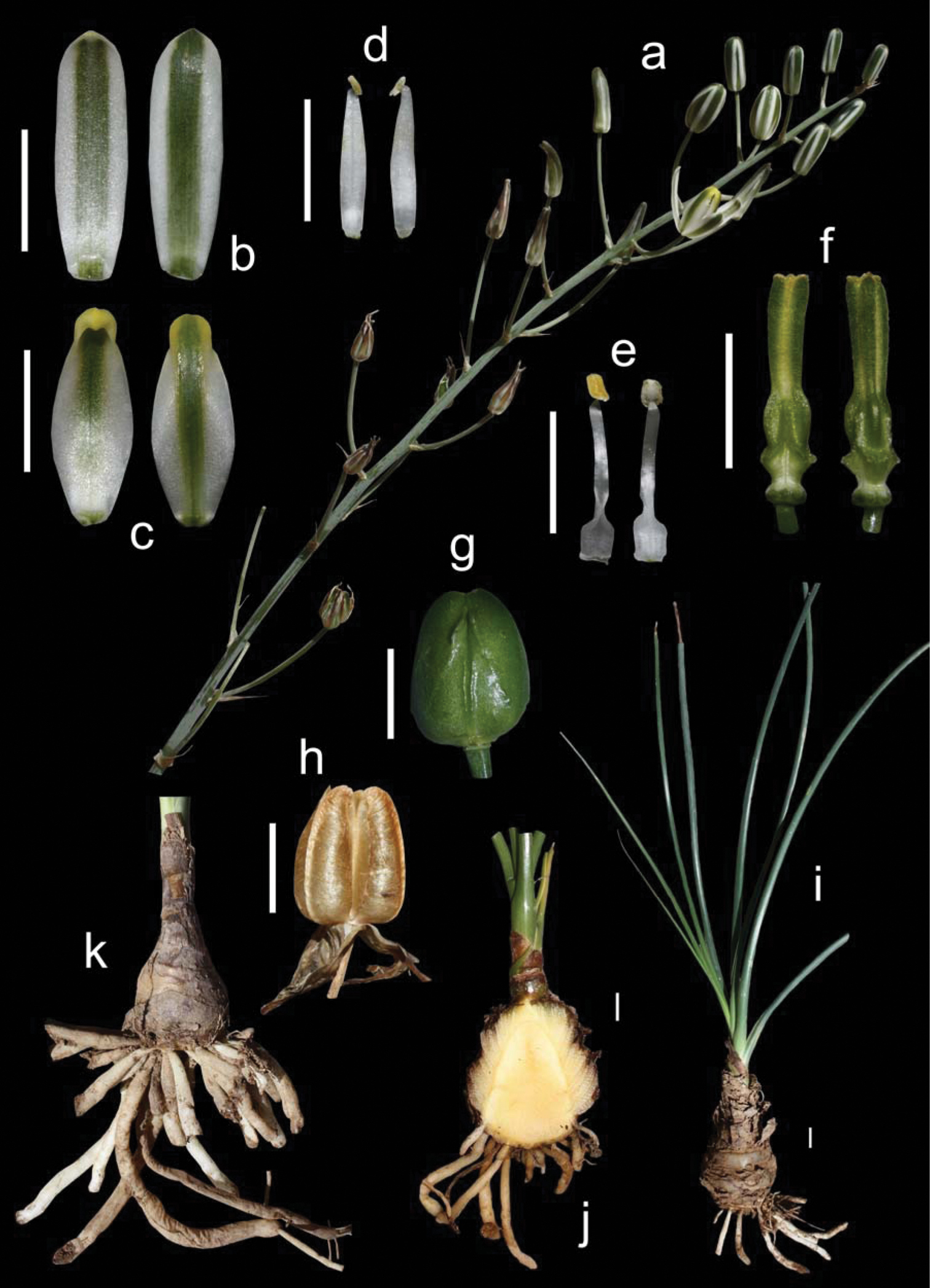
Evergreen bulbous plants. Bulb mostly solitary and hypogeal, ovoid to oblong, up to 10 × 6 cm, usually with its wide basal plate elongated into a domed axis where the fleshy scales are attached; tunics fleshy, short and usually not reaching the top of the bulb, imbricate, persistent, papery grey or brownish in the upper part, sometimes with transversal prominent dark ridges, giving a brownish multiscaled appearance to the bulb in outline. Roots fleshy, thick and usually tuberose, white, numerous, up to 200 × 4 mm. Leaves 4-10, disposed in an apical rosette, linear-lanceolate, 15-120 × 0.5-2 cm, straight up and curving down when old, infolded, canaliculate, persistent, pale bright green to glaucous, glabrous, usually minutely papillate on nerves and margins, with a terete apex evident in young leaves. Inflorescence inclined, unilateral raceme, 11–40 cm long; peduncle 12–55 cm long; pedicels 3.5–9 cm long at base becoming smaller, up to 0.2–1 cm long near top, patent and being usually all erect; bracts ovate-lanceolate to triangular, long acuminate, 11–25 × 5–9 mm, papery white with brownish