(C) 2013 Shawn E. Krosnick. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Passiflora kwangtungensis is a critically endangered Chinese species known from Guangxi, Guangdong, and Jiangxi Provinces. The species belongs to Passiflora subgenus Decaloba, supersection Disemma, section Octandranthus. Field observations decreased rapidly during the 1970s to 1980s, and it was suspected that this species might have been extirpated due to repeated deforestation events throughout southern China. In recent years, however, small isolated populations of this species have been rediscovered in Hunan Province, representing new locality records for Passiflora kwangtungensis. New herbarium collections, color photographs, and silica gel collections have provided an unexpected opportunity to examine the evolutionary significance of this species. The current study presents a revised morphological description of Passiflora kwangtungensis based on fresh material, along with an updated distribution map. Using nrITS sequence data, preliminary insights into the phylogenetic position of Passiflora kwangtungensis are presented. Molecular data support the placement of Passiflora kwangtungensis within supersection Disemma section Octandranthus. However, the exact placement of Passiflora kwangtungensis within this lineage is unclear. The nrITS data suggest that Passiflora kwangtungensis may be sister to a clade containing Passiflora from China, Nepal, India, and Southeast Asia. Morphologically, Passiflora kwangtungensis displays the most similarity Passiflora geminiflora (Nepal, India) and Passiflora henryi (China). Lastly, conservation status and recommendations are made for Passiflora kwangtungensis following the IUCN Red List Criteria, where this species is classified as CR C1+C2a(i); D.
China, Decaloba, Disemma, Passiflora, Passiflora kwangtungensis, Passifloraceae
The genus Passiflora L. consists of ca. 526 species (
The Chinese Passiflora are typically associated with limestone-rich soils and are most often found in wet, sunny openings within subtropical rainforest, along humid forest margins, or among large boulders on moist hillsides. These speciesgenerally require primary forests and are rarely found in secondary regrowth or disturbed habitat. The Asian Passiflora are found at elevations from 50 to 2000 meters but are most frequently associated with mid to upper elevations (1000–1500 meters). Population sizes are often quite small, with only a single plant observed over several kilometers (
While not often discussed in the literature, a significant factor affecting the distribution of the native Chinese Passiflora has been deforestation that has occurred within the forests of China over the past 60 years. With the establishment of the People’s Republic of China in 1949, country-wide deforestation and forest degradation accelerated rapidly (
Because the Chinese species of Passiflora require primary forest and undisturbed habitats, deforestation and deterioration of forests throughout the subtropical southern provinces of Guangdong, Guangxi, Yunnan, Hainan, Jianxi, and Hunan would have been especially detrimental to these species. One species that appears to have been vulnerable to the effects of rapid deforestation is Passiflora kwangtungensis Merr. This species, originally described by
The recent high quality herbarium collection and photographs of fresh material that Yu made of Passiflora kwangtungensis, used in conjunction with herbarium material collected over the last 80 years, allow for the revision of Merrill’s original description to more accurately reflect this species with regard to morphology, ecology, and geographical distribution. Fresh DNA material collected from this specimen provides a new opportunity to examine the phylogenetic position of Passiflora kwangtungensis within supersection Disemma using ITS sequence data. In addition, conservation status assessments and recommendations are made for Passiflora kwangtungensis based on current distribution information according to ICBN criteria.
In 2004, botanical field work in Guangdong Province was completed by Krosnick and Deng. All known localities for Passiflora kwangtungensis in Guangdong were visited based on available herbarium specimen information at the time. Between the years of 2007–2010, Yu and accompanying students conducted field studies in the Nanling Mountains spanning four counties in south Hunan Province: Rucheng (Jiulongjiang National Forestry Park), Shuangpai (Wuxinling Forest Farm), Jingzhou (county nature reserve), and Jiangyong (provincial nature reserve), where they observed ca. 14 individual plants of Passiflora kwangtungensis. The greatest number of plants were observed at Rucheng (10 individuals), with just one or two individual plants seen at the Shuangpai, Jingzhou, and Jiangyong locations. Due to the rarity of the species, photos of Passiflora kwangtungensis were taken in lieu of herbarium specimens. A single herbarium specimen was collected in May 2010 from Jiulongjiang National Forestry Park (Yu & Tan s.n., MO), as a voucher for morphological study and to provide tissue for DNA analysis.
