Citation: Robinson H, Skvarla JJ (2014) Pantoporate pollen in the Asteraceae (Vernonieae). PhytoKeys 38: 1–13. doi: 10.3897/phytokeys.38.7495
Pantoporate pollen, which occurs sporadically in the Monocots and Dicots, has now been found in Asteraceae in two apparently related genera of the tribe Vernonieae, Polydora Fenzl and Oocephala H.Rob. Disposition of pores in Polydora seems more asymmetric than in Oocephala. Based on the known relationships within the Vernonieae, some conjectures are made regarding development of the pantoporate condition in the group.
Asteraceae, Compositae, Oocephala, pantoporate pollen, Polydora
The tribe Vernonieae is notable for remarkable variations in pollen structure, variations in sublophate and lophate forms with varying degrees of reduced perforated tectum in the exine and variations in the attachments of the outer exine to the footlayer. Now a new variation has been encountered during a study of South African Vernonieae that involves the first known examples of pantoporate pollen with non-equatorial pores in the Asteraceae. It is of some interest that one of the genera involved, Polydora (as Crystallopollen) was one in which pollen was first used as a taxonomic character in the Asteraceae by
Already known in the Tribe Vernonieae are the only examples of pollen in the Asteraceae with perforated tectum partially or completely lacking in non-colpar areas. Also unique to the tribe in the family is the six-porate form of pollen in the southeast Asian genus Camchaya Gagnep., where the pores occur equatorially in three pairs (
Specimens examined are from the U.S. National Herbarium in Washington, D.C. Examination of pollen with a light microscope was insufficient to show the position of the pores. Pollen grains in most illustrations were treated with acetolysis (Erdtman 1960), followed by staining with osmium thiocarbohydrazide solutions and sputter coating with gold-palladium (
The two genera in which the pantoporate pollen is now being reported are both restricted to sub-Saharan Africa. The two genera, Oocephala and Polydora, are evidently closely related to each other according to the pollen similarity, and both have pedunculate heads, a characteristic of many genera. Nevertheless, the two genera are easily distinguished from each other. Polydora has an ordinary broadly campanulate capitulum, and an ordinary capillary pappus. Oocephala (egghead) has an egg-shaped capitulum and a plumose pappus.
Examined material (Table 1) includes three species of Oocephala, Oocephala centauroides (Klatt) H.Rob. & Skvarla, comb. nov. [basionym: Vernonia centauroides Klatt, Bull. Herb. Boissier 4: 824. 1896] (Fig. 1A–F); Oocephala staehelinoides (Harv.) H.Rob. & Skvarla, comb. nov. [basionym: Vernonia staehelinoides Harv., Thes. Cap. 2: 36. 1863, “stahelinoides”] (Fig. 2A–F); Oocephala stenocephala (Oliv.) H.Rob. (Fig. 3A, B); and eight species of Polydora, Polydora angustifolia (Steetz) H.Rob. (Figs 3C, 4); Polydora bainesii (Oliv. & Hiern) H.Rob. (Fig. 5A–D); Polydora chloropappa (Baker) H. Rob; Polydora jelfiae (S. Moore) H.Rob.; Polydora sylvicola (G.V. Pope) H.Rob. (Fig. 5E, F); Polydora poskeana (Vatke & Hildebr.) H.Rob. (Fig. 6A–C); Polydora steetziana (Oliv. & Hiern) H.Rob. (Fig. 7A–C); and Polydora serratuloides (DC.) H.Rob. (Fig. 6D–E).
Specimens studied.