distant nerves that converge at the tips, much shorter than pedicels in the lower part of the inflorescence. Flowers erect; tepals white with a green median stripe 2–4 mm wide, sometimes with the tips yellowish; outer tepals oblong, 18–28 × 4–7 mm, apex slightly cucullate; inner tepals ovate, 15–24 × 4–10 mm, with apex strongly cucullate. Stamens all six bearing fertile anthers; outer anthers 1.5–2.5 mm long; inner anthers 3–4 mm long; outer filaments 10–16 × 1.5–2 mm, linear lanceolate to narrowly oblong, not pinched down; inner filaments 10–17 × 1.5–3 mm, linear oblong, wider and pinched in the lower half. Ovary oblong to obovate, up to 6–8 × 2–3.5 mm, stipitate, with prominent paraseptal crests that are divergent in the lower part and form three prominent ridges; style subobpyramidal or clavate, trigonous, up to 7–10 × 2 mm, stigma yellowish green. Capsule ovate, 14–20 × 10–14 mm, trigonous to subsphaerical in section, pale-brown when mature; valves splitting in the upper quarter. Seeds flat, c. 5–6 × 4–5 mm, dark brown to black, flattened and semidiscoidal, biseriate and horizontally stacked in each locule. (Fig. 3)
September to November; capsules dehiscing at the end of November and December.
Plants of Albuca caudata are often associated with bush-clumps, where the inclined inflorescence is supported by woody plants.
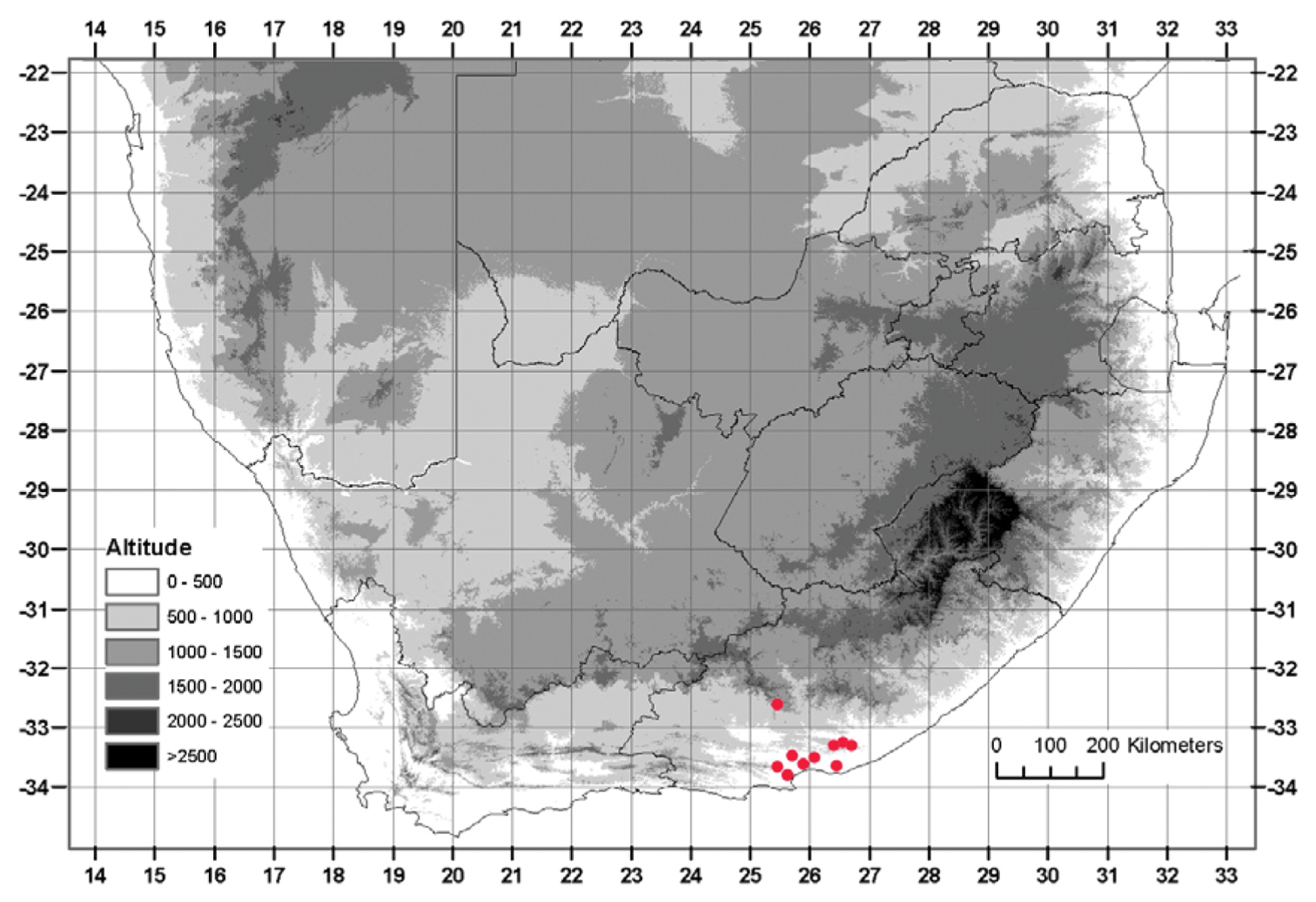
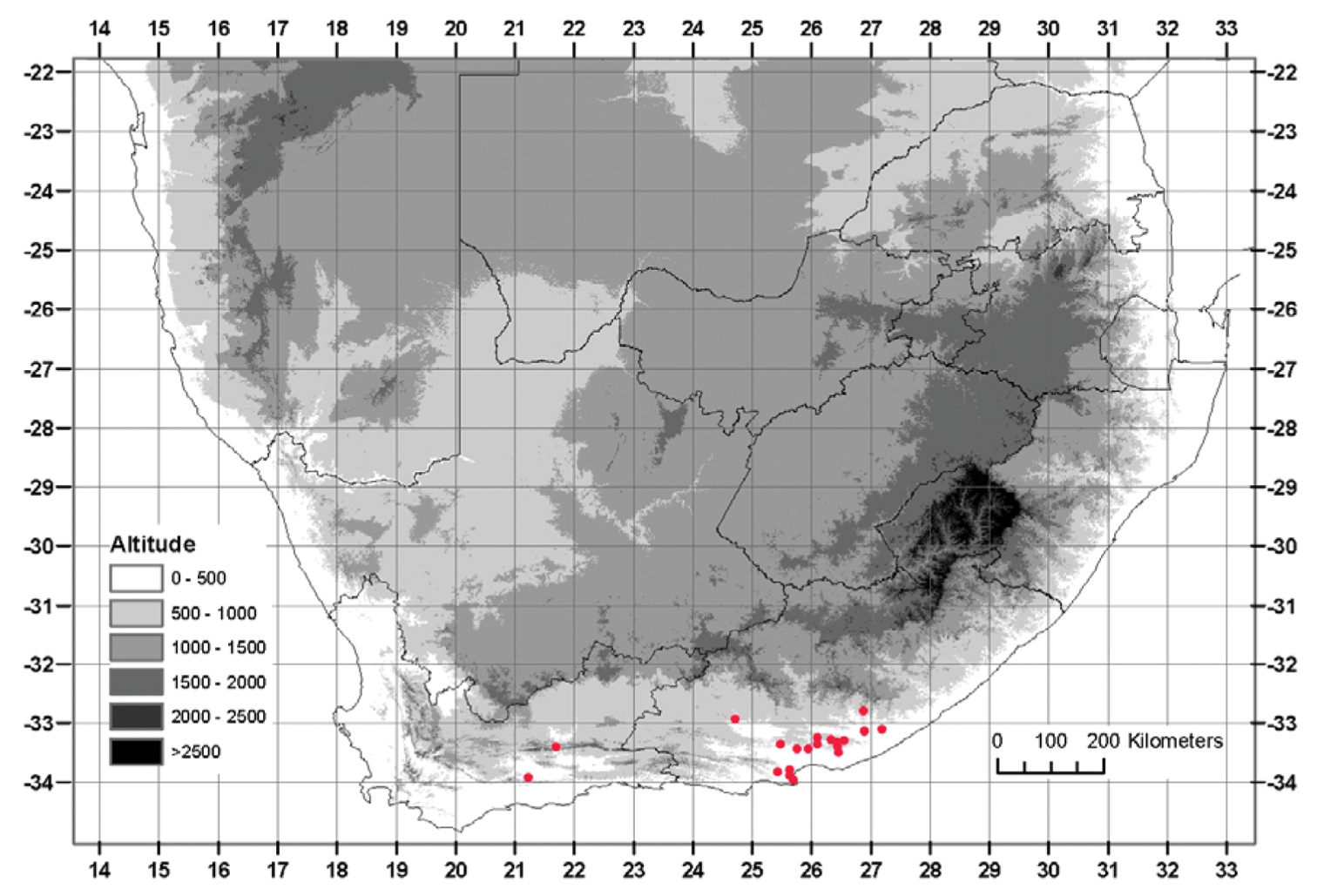
Currently known from Addo in the west to Grahamstown in the east, below 600 m, with an outlying population as far inland as Somerset East, reaching 900 m (Fig. 4).
Albuca caudata can be easily identified by its bulb mostly solitary covered by brownish papery scales usually disposed at different heights and bearing long thick tuberose roots, its long and narrow canaliculate or infolded leaves, its inclined raceme, with usually all pedicels patent and erect, giving a unilateral appearance to the inflorescence, and its white erect flowers with a median green stripe (Fig. 3).
The specific epithet ‘caudata' presumably refers to the rather pointed, tail-like leaves, although Jacquin did not specifically mention it (E.E.A. Gledhill, unpubl. ms. in NBG).
The recently described Albuca batteniana Hilliard & B.L. Burtt (