As none of the herbarium specimens examined contained primary GPS coordinates, an updated species distribution map was generated by inferring latitude and longitude coordinates using GOOGLE EARTH (
The monophyly of supersection Disemma was established using molecular data by
Total genomic DNA was isolated from fresh leaf material or tissue preserved in silica gel and extracted using the CTAB method (
Unweighted Maximum Parsimony (MP) analyses were performed using WINCLADA (Beta) ver. 0.9.9 (
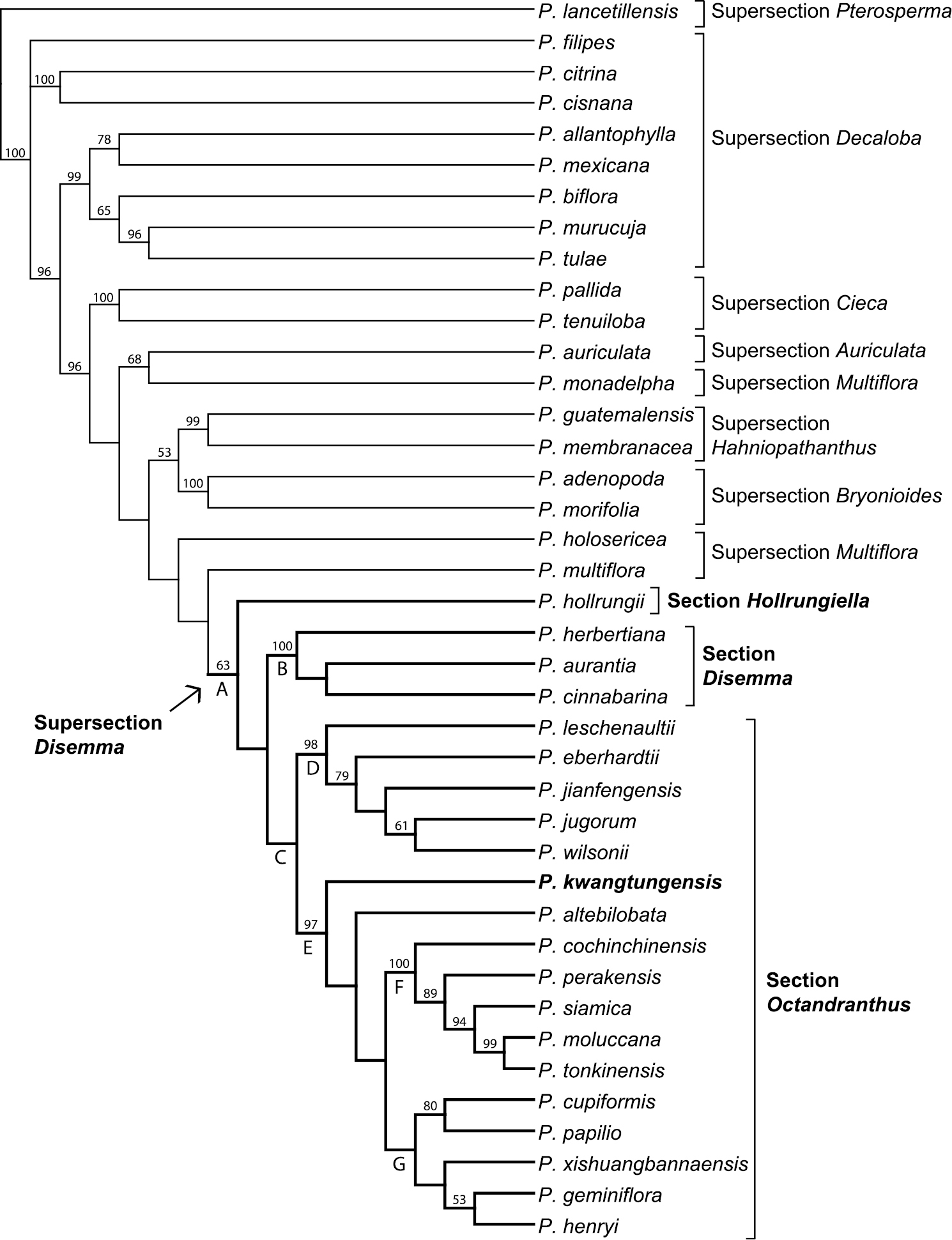
The aligned ITS dataset consisted of 801 characters, of which 272 were parsimony informative. The heuristic searches resulted in two most parsimonious trees (L=1, 070 steps, CI=0.54, RI=0.71). One branch collapsed in the strict consensus of the two MP trees (Fig. 1). Results of the phylogenetic analysis support the monophyly of supersection Disemma, though with low jackknife support (63%; Fig. 1, clade A). Monophyly was strongly supported for supersections Cieca (100%), Hahniopathanthus (99%), and Bryonioides (100%). Supersection Decaloba is resolved as polyphyletic in this analysis, with one strongly supported (99%) clade containing Passiflora allantophylla Mast., Passiflora mexicana Juss., Passiflora biflora Lam., Passiflora murucuja L. and Passiflora tulae Urb., a second clade consisting of Passiflora citrina J.M. MacDougaland Passiflora cisnana Harms (100%), and a single unresolved Passiflora filipes Benth. Supersection Multiflora is also polyphyletic, with Passiflora holosericea L. resolved as sister to Passiflora multiflora L.