Citation: Ortiz RdC, MH Nee (2014) A new species of Cissampelos (Menispermaceae) from Bolivia and Paraguay. PhytoKeys 38: 89–99. doi: 10.3897/phytokeys.38.6504
The new species Cissampelos arenicola M. Nee & R. Ortiz, from the Bolivian and Paraguayan Chaco is described, its affinities are discussed, and its preliminary conservation status is evaluated. The species is at present known from 13 collections from sand dunes or dry forests. Cissampelos arenicola is distinguished from all other American species in the genus by its ovate- to subreniform-trilobed leaves, 8-locular synandria, and relatively large, and scarcely ornamented endocarps. The most common perianth condition in the pistillate flowers of Cissampelos is one sepal and one antesepalous petal, and while these may vary in number, they are always found adaxial to the carpel, and although the southern African taxon called Cissampelos capensis, whose generic position is uncertain, superficially resembles Cissampelos arenicola, its sepals and petals are consistently lateral to the carpel and not adaxial.
Bolivia, Cissampelos, conservation status, IUCN, Menispermaceae, Paraguay, sand dunes
The pantropical genus Cissampelos L., together with African Antizoma Miers and the mostly Asian Cyclea Arn. ex Wight, were placed in subtribe Cissampelinae, one of the three subtribes in tribe Menispermeae, which together with seven other tribes were recognized by
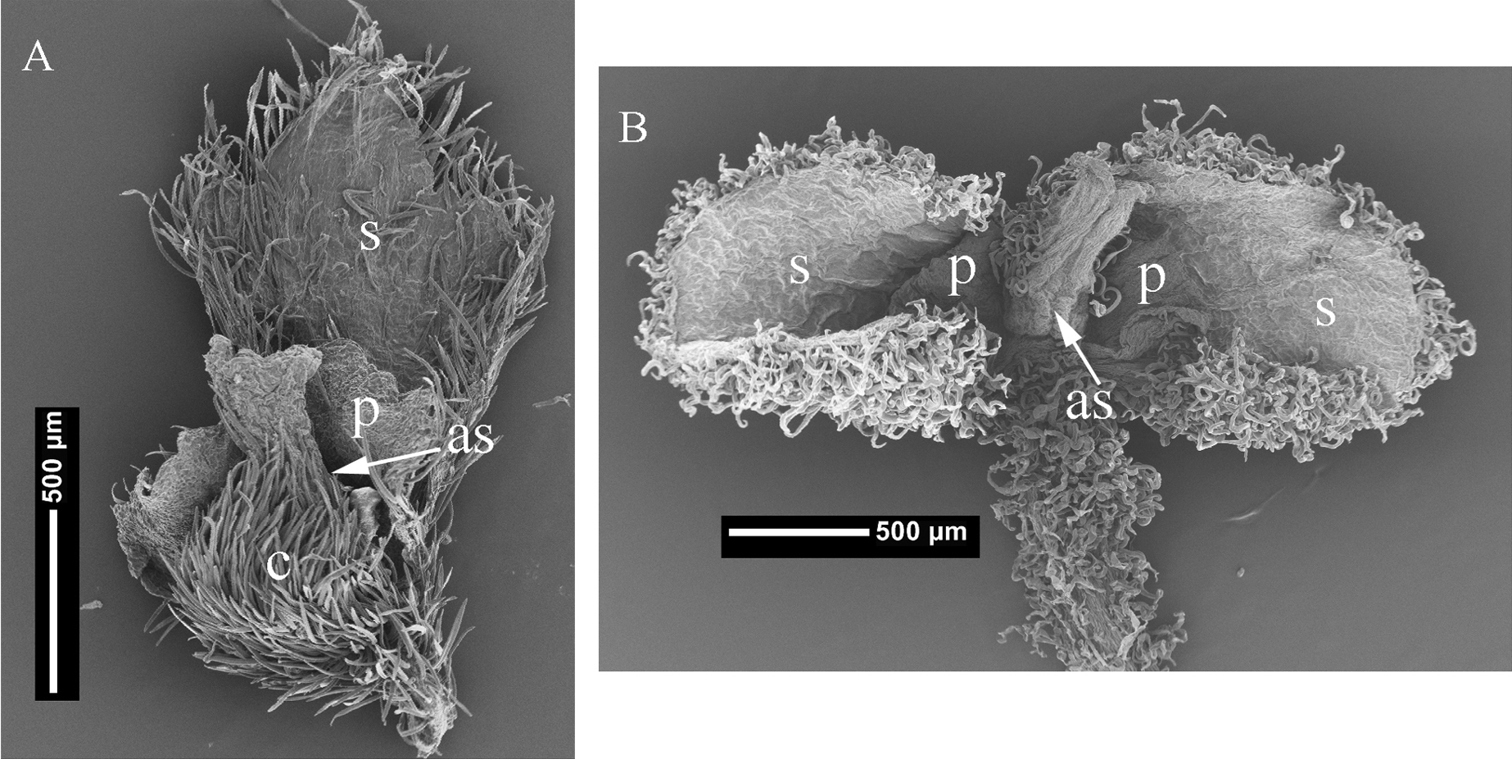
Pistillate flowers of A Cissampelos arenicola M. Nee & R. Ortiz, (M. Nee 49044, MO) B Cissampelos capensis L. f., (S.L. Williams 295, MO) showing locations of sepals and petals; s = sepal; p = petal; c = carpel; as = adaxial suture.
At present, the taxonomic status and phylogenetic affinities of Cissampelos capensis are unclear, similarly, the generic boundaries and relationships between Cissampelos, Antizoma, and Cyclea remain unresolved, and a re-assessment of all the genera in subtribe Cissampelinae is warranted, but is beyond the scope of this paper. Until further studies clarify the taxonomy and affinities of Cissampelos capensis, here it is still provisionally referred to as Cissampelos and we compare it to the new species Cissampelos arenicola as they both share superficial morphological resemblance in habit and foliage.