| 433 | Oocephala centauroides (Klatt) H.Rob. & Skvarla, Regio oriente et Mosambique, Delagoa Bay, Schlechter 18138 (US). |
| 474 | Oocephala staehelinoides (Harv.) H.Rob. & Skvarla, Transvaal, Mar. 1972, Liebenberg 8848 (US). |
| 475 | Oocephala stenocephala (Oliv.) H.Rob., Zambia, Christensen & Chisumpa 1508 (US). |
| 451 | Polydora angustifolia (Steetz) H.Rob. (as Polydora erinacea), Malawi, Zomba District, Likangala, Phalombe Road, 3000 ft., 3/ 12/ 1984, Christensen & Patel GMC 1457 (US). |
| 461 | Polydora angustifolia (Steetz) H.Rob., Brass 16090 (US), isoneotype. |
| 462 | Polydora bainesii (Oliv. & Hiern) H.Rob., Zimbabwe, West 7266 (US). |
| 463 | Polydora bainesii (Oliv. & Hiern) H.Rob., Zambia, C. Earle Smith & Richards 4671 (US). |
| 464 | Polydora bainesii (Oliv. & Hiern) H.Rob., Malawi, Salubeni & Kaunda 4187 (US). |
| 465 | Polydora chloropappa (Baker) H.Rob., Tanzania, Bidgood & Vollesen 7082 (US). |
| 466 | Polydora chloropappa (Baker) H.Rob., Zambia, Loveridge 845 (US). |
| 467 | Polydora jelfiae (Moore) H.Rob., Zambia, Christensen & Chisumba 1512 (US). |
| 473 | Polydora poskeana (Vatke & Hildebr.) H.Rob., Tranvaal, Koekemoer 234 (US). |
| 468 | Polydora serratuloides (DC.) H.Rob., Burundi, Lualla 4640 (US). |
| 469 | Polydora serratuloides (DC.) H.Rob., Tanzania, Groomany 7869 (US). |
| 470 | Polydora steetziana (Oliv. & Hiern) H.Rob., S. Africa, Koekemoer 2189 (US). |