+ supersection Disemma (<50%), and then Passiflora monadelpha P. Jørg. & Holm-Niels. as sister to Passiflora auriculata Kunth (68%). Within supersection Disemma, Passiflora hollrungii K. Schum. is resolved as sister to the rest of the clade, which consists of two lineages, section Disemma (100%; Fig. 1, clade B), and section Octandranthus (<50%; Fig. 1, clade C). Within Octandranthus, two lineages are well supported: a clade of five species (98%; Fig. 1, clade D), and a second clade with the remaining 12 species (97%; Fig. 1, clade E). Although Passiflora kwangtungensis is resolved as sister to the remaining species in clade E, jackknife support for the position of Passiflora kwangtungensis and Passiflora altebilobata Hemsl. relative to remaining species is <50%. To further explore the placement of Passiflora kwangtungensis as sister to the remainder of clade E, another heuristic search using the same parameters was performed with Passiflora altebilobata removed from the dataset (data not shown). In that analysis, Passiflora kwangtungensis was still resolved as basal within clade E, suggesting that while jackknife support is low for its placement, the position of Passiflora kwangtungensis was not affected by the presence of Passiflora altebilobata. Within the remaining 10 species, two subclades appear: a Southeast Asian clade (100%; Fig. 1, clade F), and a Chinese clade (<50%; Fig. 1, clade G).
Strict consensus of two most parsimonious trees using ITS sequence data. Jackknife support above 50% listed above branches.
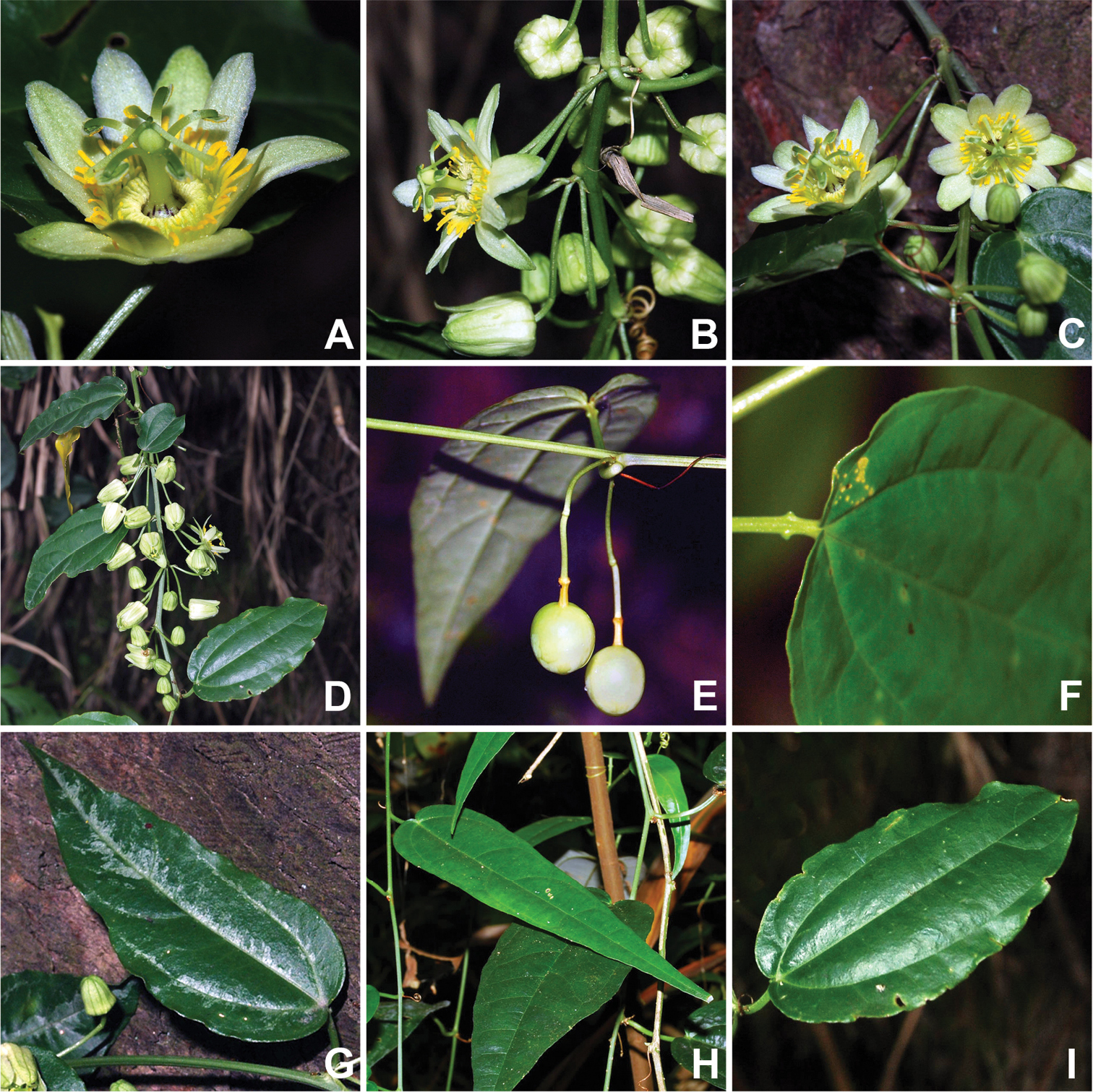
Based on the high quality photographs of living material (Fig. 2) and the additional herbarium specimens incorporated in the present study, a morphological description that more accurately reflects Passiflora kwangtungensis is presented here. Of particular note are color details that were not visible in the older herbarium specimens.
