(C) 2010 Stephen Stern. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Two new species of Solanum subgenus Leptostemonum from southern Ecuador and northern Peru are described here. Solanum rubicaule S. Stern, sp. nov., is a member of sect. Torva and is characterized by a festooning, scandent growth form and fruits held horizontally on recurved pedicels. Solanum achorum S. Stern, sp. nov., is a member of sect. Erythrotrichum and is characterized by 4–12-flowered inflorescences, small seeds, and a small calyx. Both species are distributed in the Amotape-Huancabamba Zone of the Andes in northern Peru and southern Ecuador.
Amotape-Huancabamba Zone, Andes, Ecuador, Huancabamba Depression, new species, Peru, Solanum
The Amotape-Huancabamba Zone of northern Peru and
adjacent southern Ecuador, also known as the Huancabamba Depression due
to the low summits of the Andes in this area, is one of the most
biodiverse regions of the neotropics (
Both of these new species belong to Solanum subgenus Leptostemonum
(Dunal) Bitter, a group characterized by long, attenuate anthers,
stellate hairs, and epidermal prickles. An extensive phylogenetic study
of this group resolved subgenus Leptostemonum as monophyletic and delimits 12 to 15 major subclades (
The second new species, Solanum achorum S. Stern, belongs to Solanum section Erythrotrichum Child. This group has approximately 23 species and is characterized by plurifoliate sympodial units (see
Morphological and molecular work has revealed Solanum rubicaule and Solanum achorum to be distinct species within in their respective clades. (S. Stern and L. Bohs, unpubl. data).
Taxonomic treatments Solanum section TorvaSolano subinermi Jacq. et S. asperolanato Ruiz & Pav. similis sed a S. subinermi pedicellis fructiferis curvatis, a S. asperolanato habitu scandenti differt.
Peru: Cajamarca: Prov. San Ignacio, road from San Ignacio to El Chaupe, 2–3 km hike in from trailhead to El Chaupe, 5°11'56"S, 79°03'51"W, 1775 m, 17 December 2007 (fl, fr), S. Stern et al. 181 (holotype: USM!; isotypes: BM001016784!, HAO [destroyed], NY00986627!, NY00986637!, UT!).
Scandent shrub, often festooning over other
plants, 1–3 m tall. Stems armed with recurved, tan to orange roselike
prickles to 3 mm in length, the base 2–3 × 0.5–1 mm, moderately to
densely pubescent with tan to rusty, porrect-stellate hairs, the
stalks 0.5–1 mm, multiseriate, the rays 5–10, 0.1–0.2 mm,
unicellular to multicellular, the midpoints nearly absent, the lateral
rays often partially proximally fused (see
Figure 1. Isotype of Solanum rubicaule S. Stern [Stern et al. 181 (UT)].
Figure 2. Photos of type collection of Solanum rubicaule S. Stern. A Collecting party in front of type collection, indicated by arrow, at trailside habitat in San Ignacio, Dept. Cajamarca, Peru (from left to right: Segundo Leiva, Stephen Stern, Mario Zapata, and Eric Tepe). B Fruiting inflorescence; note recurved pediels. C Hermaphroditic flower. D Functionally male flower; note the absence of exserted style. Scale bars = 1 cm.
Known only from northern Peru in Dept. Cajamarca and southern Ecuador in Prov. Zamora-Chinchipe in open places in disturbed montane tropical forest, 1650–2200 m in elevation.
The flowering specimen was collected in December. Fruiting specimens were collected in December–January and March–April.
The name Solanum rubicaule is derived from the festooning growth form, reminiscent of the genus Rubus L. and the Latin “caulis” for stem.
According to the IUCN Red List Categories (
Ecuador: Zamora-Chinchipe: Cantón Chinchipe, Parroquía Zumba, trail from Guaramizal to cabin of Sandy León, W of Escuela Byron Jiménez, just S of Las Pircas, 4°46'60"S, 79°12'18"W, 2100 m, 28 March 2005 (fr), L.Bohs et al. 3336 (QCNE, UT); same locality, same date (fr) L.Bohs et al. 3338 (QCNE, LOJA, UT); same locality, 4°46'50"S, 79°12'33"W, 2000 m, 29 March 2005 (fr), L.Bohs et al. 3357 (QCNE, UT); Fundación Arco Iris, between Loja and Zamora, trail from field station to Río San Francisco, 3°59'20"S, 79°05'35"W, 2200 m, 5 April 2005 (fr), L.Bohs et al. 3425 (LOJA, QCNE, UT). Peru: Cajamarca: Prov. San Ignacio, above San Francisco (ca. a El Chaupe), 1650 m, 5 January 1995 (fr), S.Leiva et al. 1621 (HAO [destroyed], NY).