The last comprehensive revision of the genus Cissampelos recognized 20 species, nine of them distributed in the Americas, nine in Africa, one in Asia, and the pantropical Cissampelos pareira L. (
We studied all 13 herbarium collections of the new species housed at MO and at NY. Most of the collections have duplicates in several other herbaria but these were not available during this study. The specimens studied include male, female, and fruiting individuals, an infrequent situation in dioecious plants, such as Menispermaceae. Additionally, for comparison, we examined specimens for two other species that share some morphological similarities with the new species. These included: the Paleotropical Cissampelos mucronata A. Rich. (five specimens) and the southern African Cissampelos capensis (seven specimens). Measurements given in the species description refer to ranges of the mean values stemming from two to three replicate measurements of each structure and organ per individual voucher specimen. Before measuring, floral parts were first rehydrated, and endocarps were first boiled in water for a few minutes and the fleshy part of the fruit removed. Menispermaceae fruits develop asymmetrically so that the long axis do not necessary correspond with fruit length; endocarp measurement follow the convention of
urn:lsid:ipni.org:names:77139692-1
http://species-id.net/wiki/Cissampelos_arenicola
Fig. 2Cissampelos arenicola is distinguished from the remaining American species by its small, ovate- to subreniform-trilobed leaves and by its (6)8(10)-locular synandria. Its small leaves and viny habit superficially resemble the southern African Cissampelos capensis but differs by its (6)8(10)-locular synandria (vs. 4) and by sepals and petals located adaxial to the ventral slit of the carpel. The (6)8(10)-locular synandria of Cissampelos arenicola resembles that of African Cissampelos mucronata, from which it differs by its smaller (0.8–3 × 1.3–4 cm vs. 3.3–4.3 × 4.5–6.6 cm) leaves and its larger endocarp (6 × 7 mm vs. 4.3 × 4.7 mm).