The observation of fresh material has provided additional insights into Passiflora kwangtungensis with regard to vegetative characters.The petiole has two paired papillate nectaries near the apex of the petiole (Fig. 2F). While the original description did note that the leaves are 3-nerved (Fig. 2F–G), the fresh material reveals a unique mottled variegation along the veins in younger leaves. Two distinct leaf shapes, lanceolate and ovate, are observed in the fresh material (Fig. 2G–I). The more lanceolate shape is associated with the juvenile growth form, while the ovate shape is observed on older portions of the plant.
As part of the revised species description presented below, information on phenology, ecology, and geographical distribution is presented to facilitate identification and conservation of this species in the field.
Floral and vegetative features of Passiflora kwangtungensis. A flower at anthesis B side view of open flower within inflorescence, three axillary inflorescences shown C arrangement of two flowers within axillary inflorescence D congested arrangement of individual axillary inflorescences, each with two to six flowers per inflorescence E immature fruit F papillate petiolar nectaries G mottled variegation along major veins in young leaf H lanceolate leaf shape associated with young growth I ovate leaf shape observed on older tissues. Photo credits: Xun-Lin Yu.
http://species-id.net/wiki/Passiflora_kwangtungensis
Figures 1 and 2CHINA. Guangdong: Tsungfa-Lungmoon Districts, Sam Kok Shan, Ka Wong Kwa, 29 May 1932, Tsang 20609 (holotype: NY! [NY-110492], isotype: NY! [NY-110491], PE! [PE-25522]).
Slender climber, glabrous throughout; stems terete. Stipules 1.0 × 0.5 mm, setaceous; petioles 1.0–2.0 cm long, biglandular in the upper half, the nectaries 0.3–1.0 mm in diam., papillate; laminas 9.0–13.0 cm × 2.0–5.0 cm, lanceolate to ovate, cordate at the base, apex acute to acuminate, midvein with a 1 mm mucro, margins entire, diffuse white variegation sometimes present along major veins; laminar nectaries 0.2–0.5 mm in diam, (0–) 2–7, scattered submarginally on abaxial surface. Tendrils well developed in mature shoots, green; inflorescences cymose, branched through the third order, (1–) 4–6 flowered; peduncle absent, pedicels 1.3–2.5 cm long, with an articulation 1.0–2.0 cm from the base; inflorescence bracts 1.0 mm × 0.5 mm, linear. Flower buds ovoid, the largest buds 5.0 mm × 3.0 mm; flowers erect; hypanthium 5.0 mm in diam.; sepals 5, 5.0–7.0 mm × 2.5–3.0 mm, lanceolate, glabrous, greenish-yellow, apex acute; petals 5, 4.0–6.0 mm × 2.0 mm, narrowly oblong-lanceolate, greenish-white, apex acute; coronal filaments in two series, outer series 3.0–5.0 mm long, filiform, yellow-green in lower half, yellow in upper half, inner series 1.0–2.0 mm long, filiform, clavate at apex, yellow-green throughout; operculum 1.0–2.0 mm tall, membranous, plicate, incurved towards the androgynophore, yellow-green, the inner margin fimbriate; limen 3.0 mm in diam., outer perimeter with 1 mm tall rim; nectar ring 1.0–2.0 mm wide; stamens 5, staminal filaments connate 4.0 mm along androgynophore, the free portions 4.0 mm long, green, the base flecked with brown spots 0.5–1.0 mm long; anthers 2.0 mm × 1.0 mm, green; ovary 3.0 mm × 1.5 mm, ovoid, sessile on the androgynophore, glabrous, green; styles 3, 3.0 mm long excluding stigmas; stigmas ovoid, 0.5 mm in diam. Fruit 1.0 cm in diam., globose, blue at maturity; arils unknown. Seeds unknown.