| 471 | Polydora steetziana (Oliv. & Hiern) H.Rob., Namibia, Seydel 2828a (US). |
| 472 | Polydora sylvicola (Pope) H.Rob., Zambia, Christensen & Chisumpa GMC 1526 (US). |
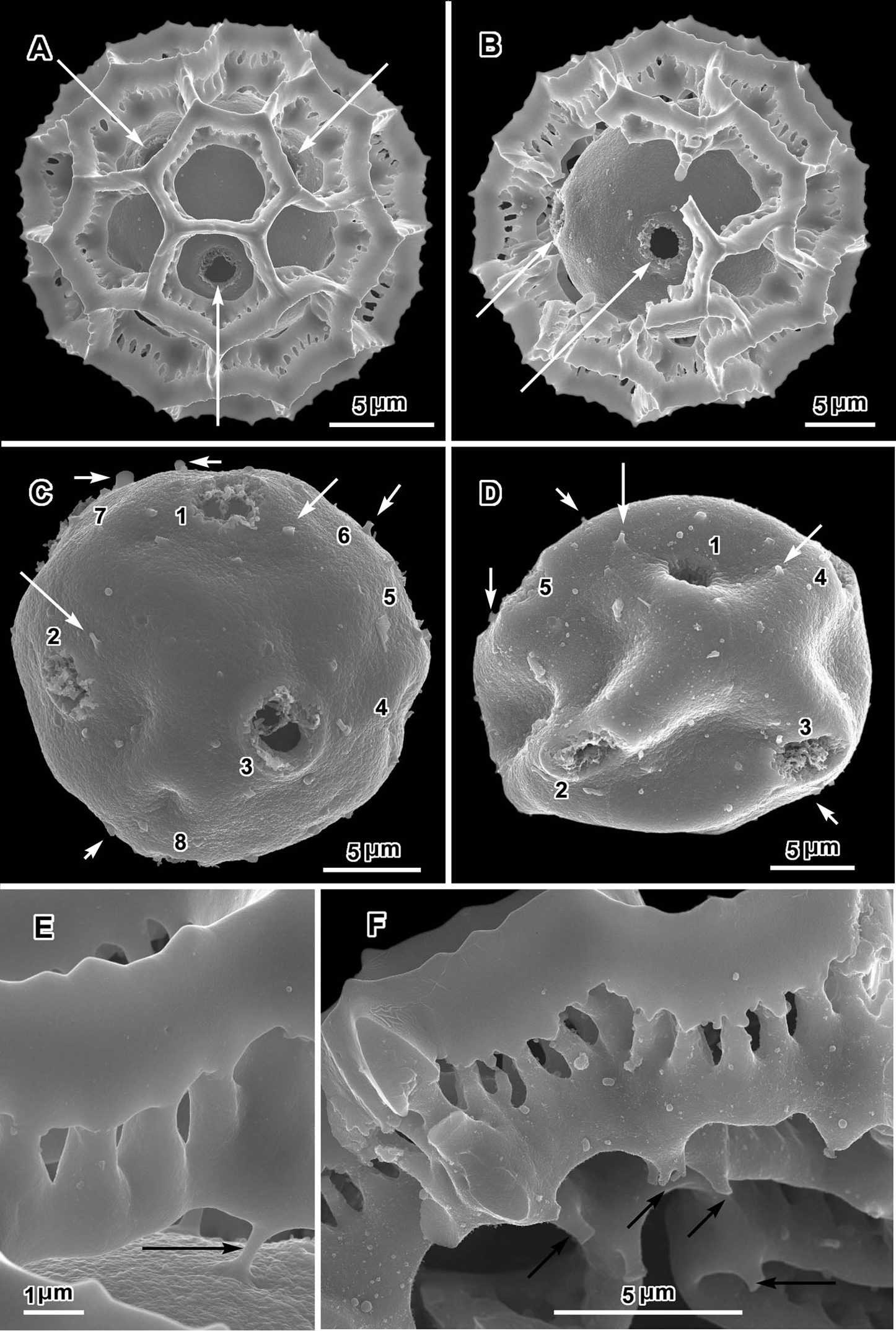
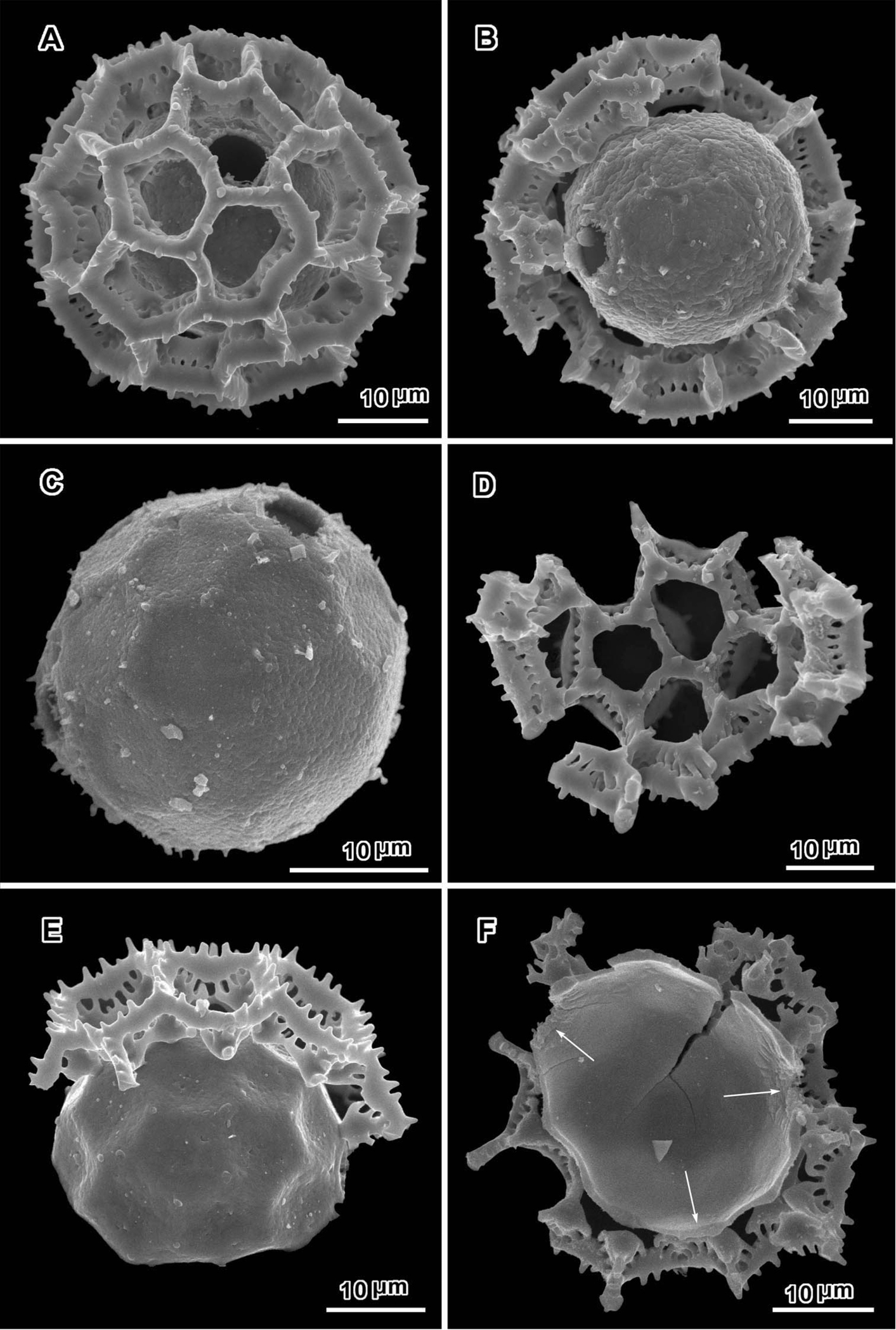
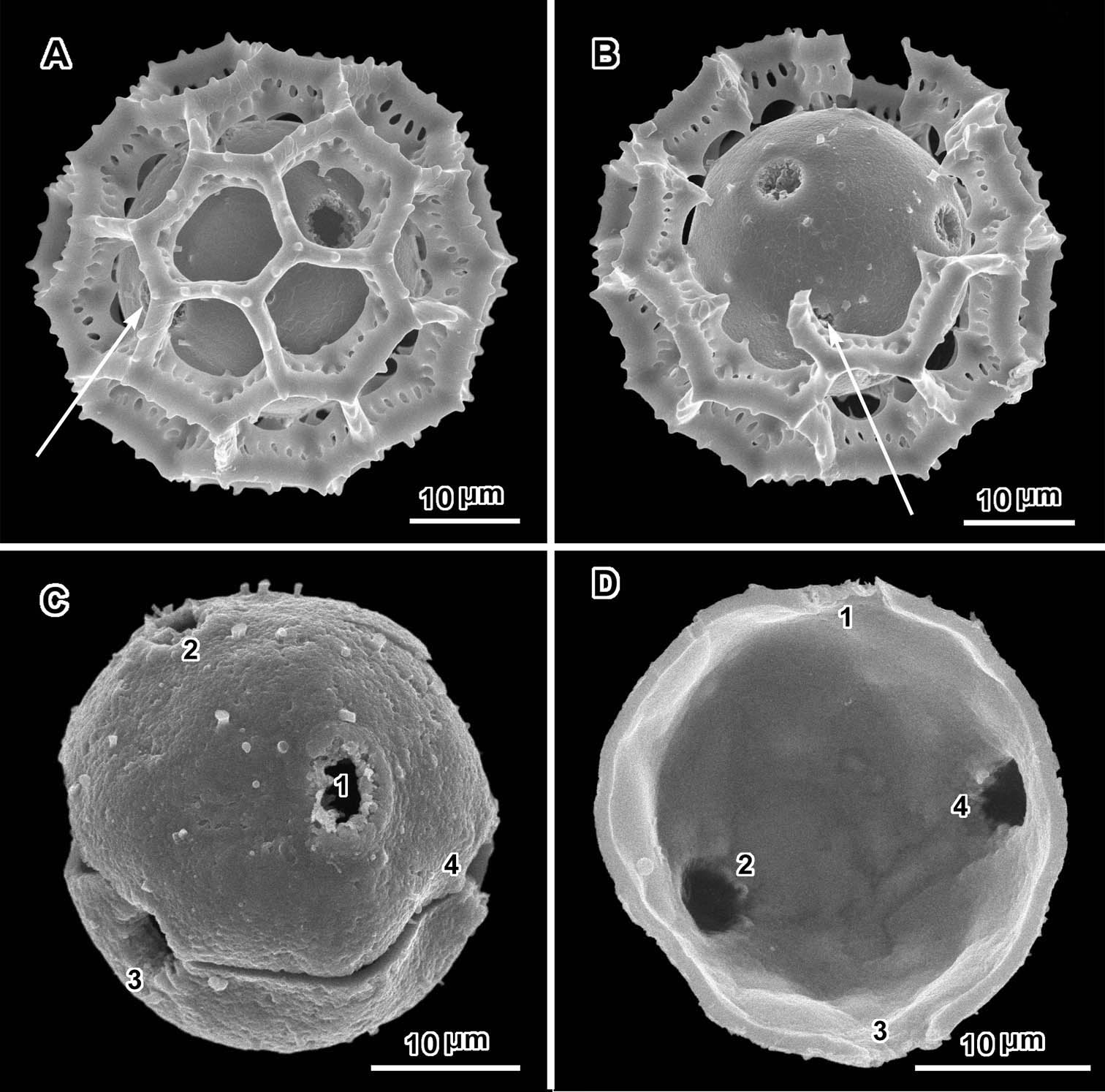
Scanning electron micrographs of Oocephala centauroides (Schlechter 18138). A, B pollen grains with muri of outer exine mostly ot totally intact, arrows showing visible pores C, D grains stripped of muri, with pores or possible pores numbered, arrows showing scars of muri attachment D distorted E, F pieces of muri showing rhizomate structure and stubs of attachments to footlayer.