BOLIVIA. Dpto. Santa Cruz. Prov. Andrés Ibáñez: along hwy from Santa Cruz to Abapó, 3 km S of crossing of railroad and 2 km S of bridge over Quebrada Peji, 17°58'00"S, 63°11'18"W, 450 m, 1 May 2001 (♂ fl), M. Nee, S. Knapp & J. M. Mendoza 51717 (holotype USZ; isotypes LPB, MO-6393940, NY, and to be distributed to K).
Isotype of Cissampelos arenicola M. Nee & R. Ortiz (Nee et al. 51717, MO).
Twining, perennial; stems striate, the older ones woody and glabrous, the younger ones subherbaceous and sparsely silvery-pilose, unarmed, growing in tangled viny masses to at least 5 m high in shrubs and small trees, to 5 mm in diameter; plants dioecious or infrequently monoecious. Leaves spiral, ovate- to subreniform-trilobed, usually broader than long, 0.8–3 × 1.3–4 cm, leaves associated with the inflorescences usually much smaller, lateral lobes divergent, rounded at apex, terminal lobe rounded and apiculate to aristate at the apex, chartaceous to subcoriaceous, glaucous, sparsely silvery-sericeous on both surfaces to nearly glabrous, palmately 6-nerved, basifixed or subpeltate with petiole inserted to 0.1–0.4 mm from the margin; petiole 5–17 mm long, pulvinulate at both ends. Staminate inflorescences: dichasium or monochasium, 1-2 from axils on adult or young leaves along the main stem or on young leaves of secondary axillary branches, silvery-pilose, peduncle 1.3–4.9 mm long, main axis of monochasium 1.2–3.2 mm long, bracts linear, 0.4–1.5 mm long; staminate flowers 5–6(–17); pedicels 0.5–1.6 mm long; sepals 4–6, 1.0–1.5 × 0.7–1.2 mm, obovate, shortly connate at base, concave, slightly spreading at anthesis, light cream-colored throughout, silvery-pilose abaxially, glabrous adaxially; petals usually 1, patelliform to barely cupuliform, 0.9–1.1 mm in diameter, or less frequently 3–4 and obovate, 0.7 × 1.1 mm, free, light cream-colored throughout, sparsely silvery-pilose abaxially, glabrous adaxially; synandrium 0.1–0.2 mm high, (6–)8(–10)-locular, loculi connate, transversely dehiscent, and radiating from a peltiform connective; carpellode absent. Pistillate inflorescences: 3–5 flowers fasciculate in the axils of adult or young leaves, sparsely silvery-pilose; pistillate flowers with pedicels 1.3–1.8 mm long; sepals and petals adaxial to the carpel; sepal 1(2), 1.2–1.5 × 0.9–1.2 mm, obovate, light cream-colored throughout, moderate silvery-pilose abaxially, less densely so adaxially; petals 1 (–3), subreniform, when 2 or 3, the petals free or partly to fully connate, opposite to the sepal, 0.7–0.9 × 0.9–1.4 mm, light cream-colored throughout, silvery-pilose abaxially, glabrous adaxially; sepal and petal soon deciduous, staminodes absent; carpel 1, gibbous, obliquely borne on pedicel, silvery-pilose, style 0.2–0.3 mm long, stigma 3–5-lobed, erect to spreading. Drupe globose, ca. 8 mm in diameter, glabrous, turning orange then red at maturity; mesocarp juicy; endocarp 6 × 7 mm, suborbicular-bilaterally compressed, with one tiny circular perforation on the lateral faces, ornamentation obscure, consisting of a very low medial ridge and obscurely transverse ridges (Fig. 3A); condyle bilaterally compressed, septiform (sensu
Endocarp ornamentation of A Cissampelos arenicola M. Nee & R. Ortiz (F. Mereles & R. Degen 5075, MO) B Cissampelos capensis L. f., (W. Giess et al. 5247, MO) C Cissampelos mucronata A. Rich., (Muller & Biegel 2281 MO). Scale bars = 3 mm.