Solanum rubicaule has a festooning growth form, meaning that it is often arched and draping over other vegetation. This growth form is similar to members of Solanum sect. Micracantha Dunal, a group of vining species from the New World tropics that climb using recurved prickles. This superficial similarity explains why specimens of Solanum rubicaule are often annotated as “Solanum sect. Micracantha.” However, other morphological and molecular characters place Solanum rubicaule in Solanum sect. Torva, including flowers with triangular corolla lobes with abundant interpetalar tissue and typically branched inflorescences. Parsimony analyses of sequence data from three molecular markers (nuclear ITS and waxy or GBSSI and chloroplast trnT-F) also place Solanum rubicaule in sect. Torva; however, the relationships within the section are not well-resolved and require further study (S. Stern and L. Bohs, unpub. data).
Following the definition of
Within sect. Torva, Solanum rubicaule is similar to Solanum subinerme Jacq., a species found throughout northern South America from the Guianas to central Peru, both of which have a scandent growth form and few-branched inflorescences. However, Solanum rubicaule has a distinctive infructescence with fruits held horizontal to the rachis due to pedicels that curve downward (see Fig. 2b) while Solanum subinerme has fruits held upright on erect pedicels. The adaxial leaf surface of Solanum rubicaule is unarmed, while the adaxial leaf surface of Solanum subinerme often has straight prickles to 1.5 cm long. Both species have multiseriate stalked hairs on the adaxial leaf surface but those Solanum subinerme are nearly sessile to short stalked (to ca. 0.4 mm) and very thin (ca. 0.1 mm in diameter) while those of Solanum rubicaule reach 0.6 mm with greatly thickened stalks (to 0.3 mm in diameter). Herbarium specimens of Solanum rubicaule and Solanum asperolanatum Ruiz & Pav. are very similarwith regard to pubescence and flower appearance, but the latter species has upright inflorescences that are more than twice branched, typically has >12 flowers, is a large shrub or small tree and does not have the festooning growth form of Solanum rubicaule.
Solanum section Erythrotrichum
The second new species, Solanum achorum, has morphological and molecular characters that place it in Solanum sect. Erythrotrichum (S. Stern and L. Bohs, in prep). Morphologically, this species shares the plurifoliate sympodial units, recurved prickles, ferruginous tomentum with stellate-glandular hairs, and berries 1.5–2.5 cm in diameter with a pubescent exocarp typical of other members of sect. Erythrotrichum. This group appears to have three distinct centers of diversity, in Central America, northeastern Brazil, and the Andes of Peru and Ecuador (Agra 2008).
Solano megaspermo Agra et S. velutino Dunal affinis sed a S. megaspermo inflorescentiis paucifloribus et seminibus parvioris, a S. velutino calycibus parvioris differt.
Peru: Amazonas: Prov. Chachapoyas, road from Leimebamba to Chachapoyas, about 15 km N of Leimebamba along Río Utcubamba, 6°37'39"S, 77°48'44"W, 2050 m, 13 December 2007 (fl, fr), S. Stern et al. 129 (holotype: USM!; isotypes: BM001016783!, HAO! [destroyed], NY00986767!, UT!).