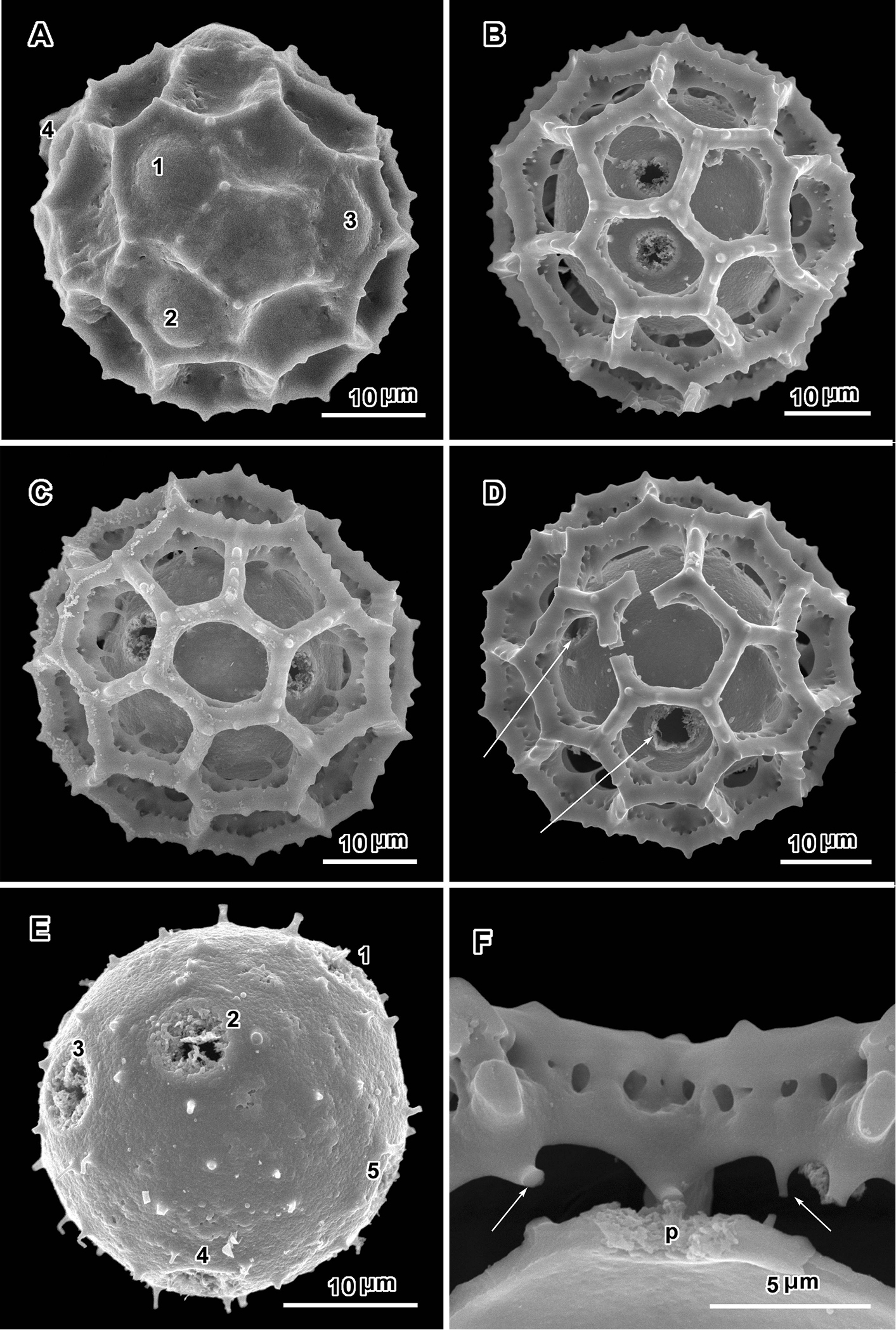
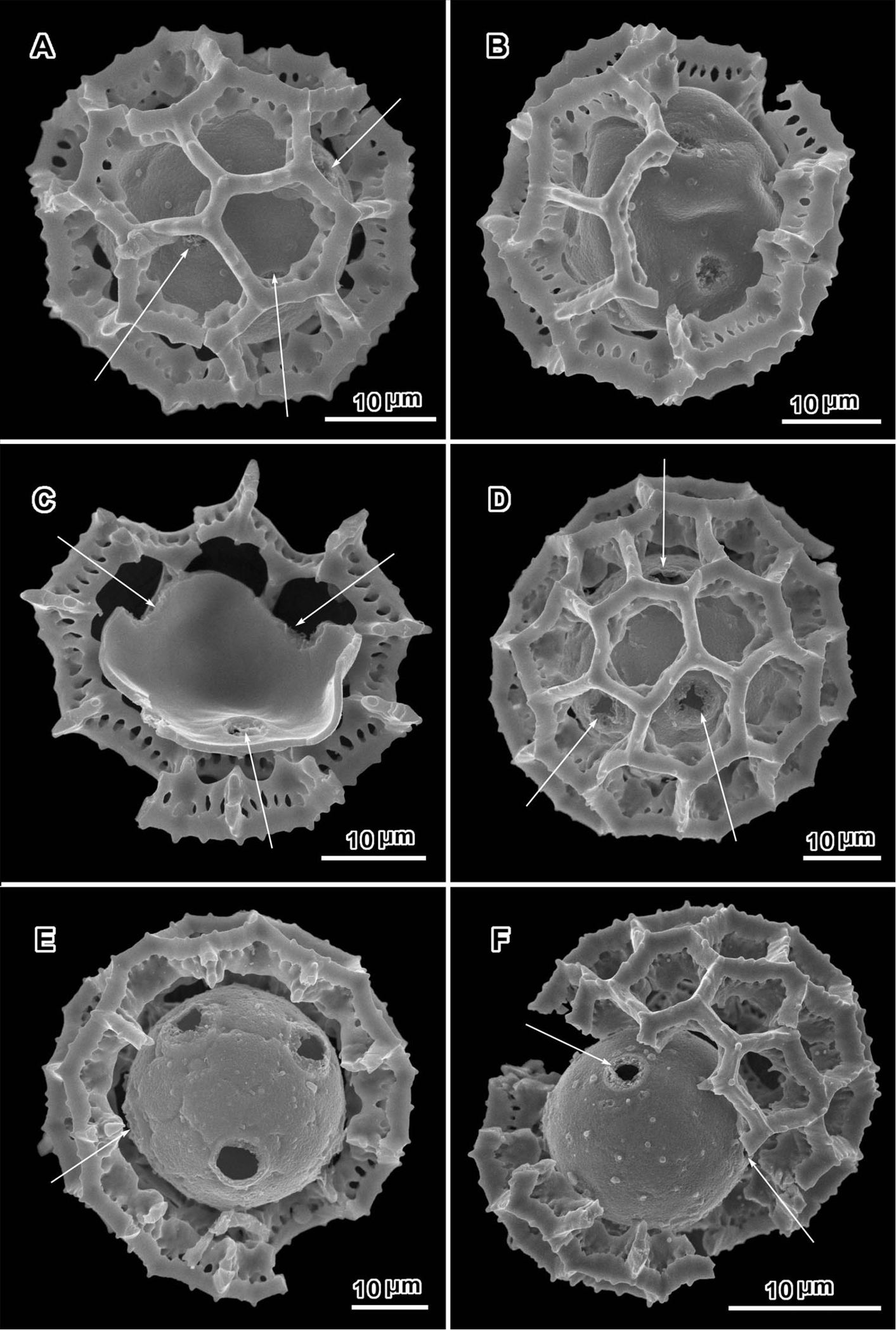
Scanning electron micrographs of Oocephala staehelinoides (A–D and F from Liebenberg 8848; E from Liebenberg 8843). A unacetylized grain showing three pores with caps intact, two pores in adjacent lacunae B–D intact or nearly intact grains showing pores in both pentagonal and hexagonal lacunae B with pores in adjacent lacunae E grain stripped of muri showing five pores and stubs of muri attachments F segment of muri showing rhizomate structure and remnants of weak basal attachments to footlayer.
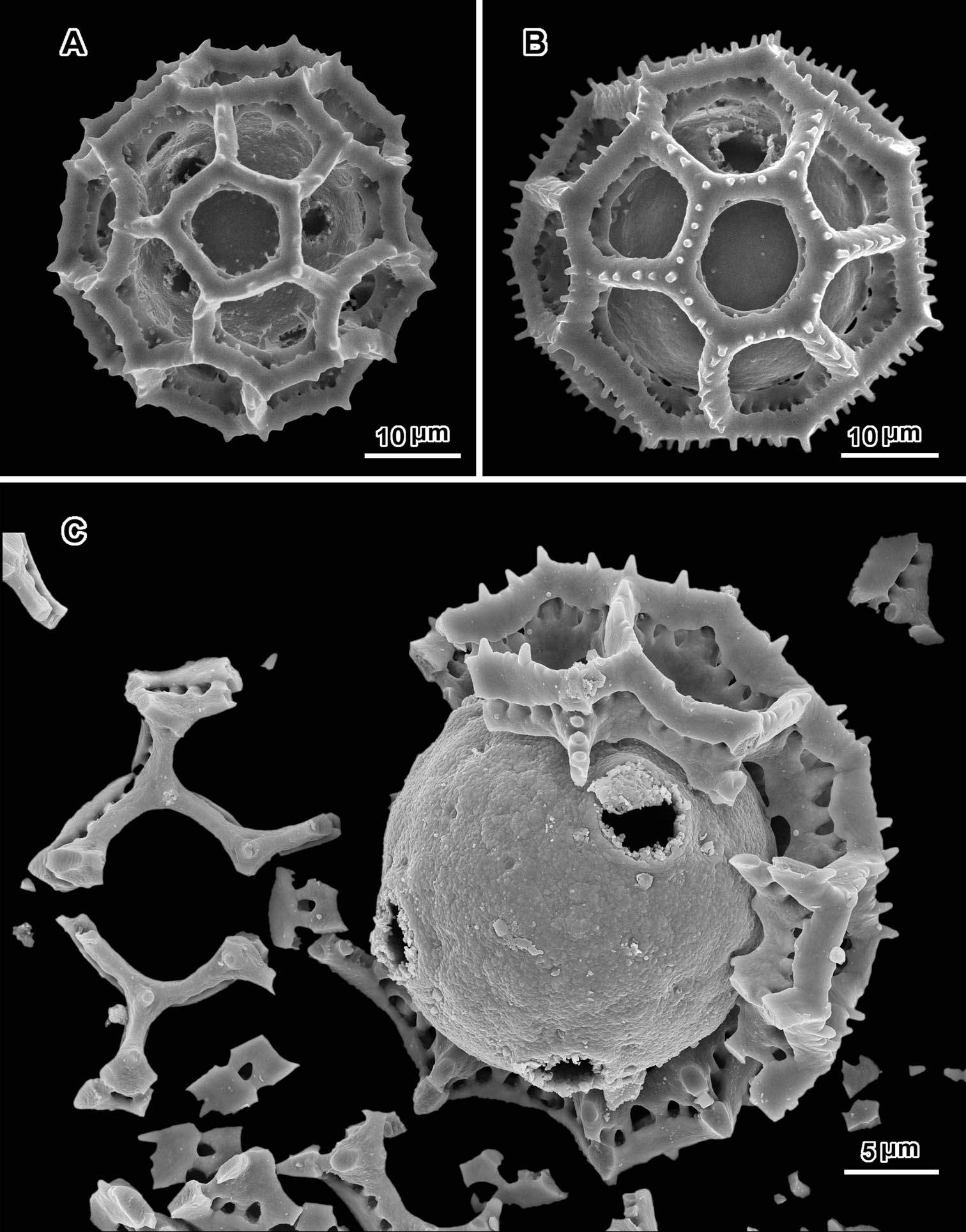
Scanning electron micrographs of Oocephala stenocephala (A–B from Christensen & Chisumpa 1508) and Polydora angustifolia (C from Christensen & Patel 1457). A intact grain with four visible pores B intact grain with one visible pore C grain partially stripped of muri showing three pores in pantoporate positions.
Scanning electron micrographs of Polydora angustifolia (A from Brass 16090; B–C from Christensen & Patel 1457). A intact grain with visible pore B intact grain with two visible pores in adjacent lacunae C grain with muri partially removed showing distorted inner surface and five pores.