Flowering May; fruiting May–June.
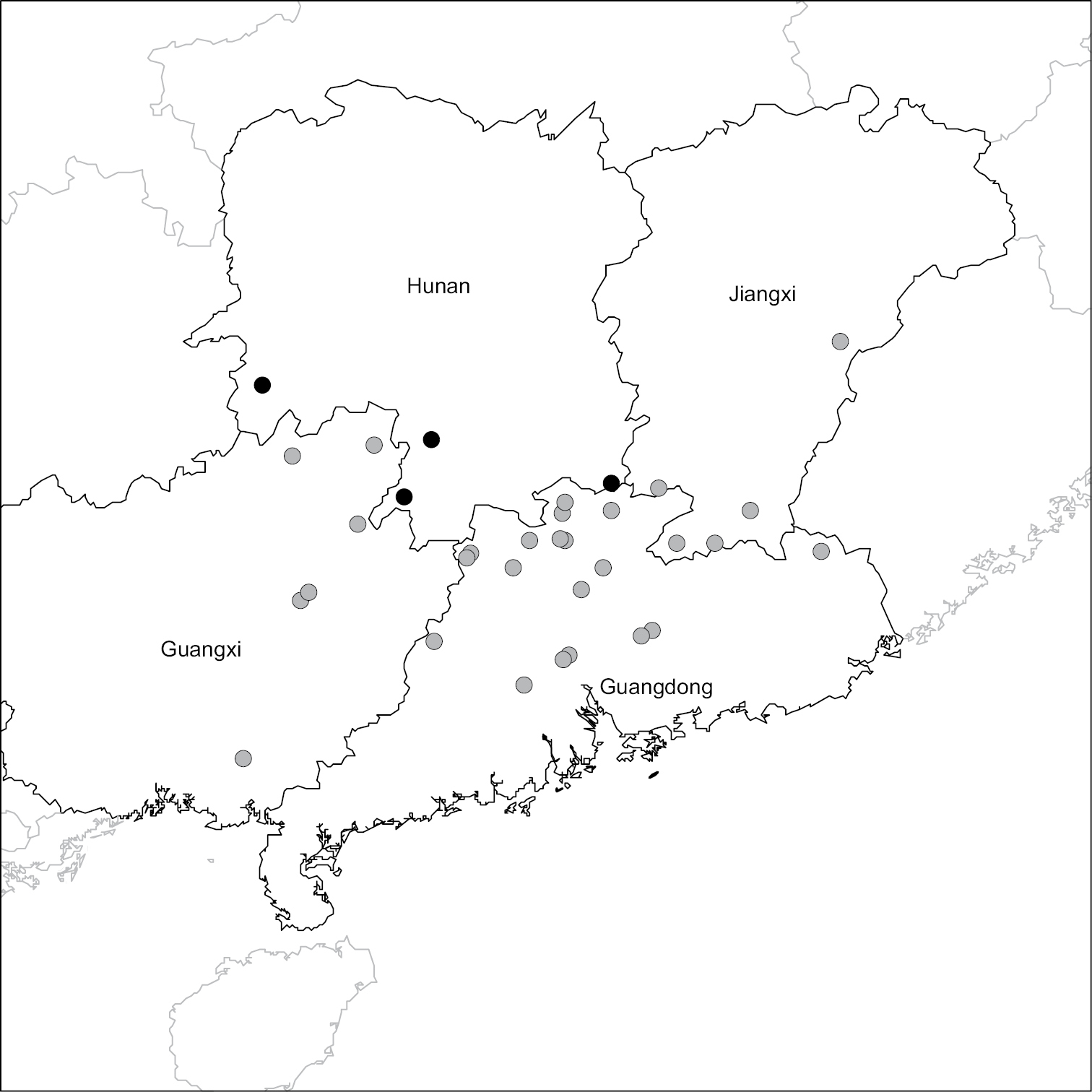
(Figure 3). Endemic to China in Guangdong, Guangxi, Jiangxi and Hunan Provinces; rare.
Distribution of Passiflora kwangtungensis in China. Grey circles indicate localities taken from herbarium specimens. Black circles indicate populations observed by Yu from 2007–2010.
Passiflora kwangtungensis is observed most frequently on hillsides in thickets, along roadsides in forest valleys, or along primary forest margins. This species prefers wet, sandy soils, and is scandent along the ground, sometimes climbing onto low shrubs or tree trunks. Elevation ranges from 500–1000 m.