Erect to scandent shrub, 1–3 m tall. Stems
armed with recurved, tan to orange roselike prickles to 2.5 mm in
length, the base 2–3 × 0.5–1 mm, sparsely to moderately pubescent with
rusty, porrect-stellate hairs, the stalks nearly absent to 0.2 mm,
multiseriate, the rays 5–10, 0.2–0.3 mm, unicellular to
multicellular, the midpoints nearly absent. Flowering portions of stem
consisting of plurifoliate sympodial units, the leaves apparently not
geminate. Leaves simple, the blades 6–20 × 2.5–11 cm, elliptic to
ovate, chartaceous, discolorous, adaxially dark green, abaxially
whitish green, the adaxial surface sparsely stellate-pubescent with
hairs like those of the stem, the abaxial surface moderately to densely
stellate-pubescent with hairs like those of the stem but white and with
midpoints often gland-tipped, these mixed with multicellular,
uniseriate glandular hairs 0.3–0.6 mm long; venation pinnate, the
secondary veins 4–5 on both sides of the midvein, the midrib abaxially
occasionally with sparse recurved spines like those of the stem; base
obtuse, often asymmetrical; margin entire; apex acute; petioles 0.5–3
cm, moderately pubescent with hairs like those of the stem,
occasionally armed with sparse recurved spines. Inflorescences 2–15 (20)
cm, 2–5-branched, with 4–12 flowers, the plants andromonoecious,
specifically androgynoecious (
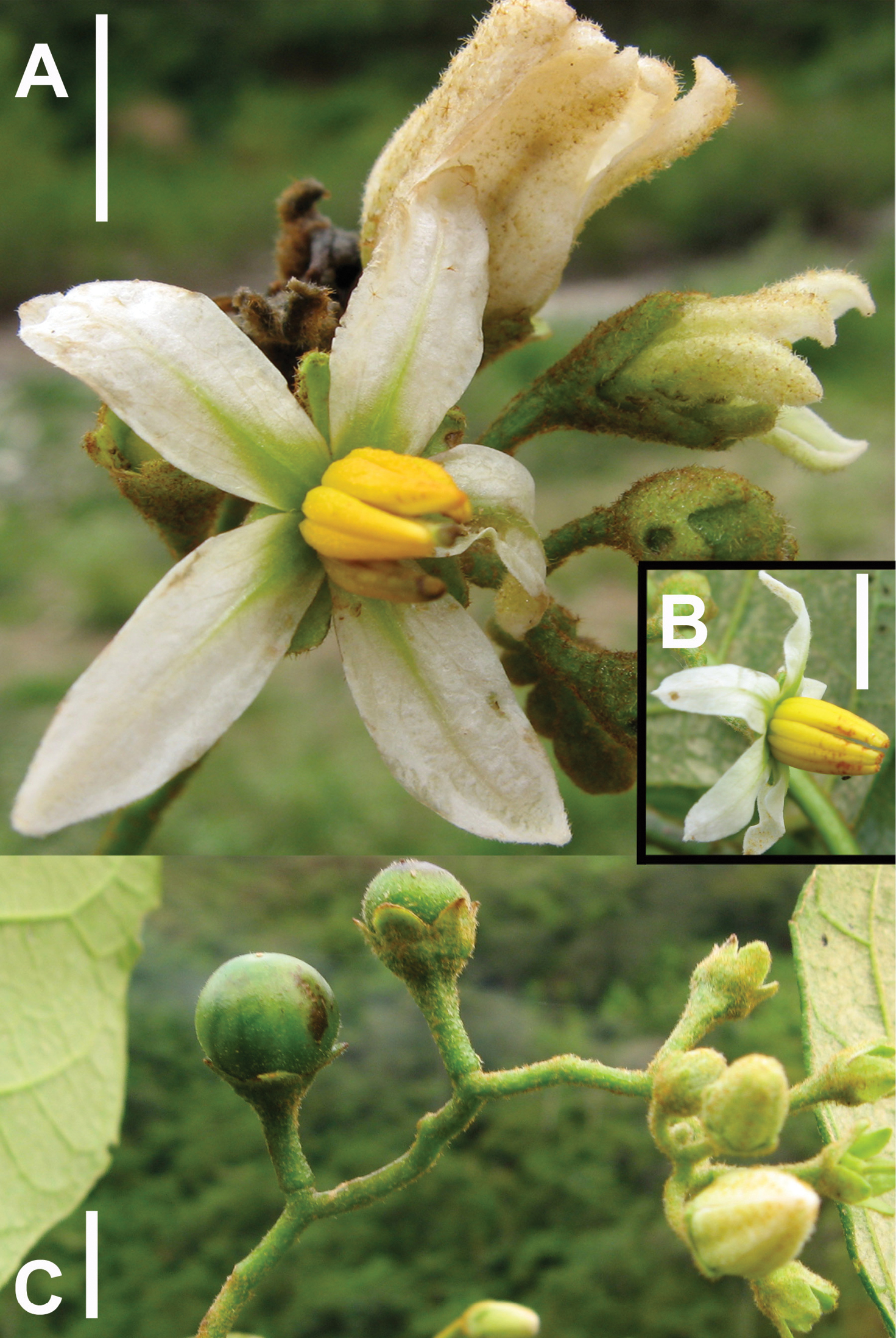
Figure 3. Isotype of Solanum achorum S. Stern [Stern et al. 129 (UT)].
Figure 4. Photos of type collection of Solanum achorum S. Stern. A Hermaphroditic flower and buds. B Functionally male flower; note the absence of exserted style. C Inflorescence and immature fruits. Scale bars = 1 cm.
Known from northern Peru in Depts. Amazonas and Cajamarca and southern Ecuador in Prov. Zamora-Chinchipe in disturbed open places in montane tropical forest, 700–2100 m in elevation.
Flowering specimens were collected in December; fruiting specimens were collected in July, October, and December.