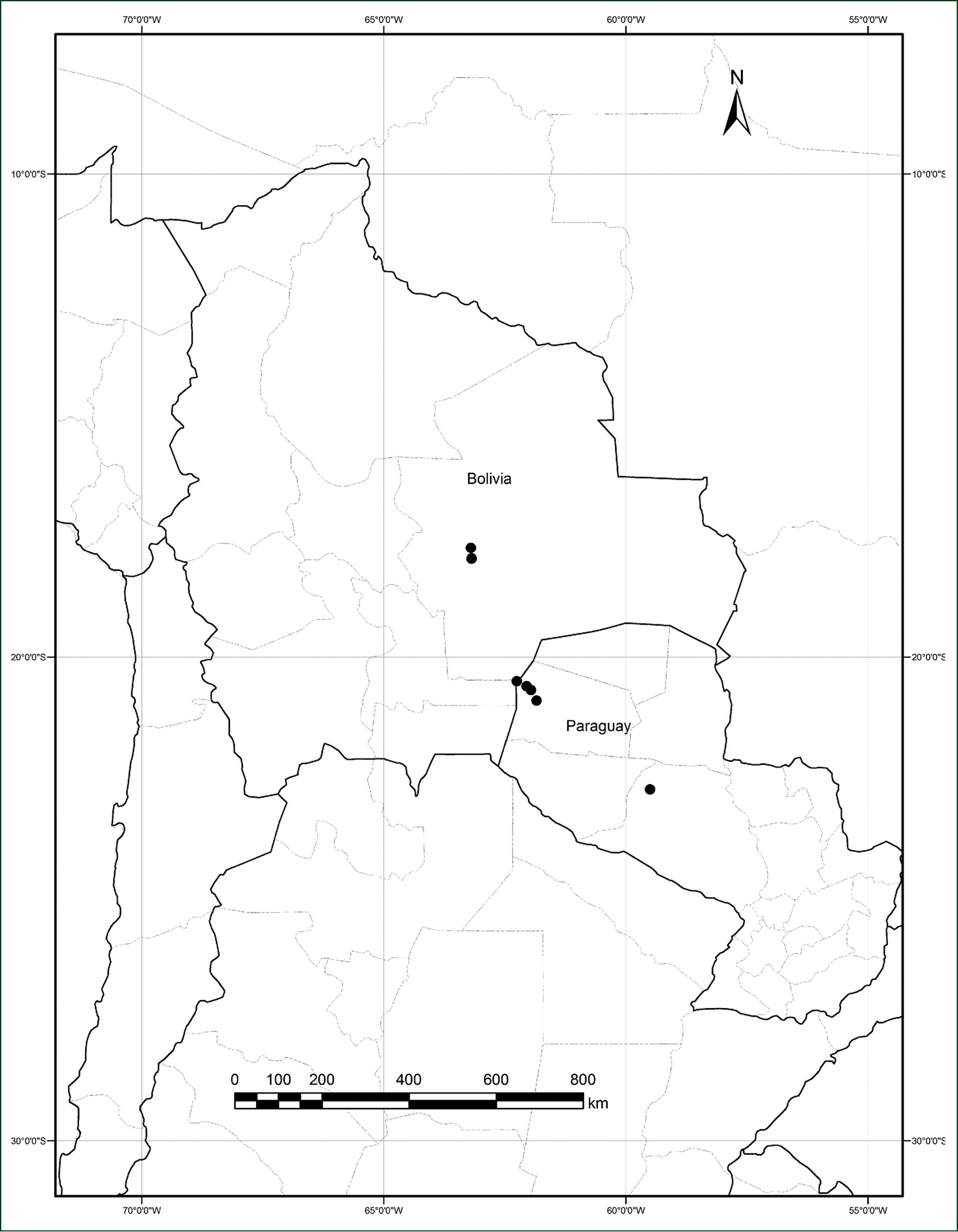
The species is at present known from southern Bolivia and northwestern Paraguay (Fig. 4). All Bolivian collections are from similar habitats of the sandy dune systems southwest of the city of Santa Cruz on the main highway which runs south to Abapó and on to Camiri and to Yacuiba on the Argentinian border. In Paraguay the species was collected along ruta Transchaco and also near the border with Bolivia in the proposed National Park Médanos del Chaco, where it has been found in seasonal forests and in dunes. In Bolivia there are extensive sandy savannas and large active dunes, the most well-known being the “Lomas de Arena” recreation area 15–20 km SSE of the center of Santa Cruz de la Sierra. The area where the species has been collected is on the western edge of this dune field. Plants were collected from 300–470 m elevation.