Albuca caudata shows some variability in the colour of the membranous bulb scales, being pale coloured with orange transversal ridges in some inland populations whilst those from the coastal areas are usually brown coloured with darker transversal ridges.
SOUTH AFRICA. Alexandria, 4 miles east of Sandflats, 1000 feet, 17/12/1953, E.E.A. Archibald 5431 (GRA); Eastern Cape, along road in Springs Reserve, north of Uitenhage, 39 m, 21.X.2009, A.B. Low 16732 (GRA); Addo Elephant National Park, in main Botanical Reserve, 20.X.1996, K. Johnson 241 (GRA); Alexandria, Nanaga, opposite Glen Rosa turn-off, 1100 feet, 23.X.1953, E.E.A. Archibald 5315 (GRA); Eastern Cape, Albany, Queen´s Road, 10 miles north of Grahamstown, 2000 feet, 05.X.1953, S. Johnson 774 (GRA); Eastern Cape, Albany, a few yards from Archibald 5636, Pluto´s Vale, 2000 feet, 22.IX.1954, E.E.A. Archibald 5636 (GRA); Eastern Cape, Grahamstown, c. 5 miles on Cradock road, 626 m, 11.XII.2009, M. Martínez-Azorín & A.P. Dold 85 (GRA); Eastern Cape, Redhouse, thicket west of village, 6 m, 27.XI.2009, M. Martínez-Azorín, A.P. Dold & A. Martínez-Soler 45 (GRA).
Albuca caudata Jacq. from Jacquin, Ic. Pl. Rar. 2(16): 20, t. 442. 1795.