The name Solanum achorum is derived from the Greek “achoros” meaning “homeless.” This name was chosen because of disagreement as to which group within Solanum subg. Leptostemonum this species belongs.
According to the IUCN Red List Categories (
Ecuador: Zamora-Chinchipe: Cantón Chinchipe, Parroquía Zumba, Quebrada Tarrangamí, near cabin of Sandy León, W of Escuela Byron Jiménez, just S of Las Pircas, region of Guaramizal, 4°46'50"S, 79°12'33"W, 2000 m, 29 March 2005 (fr), L.Bohs et al. 3356 (LOJA, QCA, QCNE, UT); same locality, same date (fr), L.Bohs et al. 3358 (QCNE, UT); Cantón Valladolid, Parroquía Vallodolid, road between Valladolid and El Porvenir del Carmen, ca. 3 km from Valladolid en route to Tapala, 1600–1650 m, 4°33'27"S, 79°07'50"W, 1 April 2005 (fl), L.Bohs et al. 3380 (QCNE, UT); road between Zumba and Amaluza, 8–10 km W of Zumba, 1500–1700 m, 4°50'07"S, 79°09'50"W, 31 March 2005 (fl, fr), L.Bohs et al. 3367 (QCNE, LOJA, UT); along road between Zumba and Vilcabamba, 57.9 km N of Zumba, 9.2 km S of Santa Ana, 6.3 km N of Palanda, 4°36'39"S, 79°07'42"W, 1243 m, 28 July 2004 (fr), T.Croat 92480 (BM). Peru: Cajamarca: Prov. San Ignacio, Dist. San José de Lourdes, caserio Rumichina, limité con caserio Naranjos, 5°54'04"S, 78°36'09"W, 1811 m, 24 June 2006 (fr), J.Perea & V.Flores 2407 (BM); Prov. San Ignacio, Dist. San José de Lourdes, bosque alrededor de la comunidad, 5°06'16"S, 78°51'11"W, 1860 m, 10 October 2006 (fr), J.Perea & V.Flores 2799 (BM); Prov. San Ignacio, approximately km 115 on road from Jaen to San Ignacio, east side of hills dividing San Ignacio and Rio Chinchipe, 5°06'53"S, 78°59'16"W, 711 m, 17 December 2007 (fl, fr), S. Stern et al. 177 (BM, NY, USM, UT).
The plurifoliate sympodial units, ferruginous to reddish tomentum with stellate-glandular hairs, and large berries with large seeds and a pubescent exocarp identify Solanum achorum as a member of Solanum sect. Erythrotrichum. Additionally, parsimony analyses of sequence data from three molecular markers (nuclear ITS and waxy or GBSSI and chloroplast trnT-F) place Solanum achorum in this section; however, the relationships within the group are incompletely resolved due to a lack of taxon sampling and require further study (S. Stern and L. Bohs, unpub. data). The inflorescence structure of Solanum achorum, being branched with both hermaphroditic and staminate flowers, would place it in Agra’s (2008) subsect. Rhytidoandrum Agra; however, this relationship has not been tested phylogenetically using molecular data.
Of the 23 species of sect. Erythrotrichum that Agra (2008) recognized, only three occur in Peru, with one species, Solanum urubambaense Agra, endemic to southern Peru in the area around Cuzco. Solanum achorum can be distinguished from the two members of sect. Erythrotrichum occurring in northern Peru and southern Ecuador by a number of characters. It shares a similar vegetative appearance with Solanum megaspermum Agra, especially regarding habit, pubescence, and leaf shape; however, Solanum megaspermum has more robust inflorescences (> 30 flowers vs. 4–12 flowers in Solanum achorum) and larger seeds (5–5.5 × 2–3 mm vs. 3–5 × 1.5–3.5 mm in Solanum achorum). Solanum achorum also shares many characteristics with Solanum velutinum Dunal, including a scandent habit, similar pubescence, and similar-sized white corollas, but Solanum achorum has a branched inflorescence that can reach 20 cm versus inflorescences to 6 cm long in Solanum velutinum. The calyx lobes in Solanum achorum are 2–4 mm in length and not foliaceous while those of Solanum velutinum are commonly over 10 mm long and foliaceous.
We thank E. Tepe, S. Leiva, and M. Zapata for field assistance; S. Knapp for discussion about the new species; S. Knapp and two anonymous reviewers whose comments greatly improved this manuscript; and the following herbaria for loans of specimens: BM, HAO, LOJA, QCA, QCNE, NY, and USM. This work was supported by NSF grant DEB-0316614 (PBI Solanum: A worldwide treatment) to LB.