Scanning electron micrographs of Polydora bainesii (A–D from Smith & Kaunda 4187) and Polydora silvicola (E–F from Christensen & Chisumpa 1526). A intact grain with one visible pore B, C grains partially and completely stripped of muri D segment of outer exine showing how muri can detach in large units E, F broken grains F split grain with three pores (arrows) B, C, E grains showing asymmetry with large areas lacking pores.
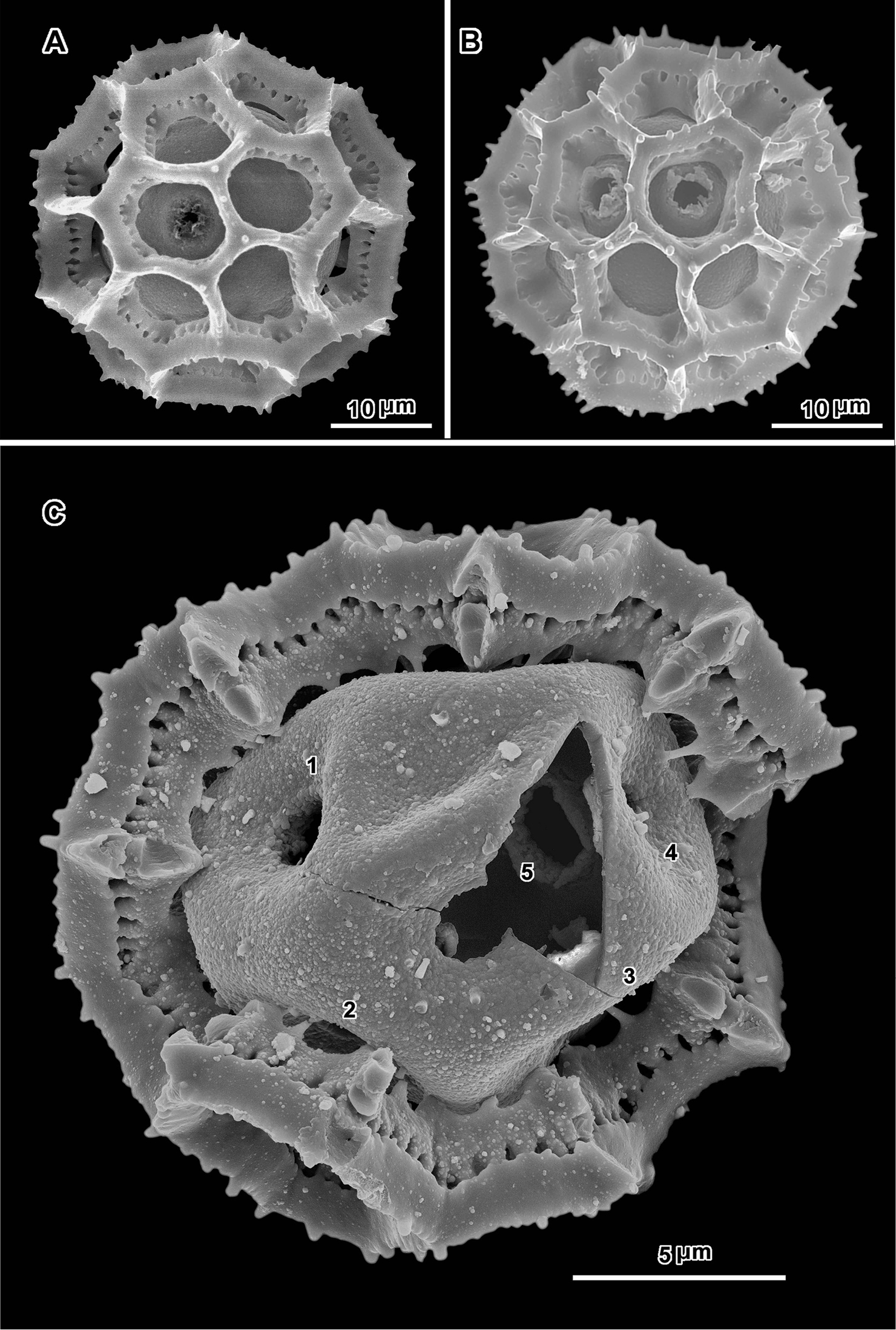
Scanning electron micrographs of Polydora poskeana (A–C from Koekemoer 234) and Polydora serratuloides (D–F, D and E from Lualla 4640, F from Groomany 7869). A intact grain with pore and incipient pores (arrows) B grain partially stripped of muri showing three visible pores C grain stripped of muri with three visible pores D intact grain with three visible pores, two in adjacent lacunae E, F broken grains with four and two pores visible.
Scanning electron micrographs of Polydora steetziana (A and B from Seydel 2828a, C and D from Koekemoer 2189). A intact grain with two visible pores B broken grain with three visible pores C grain stripped of muri with four pores visible D broken grain with four pores visible from inside.
The pollen of both genera has a lophate exine pattern that is nearly spherically symmetrical. The pattern consists of a mixture of pentagonal and hexagonal lacunae. Perforated tectum is essentially absent leaving totally exposed muri and their subtending columellae. Both genera also share a mural structure that has been referred to as rhizomate (