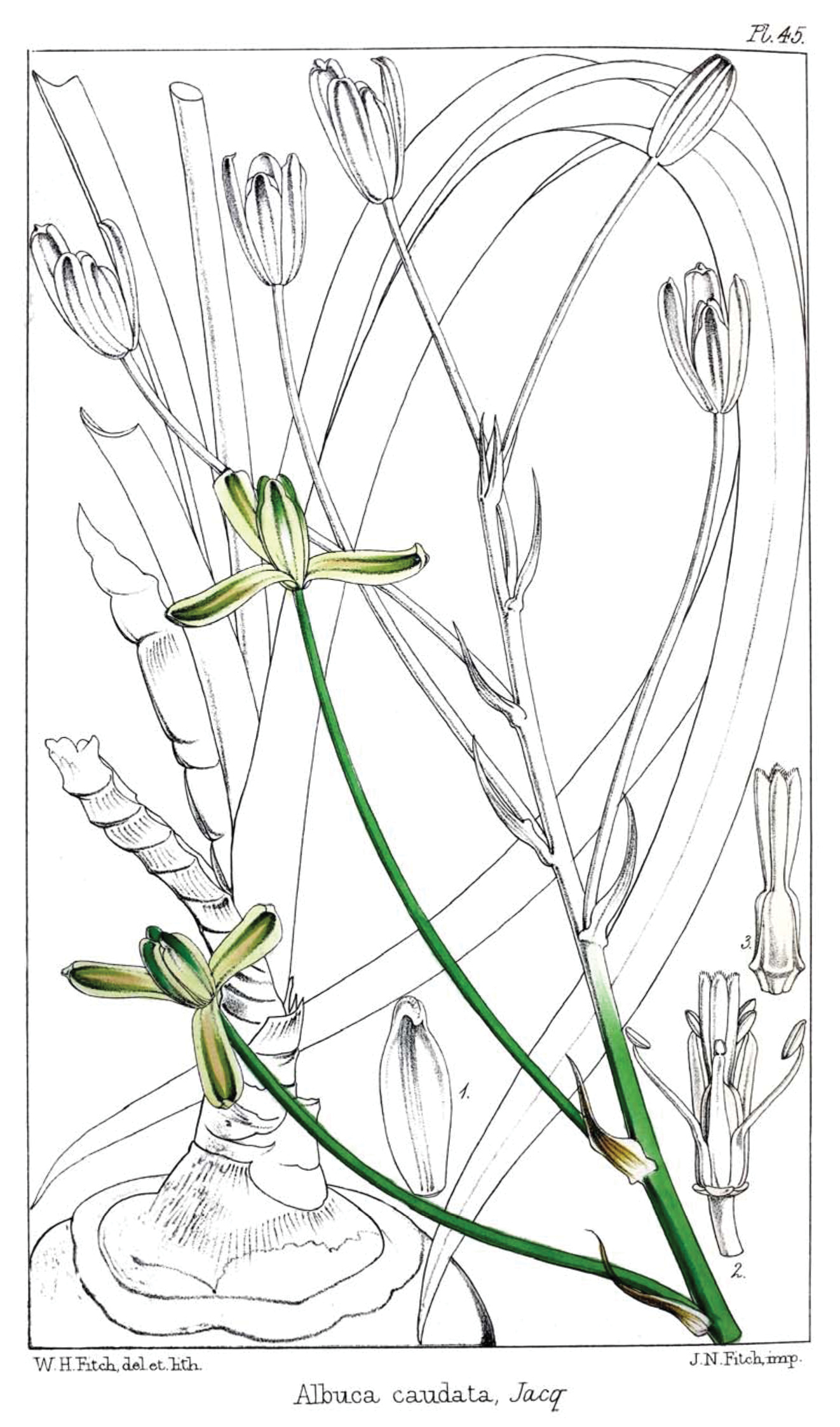
Albuca bakeri Mart.-Azorín & M.B. Crespo from Baker, Refug. Bot. (Saunders) vol. 1, t. 45. 1869 (as Albuca caudata).
Albuca caudata Jacq. Eastern Cape, Redhouse (M. Martínez-Azorín, A.P. Dold & A. Martínez-Soler 45 GRA) a Inflorescence b Outer tepals c Inner tepals d Outer stamen e Inner stamen f Ovary, lateral views g Mature capsule h Dehiscing capsule i Bulb and leaves j Bulb in longitudinal section k Bulb with tuberose roots. Scales 1 cm.