Distribution of Cissampelos arenicola M. Nee & R. Ortiz, based on examined collections with coordinates.
No observations of pollinators are available, nor notes on any possible odor. The extremely small size of the flowers suggests that a very small insect must be involved in pollination. Label note of Nee 51401: “It is difficult to separate the sparse and inconspicuous flowering and immature fruiting material from the vegetative mass”.
Male flowers were collected in February, May, November, and in December, pistillate flowers and mature fruits in February, April, June, and in December.
From the Latin, arenicola, dweller in sand, as the species seems to be restricted to sandy savanna soils and edges of dunes.
Cissampelos arenicola is at present known from 13 collections at nine localities from southern Bolivia and northwestern Paraguay. The sandy savannas in the area surrounding Santa Cruz de la Sierra are frequently burnt and heavily grazed by cattle. This appears to have little effect on the native vegetation, but development of the land for subdivisions and chicken ranches is a greater threat and is destroying much of the original vegetation. Although the Viru-Viru pampa surrounding the international airport north of the city of Santa Cruz is maintained as a natural savanna, Cissampelos arenicola has never been collected there. In Paraguay, Cissampelos arenicola has been collected in habitats described as seasonal forest, but also in areas with nomadic sand dunes. Thus, the “ephemeral” condition described on the labels may refer to its dynamic and transient habitat and not to the plant per se. Because of the fragmented and threatened habitat in Bolivia, its reported ephemeral condition of its habitat in Paraguay, and its usually small population sizes, Cissampelos arenicola may be considered as vulnerable.
On the other hand, by applying the IUCN Red List Criteria (
Bolivia. Santa Cruz: Prov. Andrés Ibáñez, along Quebrada Peji, vic. bridge of new hwy from Santa Cruz to Camiri and railroad bridge, 17°57'30"S, 63°11'00"W, 440 m, 11 Dec 1994 (imm & mat fr), Nee 45861 (LPB, NY!, USZ); along hwy from Santa Cruz to Abapó, 3 km S of crossing of railroad and 2 km S of bridge over Quebrada Peji, 17°58'S, 63°11.3'W, 450 m, 27 Feb 1998 (♂ fl, imm fr), Nee et al. 48487 (LPB, NY!, USZ); 15 Nov 2000 (♂ fl, imm fr), Nee 51401 (NY); along hwy from Santa Cruz to Abapó, 5.4 km S of turnoff at “km 13”, 17°55'S, 63°15'W, 470 m, 18 Apr 1998 (imm fr), Nee 49044 (LPB, NY!, USZ); Prov. Cordillera, a 3–4 km al S de Puerto Guaraní, al norte de la frontera Paraguaya, sabanas planas, partes húmedas, 20°30'S, 62°15'W, 400 m, 19 Jun 1992 (♀ fl & mat fr), Mostacedo et al. 385 (MO!, USZ). Paraguay. Alto Paraguay: Fortín Teniente Montania, seasonal forests and swamps on clay soils, 22°03'15"S, 59°57'14"W, 5 Feb 2002 (♂ fl), Zardini & Guerrero 57845 (MO!). Boquerón: Ruta Transchaco, a partir del km 702, entre PN Tte. Enciso y PN Médanos del Chaco, ambiente ruderal, 20°36'19"S, 62°02'54"W, 22 Feb 2006 (♂ fl), De Egea et al. 923 (FCQ, BM, CTES, G, MO!, PY, SI, UNR); proposed National Park Médanos del Chaco, ephemerals on dunes, 20°41'03"S, 61°57'37"W, 300 m, 12 Dec 1998 (♂ fl), Zardini & Duarte 49600 (AS, MO!); (mat fr), Zardini & Duarte 49630 (AS, MO!); 13 Dec 1998 (♀ fl & imm fr), Zardini & Duarte 49829 (AS, MO!). Nueva Asunción: 5 km después del Destacamento, sobre vegetación, 19 Mayo 1993 (♂fl), Degen & Mereles 2969 (MO!). Presidente Hayes: Tyto Villazón, Fortín Guaraní, en espatillar arenoso, [22°44'S, 59°30'W], 2 Feb 1993 (♀ fl & mat fr), Mereles & Degen 5075 (FCQ, MO!).