Positions of the pores are often difficult to determine in lophate pollen of the Centrapalinae and Erlangeinae. The lacunae in which the pores occur are totally undifferentiated in any other way, being either pentagonal or hexagonal, even in the polar regions. The depths of the lacunae usually prevent seeing more than one pore at a time unless the grains are unacetylized and the caps on the pores are preserved (Fig. 2A) (
An exact count of the pores can never be certain, since there is evidence of some uneven distribution of pores in some grains. In a few cases, in Oocephala, even in grains with the muri present, pores can be seen in adjacent lacunae (Figs 2A, B, 3A, 4B, 6D). In other cases in Polydora (Figs 3C–7C), expanses can be seen that have no pores (Fig. 5B, C, E). The total number of pores may never be more than the five or six that can be seen in some views of Oocephala (Figs 1C, D, 2E), and asymmetry is probably even more extreme in Polydora (Fig. 5C, E), but pantoporate non-equatorial distribution of pores is certain in both genera, and at least some asymmetry is certain.
Two basic porate conditions of Angiosperm pollen are well known (
There have been no previous reports of pantoporate pollen in the Asteraceae, and suggestions here are based on observations of the pollen of related members of the family in the tribe Vernonieae.
The Asteraceae has pollen that is basically tricolporate, a condition that seems basic to all the tribes including the Vernonieae (
There is even a developmental basis for the asymmetry in the distribution of the pores, more obvious in Polydora and less evident in the more specialized Oocephala. This could trace to the fact that the developing pollen grain has a distal surface that faces outward in the pollen mother cell, and a proximal surface that faces inward toward the center of the tetrad of pollen grains. Thus, the distribution of the pores is influenced by early stages in pollen development, beginning with position in the tetrads or mother cell.
We wish to thank W.F. Chissoe who operated the University of Oklahoma SEM. We also wish to thank Carol Kelloff who operated the USNM SEM, and Scott Whittaker supervisor of the USNM SEM Laboratory. March 2, 2014, after completion of this manuscription but before its submission, John Skvarla unexpectedly died. Two messages that he had send to me recently are worth citing. First looked forward to seeing the reaction to this paper, and second, he wish he had started working on the Vernoniae earlier in his career.