Known distribution of Albuca caudata Jacq.
Main diagnostic characters among Albuca caudata, Albuca bakeri and Albuca batteniana.
| Albuca caudata | Albuca bakeri | Albuca batteniana | |
|---|---|---|---|
| Bulb | Mostly solitary | Mostly solitary | Proliferous |
| Ovoid to oblong | Ovoid to spherical | Narrowly oblong | |
| Outer tunics membranous, brown to grey | Outer tunics fleshy, white to yellow | Outer tunics somewhat coriaceous, green to brown | |
| Mostly hypogeal | Hypogeal | Mostly epigeal | |
| Imbricate scales mostly ending at different heights | All scales reaching the top of the bulb | Imbricate scales ending at different heights | |
| Neck absent or short and thick, covered by brown to grey membranous scales | Neck long and thin, covered by transversally banded sheathing cataphylls | Neck usually absent | |
| Roots | Usually numerous, thick and tuberose | Thin and scarce | Thin or slightly thickened |
| Leaves | Narrow, infolded and canaliculate | Narrow, infolded and canaliculate | Wide, flattened and usually recurved |
| Inflorescence | Inclined and secund | Erect and helicoidal | Inclined and secund |
| Outer tepals | 18–28 mm | 19–23 mm | 30–42 mm |
| Seeds | 5–6 × 4–5 mm | 4–5 × 3–4 mm | 5–7 × 4–5 mm |
SOUTH AFRICA. Eastern Cape: North of Grahamstown, on Cradock Road turn off to Kwandwe, 592 m, 05.IX.2010, 33°12'39"S, 26°24'07"E, M. Martínez-Azorín & A. Martínez-Soler 218 (GRA Holo.; ABH, K, NBG, PRE Iso.).
Diagnosis: Species insignis ex Albuca subg. Mitrotepalum characteribus floralibus ad Albucam caudatam accedit, sed valde differt et facile distinguitur bulbo hypogaeo solitario carnoso tunicis omnibus apicem attingentes in collum angustum supra solum desinentes, e basi cataphyllis albido-membranosis manifeste transversaliter fusco-striatis obtectum qui habitum pulchre zebrinum exhibent, insuper racemo subdeltoideo non secundo floribus spiraliter dispositis.
Illustrations:
Evergreen or deciduous bulbous plants. Bulb mostly solitary, occasionally growing in small clumps, hypogeal, ovoid to spherical, 3.2–7 × 2.5–6.5 cm, with soft outer tunics that are pale and fleshy, ending in a long epigeal neck, up to 10 × 2 cm, covered with whitish open and sheathing membranous cataphylls bearing transversal sinuous ridges with their lower side pale to dark brown coloured, giving a zebra banding horizontal pattern; tunics fleshy, whitish, all reaching the top of the bulb, concentrically arranged. Roots fleshy, narrow, white, up to 90 × 2 mm. Leaves 2-6, disposed in an apical rosette, linear-lanceolate to oblong, 9-40 × 0.4-1.3 cm, erect when young and later curving downwards, infolded, canaliculate, persistent or usually deciduous, pale bright green to glaucous, glabrous, usually minutely papillate on nerves and margins, exceptionally with long papillate margins. Inflorescence an erect raceme or subcorymb, 3–15 cm long; peduncle 9–22 cm long; pedicels helicoidally disposed, 3–7.5 cm long, longer at the base, up to 0.2–0.7 cm long near top, erect-patent; bracts ovate-lanceolate to triangular, long acuminate, 9–27 × 4–10 mm, papery white with brownish separated nerves that converge at the tips, much shorter than pedicels at least in the lower part of the inflorescence. Flowers erect; tepals white with a green median stripe 2–3 mm wide, sometimes with the tips yellowish; outer tepals lanceolate-oblong, 19–23 × 5–7 mm, with apex slightly cucullate; inner tepals ovate, 13–17 × 6–7 mm, with apex strongly cucullate. Stamens all six bearing fertile anthers; outer anthers 1.5–3 mm long, inner anthers 4–6 mm long; outer filaments 10–13.5 × 1.5–2 mm, linear lanceolate to narrowly oblong, not pinched down; inner filaments 10.5–14.5 × 2–3.5 mm, linear oblong, wider and pinched in the lower half. Ovary oblong to obovate, up to 6–7 × 2–3.5 mm, stipitate, with prominent paraseptal crests that are divergent in the lower part and form three prominent ridges; style subobpyramidal or clavate, trigonous, up to 7–11 × 3.5–4.5 mm, stigma yellowish green. Capsule ovate, 14–16 × 11–12 mm, trigonous to subsphaerical in section, pale-brown when mature; valves splitting in the upper quarter. Seeds flat, c. 4–5 × 3–4 mm, dark brown to black, flattened and semidiscoidal, biseriate and horizontally stacked in each locule. (Fig. 5)
July to September; capsules dehiscing at the end of September and November.