Although, the phylogenetic relationships of Cissampelos and the other genera in the Cissampelinae are still unresolved, Cissampelos is the oldest name in the clade and will therefore stand any future generic reassessment. While Cissampelos arenicola has not yet been included in any DNA based studies and as a result its phylogenetic affinities are not known, and despite the fact that the sepals and petals are variable in number, they are consistently located on the adaxial side of the carpel, a feature that is shared by all other studied species of Cissampelos with exception of Cissampelos capensis. Hence, we are confident that our new species belongs to Cissampelos s. str.
The genus Cissampelos is characterized by its dioecy, as are nearly all Menispermaceae. However, rare instances of monoecy have been reported by
Anther cells in Cissampelos arenicola vary from 6–10 (Table 1), with about 45% of the sampled 24 staminate flowers having an 8-locular synandrium. This condition is also rarely observed in Cissampelos grandifolia Triana & Planch., but is unknown in the remaining American species of Cissampelos recognized by
Main quantitative and qualitative variables that distinguish Cissampelos arenicola M. Nee & R. Ortiz from African species that are vegetatively similar and/or with 8-locular synandria.
| Species | Habit | Leaf shape | Leaf size (cm) | Synandria locule # | Sepals & petals position regarding the carpel vs | Endocarp size (mm) |
|---|---|---|---|---|---|---|
| Cissampelos arenicola | vine | ovate-subreniform-trilobed | 0.8–3 × 1.3–4 | (6)8(10) | adaxially | 6 × 7 |
| Cissampelos capensis | shrub | ovate-triangular | 1.1–3.9 × 0.8–2.8 | 4 | laterally | 4.2 × 5.5 |
| Cissampelos mucronata | vine | ovate | 3.3–4.3 × 4.5–6.6 | (6)8(10) | adaxially | 4.3 × 4.7 |
| 1 | Inflorescences and flowers of both sexes densely to sparsely silvery-tomentose; staminate flowers with 4-locular synandria; pistillate flowers with sepals and petals located lateral to the adaxial slit of the carpel, often with staminodes | Cissampelos capensis |
| 1’ | Inflorescences and flowers of both sexes moderately silvery-pilose; staminate flowers with 6–10-locular synandria; pistillate flowers with sepals and petals located opposite to the adaxial slit of the carpel, lacking staminodes | 2 |
| 2 | Inflorescences of both sexes with well-developed, foliaceous bracts; perianth conspicuously brownish-speckled | Cissampelos mucronata |
| 2’ | Inflorescences of both sexes with small, ovate bracts; perianth pale cream-colored through | Cissampelos arenicola |
| 1 | Endocarp 6 × 7 mm, lateral faces perforated | Cissampelos arenicola |
| 1’ | Endocarp 4.2 × 5.5 mm, lateral faces not perforated | 2 |
| 2 | Endocarp pyriform, surface ornamented with ridges and tubercles | Cissampelos capensis |
| 2’ | Endocarp subglobose, surface ornamented with well-developed transverse ridges | Cissampelos mucronata |
We acknowledge John F. Pruski, Peter F. Stevens, and Charlotte M. Taylor, researchers from (MO) who kindly read earlier versions of the manuscript, Burgund Bassuner assisted with the conservation status analysis, and Stephanie Keil scanned the isotype. We extend our acknowledgment to Alina Freire-Fierro (PH) and Frédéric M.B. Jacques for their constructive comments that improved the manuscript.