China:“Guang dong xi fan lian” (
CHINA. Guangdong: 800 m, Anon. 915 (PE); Yangshan Xian, Wuyuan Xiang, Tianjingshan, Deng 1370 (IBSC); Nanxiong Xian, Baishun Xiang, Danankeng, 300–400 m, Deng 6213 (IBSC); Yingde Xian, Shakou Xiang, Huashuishan, 580 m, Liang 84483 (IBSC, PE); Lechang, Jiufeng, Lianan, Lo 1084 (IBSC); Longmen Xian, Nankunshan, Zhongping, Zhukeng, Shihuixiezi, Lo 1782 (IBSC); Lian Shan Town, Nang 659 (IBK, IBSC); Yangshan, Nanling Expedition 1349 (IBSC); Longmen, 350 m, Nanling Expedition 2006 (IBSC); Jiaoling, Nanling Expedition 2273 (IBSC); Yangshan Xian, Chengjia, Tam & Huang 359 (IBSC); Lianshan Xian, Hedong Xiang, Huangniushan, 880 m, Tam 58338 (IBSC); K’I Ravine, To & Ts’ang 12274 (A); Lung T’an Xian, To & Ts’ang 2035372 (L); Lung T’au Mtn., To Kang et al. 275 (US); Lung T’an Mtn., To Kang et al. 535 (US); Tsungfa-Lungmoon Districts, Sam Kok Shan, Ka Wong Kwa, Tso 20749 (NY [paratype]). Lianshan Xian, Shangshuai Zhen, Jinjiling, Ye 3381 (IBSC); Ruyuan, Daqiao, Yue-71 Expedition 355 (IBSC); Ruyuan Xian, Ruyang, Baimakeng, 1200 m, Yue-73 Expedition 720 (IBSC); Fengkai Xian, Qixing, Yue-74 Expedition 4958 (IBSC); Heping, Zhang 705 (IBSC); Guangxi: Jinxiu Xian, 1000 m, Dayaoshan Expedition 12445 (IBK); Jinxiu Xian, 500 m, Dayaoshan Expedition 811616 (IBK); Gongcheng, Gongcheng Expedition 0179 (IBK); Longsheng Xian, Qin & Li 70609 (IBK, IBSC); Ku Chun, Yao Shan (Dayao Shan Mtns.), Sin 21066 (IBSC); Kuchen, Sin 21283 (IBSC); Kuchen, Sin 21407 (IBSC); Quanzhou, near Baiyunan, Tsang 27737 (IBSC, US); Hunan: Jiulongjiang National Forest Park, Rucheng Xian, 520 m, Yu & Tan s.n. (MO); Jiangxi: Anyuan Xian, Huangdi, Lai 2273 (LBG); Quannan Xian, Zhushan Xiang, Yaoshan, Longwei, 800 m, Lai 768 (LBG); Lichuan County, Hong Ling Qu Kongdau Xiang, Nie et al. 2773 (KUN); Dayu Xian, Yaofu, 650 m, Yue 1297 (IBSC; KUN).
Based on the strict consensus of the ITS data presented here (Fig. 1), both supersection Disemma (clade A) and section Octandranthus (clade C) are resolved as monophyletic, though with low jackknife support. Within section Octandranthus, Passiflora kwangtungensis is strongly supported as a member of clade E, which consists of species from India, Nepal, China, and Southeast Asia. Passiflora kwangtungensis and Passiflora altebilobata form a basal grade leading to a Southeast Asian clade (clade F) and a Chinese clade (clade G). However, jackknife support values are quite low for several key nodes within the ITS phylogeny presented here, suggesting that alternative topologies may be obtained as more loci are included. Therefore, while taxon sampling for supersection Disemma is complete with regard to the ITS dataset, it is not yet possible to make strong conclusions regarding relationships within Disemma or about the phylogenetic position of Passiflora kwangtungensis in section Octandranthus. The addition of nuclear and chloroplast sequence data for Passiflora kwangtungensis will allow for more thorough insights into the evolutionary position of this species within section Octandranthus.
Supersection Disemma is a difficult lineage to study from a morphological standpoint because there are no clear synapomorphies that distinguish these 21 species as a group from the rest of subgenus Decaloba. Moreover, there seems to be a high rate of character transformation in this lineage, such that even closely related species appear quite distinct with regard to key floral and vegetative features. For example, within clade D, Passiflora eberhardtii has the smallest flowers in the supersection (ca. 1 cm or less in diameter), large cordate leaves with scattered abaxial nectaries, and flattened petiolar nectaries. This species is sister to a clade containing Passiflora jianfengensis S.M. Hwang & Q. Huang, Passiflora jugorum, and Passiflora wilsonii, all of which have flowers 3 cm or greater in diameter, leaves that are more or less truncate, abaxial nectaries in pairs, and petioles with peg-shaped glands. Similarly, placement of Passiflora kwangtungensis within supersection Disemma is challenging because while this species displays characters that might be considered plesiomorphic for clade E, it also exhibits many morphological similarities (inflorescence structure, floral coloration, and petiolar nectary shape) to both Passiflora henryi Hemsl. and Passiflora geminiflora D. Don, both of which occupy relatively derived positions in clade G. Thus, it is useful to consider the similarities of Passiflora kwangtungensis to the remainder of clade E as a whole (Passiflora altebilobata+ clades F, G), as well the similarities of this species to Passiflora henryi and Passiflora geminiflora.