Albuca bakeri is found growing singly in dry, stony, open ground at low altitude reaching c. 650 m.
from Jansenville to Alice and the Keiskamma river in the Eastern Cape, with two outlying populations near Calitzdorp in the Western Cape karroo (Fig. 6).
Albuca bakeri can be easily identified by its solitary hypogeal fleshy bulb ending in an epigeal neck, covered by whitish transversally banded membranous cataphylls, giving a conspicuous zebra banding pattern (Fig. 5). Moreover, its erect and helicoidal raceme with white and green erect flowers, and the smaller seeds (c. 4–5 × 3–4 mm), separate it from Albuca caudata.
Name honouring John Gilbert Baker (1834–1920), a leading expert on monocotyledons, who worked at the Royal Botanic Gardens, Kew, and was the keeper of the herbarium K.
No other Albuca with erect flowers have been described with the characteristic long, thin, zebra banded bulb neck of Albuca bakeri. The closest species appears to be Albuca caudata, though the structure of the bulb and inflorescence clearly distinguish them (Table 1).
The peculiar zebra banded cataphylls of Albuca bakeri are similar to those found in some other groups of Hyacinthaceae. As pointed out by
Some morphological variation has been found within Albuca bakeri. Some individuals from Janseville and Port Elizabeth have a slightly setose bulb neck with the characteristic transversally banded membranous cataphylls of this species. Other specimens from Grahamstown and Port Elizabeth showed somewhat proliferous bulbs, resulting in a small clump of plants growing together, and with shorter scales not so markedly banded.
When
SOUTH AFRICA. Eastern Cape, Alexandria, 1½ miles east of Paterson, 1000 feet, 24.VIII.1953, E.E.A. Archibald 5972 (GRA); Eastern Cape, Alexandria, south-west end of Zuurkop, Addo National Park, 1000 feet, 23.IX.1953, S.M. Johnson 751 (GRA); Eastern Cape, Albany, 5 miles north of Alicedale, on Riebeck East road, 1500 feet, 21.IX.1954, E.E.A. Archibald 5638 (GRA); Victoria East: Alice, dry stony places on Sandilis Kop on north east side, 08.IX.1934, M.H. Giffen 614 (GRA); Victoria East: Alice, Sandilis Kop western side among grass, 13.IX.1935, M.H. Giffen 618 (GRA); Hillside, Gowie´s Kloof, Grahamstown, IX.1947, Hill s.n. (GRA); Grahamstown, West Hill, Pine plantation, VIII.1956, V. van Niekerk s.n. (GRA); Cradock road, Grahamstown, 01.IX.1945, E. Barrat 28 (GRA); In graminosis prope Grahamstown, M. Daly & M. Sole 316 (BOL); In graminosis prope Grahamstown, 2000 feet, VIII.1893, Schonland s.n. (NBG); Grahamstown (3326 BC): Ecca Reserve, south near old Queens Road/Quarry, 20.VIII.1992, T. Dold 153 (GRA); Leander Beacon, VIII.1943, L. Miles s.n. (GRA); Port Elizabeth, Summerstrand, grassy roadside, IX-X.1990, H.J. Vanderplank s.n. (GRA); Port Elizabeth (3325CD): 3 km south of Uitenhage towards van Stadens, 01.IV.1978, P.L. Perry 601 (NBG); Port Elizabeth (3325CB): Kirkwood District, farm Brakleegte, 300 m, 28.VIII.1985, M.T. Hoffman 1064, 1065 (NBG); ibidem, 14.IX.1985, M.T. Hoffman 1002 (NBG); Graaff-Reinet (3224DC): District Janseville, just south of Janseville (+/- 1 km) in municipal-owned land, 11.VIII.1985, M.T. Hoffman 1063 (NBG); Ladismith (3321BC): Calitzdorp dam, 22.II.1981, P.L. Perry 1521 (NBG); Ladismith (3321CC): Sopieshoogte, north entrance to Garcia´s Pass, Riversdale, 1600 feet, 15.IX.1981, Albuca Fellingham 149 (NBG); Eastern Cape, Grahamstown, hills above Botanic Garden, 591 m, 14.XI.2009, 33°19'04"S, 26°31'15"E, M. Martínez-Azorín & A. Martínez-Soler 12 (GRA); Eastern Cape, Grahamstown, Burnkraal, 649 m, 24.XI.2009, 33°16'40"S, 26°29'41"E, M. Martínez-Azorín & A.P. Dold 34 (GRA); Eastern Cape, Redhouse, thicket west of village, 6 m, 27.XI.2009, 33°50'01"S, 25°33'56"E, M. Martínez-Azorín, A.P. Dold & A. Martínez-Soler 44 (GRA); Eastern Cape, north of Grahamstown, Table Hill farm, 587 m, 11.XII.2009, 33°15'21"S, 26°27'17"E, M. Martínez-Azorín & A.P. Dold 83 (GRA); Eastern Cape, north of Grahamstown, on Cradock road turn off to Kwandwe, 594 m, 31.I.2010, 33°12'38"S, 26°24'07"E, M. Martínez-Azorín, M.B. Crespo & A. Martínez-Soler 118 (GRA); Eastern Cape, Quamnyana, between Breakfast Vlei and Commitees Drift, 411 m, 14.VIII.2010, 33°06'56"S, 22°55'57"E, C. Peter (GRA); ibidem, 27.VIII.2010, M. Martínez-Azorín & A.P. Dold 207 (GRA); Eastern Cape, Port Elizabeth, Settler´s Park, 28 m, 03.IX.2010, 33°58'21"S, 25°36'09"E, M. Martínez-Azorín & A.P. Dold 210 (GRA); Eastern Cape, Grahamstown, Sunny side, Hillsview street, 570 m, 07.IX.2010, 33°19'08"S, 26°32'00"E, M. Martínez-Azorín & A. Martínez-Soler 221 (GRA); Eastern Cape, Alicedale, railway cross to Burchell Game Reserve, 288 m, 14.IX.2010, 33°18'51"S, 26°06'01"E, M. Martínez-Azorín & A. Martínez-Soler 226 (GRA); Eastern Cape, Alice, Fort Hare University, Sandili's Kop, 582 m, 17.IX.2010, 32°47'02"S, 26°51'38"E, M. Martínez-Azorín & A. Martínez-Soler 235 (GRA); Eastern Cape, Keiskamma River, Linedrift, 141 m, 12.XI.2010, 33°04'29"S, 27°13'02"E, M. Martínez-Azorín, A.P. Dold & A. Martínez-Soler 525 (GRA).
Albuca bakeri Mart.-Azorín & M.B. Crespo. North of Grahamstown, turn off to Kwandwe (holotype: M. Martínez-Azorín & A. Martínez-Soler 218 GRA) a Plant b Flower c Outer tepals d Inner tepals e Outer stamen f Inner stamen g–h Ovary, lateral views i Mature capsule j Capsule, longitudinal section k Capsule, transversal section l Bulb neck with membranous banded cataphylls. Scales 1 cm.
Known distribution of Albuca bakeri Mart.-Azorín & M.B. Crespo.
This work was supported by the Fundación Ramón Areces (Spain). We thank the curators of the herbaria who provided access to specimens examined and the Cacadu Department of Economic Development and Environment Affairs for permission to collect herbarium specimens. Thanks to Cameron McMaster for providing additional images of Albuca batteniana.