Considering first the placement of Passiflora kwangtungensis as basal within clade E, a number of features observed in Passiflora kwangtungensis could be viewed as plesiomorphic for this clade. Passiflora kwangtungensis has small flowers that are generally no larger than 2 cm in diameter. Seven of the 12 species in clade E have small flowers (less than 2.5 cm in diameter), while the five Southeast Asian species (clade F) have much larger flowers, generally 3–5 cm in diameter. Larger flower size could represent a synapomorphy for clade F, while for the rest of clade E flowers could have remained small. All species in clade E display inflorescence branching through at least the second order, and all species have at least two flowers per inflorescence. Branching in Passiflora kwangtungensis may be through the third order, with one to four flowers per inflorescence. However, there is great variation in the extent of branching across the species in clade E. For example, Passiflora altebilobata has branching through the fourth order and up to 11 flowers per inflorescence, while inflorescences in Passiflora cupiformis may have up to 18 flowers. Leaves in Passiflora kwangtungensis range from lanceolate to ovate, simple shapes that could easily be modified to create the various forms observed across the clade. Passiflora altebilobata (clade E) and Passiflora xishuangbannaensis Krosnick (clade G) are perhaps the most specialized with deeply bilobed leaves, but this shape could be readily achieved through truncation of the midvein if starting from an ovate leaf form. The leaves of Passiflora kwangtungensis have submarginal abaxial nectaries, a feature which is observed in all species across clade E. Should its basal position continue to be supported as additional loci are sequenced, the morphological features of Passiflora kwangtungensis described here would be consistent with character traits in the other 11 species in clade E, highlighting the notable morphological plasticity in supersection Disemma.
Alternatively, there are three morphological similarities shared among Passiflora kwangtungensis, Passiflora henryi, and Passiflora geminiflora that are suggestive of a close relationship between these species. First, Passiflora kwangtungensis (Fig. 2B–D), Passiflora henryi, and Passiflora geminiflora display many similarities with regard to their inflorescence architecture. They all have cymose inflorescences branched through the third or fourth order. Within the inflorescence, third order flowers sometimes appear to be arranged in pairs, caused when the second order bud is aborted. This condition is commonly observed in Passiflora geminiflora and somewhat less commonly in Passiflora henryi. Pedicels within the inflorescence are of more or less equal lengths and held at the widest angle possible from one another, which results in the inflorescences appearing as mirror images of one another on either side of the central tendril. This differs from other species in section Octandranthus that have fasciculate inflorescences caused by the presence of sequentially shorter pedicels as branching order increases. Second, Passiflora kwangtungensis (Fig. 2A), Passiflora henryi, and Passiflora geminiflora each exhibit narrow flecks of brown coloration ca. 1 mm in length along the androgynophore and limen surface. Third, Passiflora kwangtungensis has papillate to narrowly peg-shaped petiolar nectaries (Fig. 2F), which are also observed in Passiflora henryi and Passiflora geminiflora. Should additional data resolve Passiflora kwangtungensis with Passiflora henryi and Passiflora geminiflora, these similarities would represent synapomorphies for that clade. These features are strongly suggestive of an evolutionary connection among the three species, or at the very least, an interesting convergence of form.
Passiflora kwangtungensis was originally described based on two herbarium specimens (Tsang 20609 holotype, Tso 20749 paratype) collected in Guangdong Province. Even in the original description,
Historically, Passiflora kwangtungensis appears to have been most abundant in Guangdong Province, with 23 of the 37 localities from this province. Given the high number of deforestation events that have occurred in southern China since 1958, it seems plausible that the decreasing numbers of collections each year for Passiflora kwangtungensis was correlated to the abundance of suitable habitat available in its native range. It is possible that these declining collections may simply reflect a decrease in botanical field work in Guangdong, Guangxi, and Jiangxi Provinces. However, given the gradual decline in numbers of Passiflora kwangtungensis specimens collected from the 1960’s through the 1980’s and the complete absence of collections after 1987, it seems more likely the result of reduced available habitat for an already rare, obligately out-crossing species being pushed to the brink of extinction throughout its range. The two most recent collections made in 2000 and 2010 are along the border of Guangdong and Hunan Province in the Nanling Mountain Range (Fig. 3). Based on the field observations of Yu during 2007–2012, it appears that the ca. 14 individual plants observed in Hunan Province may be some of the last remaining extant individuals of Passiflora kwangtungensis.
Under the IUCN Red List guidelines (
Under criterion D, very small or restricted population: Passiflora kwangtungensis should be classified as D, number of mature individuals less than 50. This species, previously feared to be extinct throughout its native range, is surviving in isolated pockets along primary forest margins, or quality habitat on undamaged hillsides throughout the Nanling Mountains. There is likely very little gene flow among the subpopulations, and even if the species are self-compatible, genetic diversity would be assumed to be quite low due to inbreeding. Fortunately, three of the four locations where Passiflora kwangtungensis was observed are in county, provincial, or national park reserves. This gives them some protection from habitat destruction but cannot ensure their survival due to reproductive isolation caused by low population numbers.
While the highest Red List conservation status a species qualifies for should be used, Passiflora kwangtungensis would also qualify as endangered under criterion A2abc, where A2 specifies a ≥50% decline over the longer of 10 years or three generations, and where population reduction was observed or inferred to have occurred in the past and the causes of reduction may not have ceased, may not be reversible, and may not be understood. The “abc” is determined based on a, direct observation, b, an index of abundance appropriate to the taxon, and c, a decline in the extent of occurrence and habitat quality. If herbarium specimens are taken as evidence, a clear drop off in the number of collections made occurs from the late 1980s forward. Conservatively, the lower number of specimens collected is assumed to reflect reduced population numbers, as opposed to reduced collecting efforts by scientists in the region. As several important floristic works focused on China have emerged during the 1980’s and 1990’s (
In general, Passiflora grow quite well from stem cuttings. Both in situ and ex situ conservation methods would be recommended with immediate implementation to protect the remaining individuals of Passiflora kwangtungensis from what seems to be near-certain extinction. Seeds, if produced, should be germinated and maintained in cultivation at local botanical gardens (such as IBSC) where soil type and other ecological factors will be most favorable for their survival. Further exploration is needed in Hunan, Jiangxi, and Guangxi Provinces to see if additional refugial populations still exist; if so, particular effort should be placed on cultivation of stem cuttings and eventual cross-pollination with the Hunan material to increase the genetic diversity of the material in cultivation. The case of Passiflora kwangtungensis represents a rare opportunity where botanists have the chance to assist in bringing a plant back from the brink of extinction. We hope that the information presented here will facilitate the protection and conservation of this species. This manuscript will also be presented as part of the application for placement of Passiflora kwangtungensis as critically endangered on the IUCN Red List, a recognition that will confer additional protection and increased awareness regarding the status of this species.
The authors would like to thank Erin Tripp for the initial investigatory work that confirmed Passiflora kwangtungensis was in fact still extant in China. We would like to thank Gordon Tucker for facilitating the acquisition of Passiflora kwangtungensis herbarium specimens and DNA samples from China. We also acknowledge Douglas Goldman, Clinton Morse, Kristen Porter-Utley, John MacDougal, Ron Boender, David Hearn, Arthur Gibson, Jan Meerman, George Keeney, Wang Hong, Elma Kay, Joan Leonard, and the Missouri Botanical Garden DNA Bank for plant material used in the phylogenetic analysis presented here. We thank Sandra Namoff and Kristen Porter-Utley for help with the sequencing of Passiflora kwangtungensis. We would also like to thank Kristen Porter-Utley, John MacDougal, Peter Jørgensen, Christian Feuillet, and an anonymous reviewer for critical comments on the manuscript. Funding for this research was made possible by NSF DEB Revisionary Systematics Grant 0717151 to S. Krosnick and L. McDade and NSF DEB Dissertation Improvement Grant 0407894 to S. Krosnick.
Herbarium specimen localities with inferred geographical coordinates. (doi: 10.3897/phytokeys.23.3497.app1) File format: Microsoft Excel document (xls).
Explanation note: Localities used for mapping the geographical distribution of Passiflora kwangtungensis.
List of species of Passiflora subgenus Decaloba used in the molecular phylogenetic analysis of ITS. (doi: 10.3897/phytokeys.23.3497.app2) File format: Microsoft Word document (docx).
Explanation note: Information includes supersection, section, voucher information (collector, collection number, and herbarium acronym), and GenBank accession numbers for ITS sequences.
Aligned ITS dataset used in phylogenetic analysis of Passiflora kwangtungensis. (doi: 10.3897/phytokeys.23.3497.app3) File format: Nexus file (nex).