(C) 2013 Wendy A. Nelson. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A commonly found red alga of the upper intertidal zone of New Zealand rocky coasts is described for the first time as Pyropia plicata sp. nov. This species has been incorrectly known as Porphyra columbina Mont. (now Pyropia columbina (Mont.) W.A.Nelson) for many years. Pyropia plicata is widespread and common, and it is readily distinguished from other species of bladed Bangiales in New Zealand by its distinctive morphology, with pleated blades attached by a central rhizoidal holdfast.
Bangiales, New Zealand, Porphyra, Pyropia columbina, Pyropia plicata sp. nov.
For many years the most commonly found and widespread species of bladed Bangiales in New Zealand has been incorrectly known as Porphyra columbina Mont. Based on material collected from the New Zealand subantarctic Auckland Islands (
The combination of targeted collections of members of the Bangialesfrom throughout the New Zealand region, and analyses of sequence data coupled with morphological and anatomical investigations, has revealed many undescribed species around the archipelago (e.g.
Although the monophyly of the Bangiales had been shown to be well supported by a number of studies (e.g.
The rosette-forming species of Pyropia, previously referred to as ROS54, is formally described here.
This study is based on specimens of foliose Bangiales collected from throughout the New Zealand region, particularly from the North, South and Chatham Islands from 1987 to 2012, as part of diversity surveys. Voucher material is deposited in the herbarium of the National Museum of New Zealand Te Papa Tongarewa (WELT,
Blades circular to folded rosettes, strongly attached centrally by rhizoidal holdfast. Blades (1.5) 4–12 (42) cm in diameter. Colour purple to grey, bleaching to khaki-green on upper edges. Blades monostromatic, margin irregular bordered by pale cells. Monoecious, fertile regions marginal with intermixed sterile cells; zygotosporangia large, deep red to maroon, lozenge-shaped (a/4-8 × b/4-8 × c/4-8), spermatangia golden (a/2, b/2, c/8). Found in the upper to mid intertidal zone on open coasts.
WELT A032582 (Figure 1).
North Island, Wellington, Island Bay, W. Nelson, 22 Aug 1990.
New Zealand - North I., South I., Chatham Is.
GenBank - nSSU – AF136426, rbcL – GU046410, voucher specimen = WELT A024408.
plicata – folded or pleated.
The blades of Pyropia plicata are deeply folded and when fully extended are seen to have a circular to oval shape. The blades are very variable in size, generally in the range of 4–12 cm in diameter although reproductively mature thalli have been found to range from 1.5 cm through to 42 cm in diameter. The thalli are attached to rock substrata by a centrally located holdfast, made up of rhizoids extending from cells in the lower (central) area of the blade. The thalli are robust and very strongly attached to rock substrata in the upper intertidal zone of rocky open coasts (Figure 2). Thalli are primarily purple to grey in colour, but they become bleached particularly in summer and autumn and become khaki to yellow-green particularly on the upper edges.
Thalli are monostromatic and monoecious. Sterile regions of the blades are ca. 50-55 µm thick and the margin of the blade has a ragged or irregular appearance bordered by several layers of small pale cells (Figure 3). Fertile regions of the blade develop around the margins with sterile cells intermixed with patches of spermatangia and presumed zygotosporangia (Figure 4).
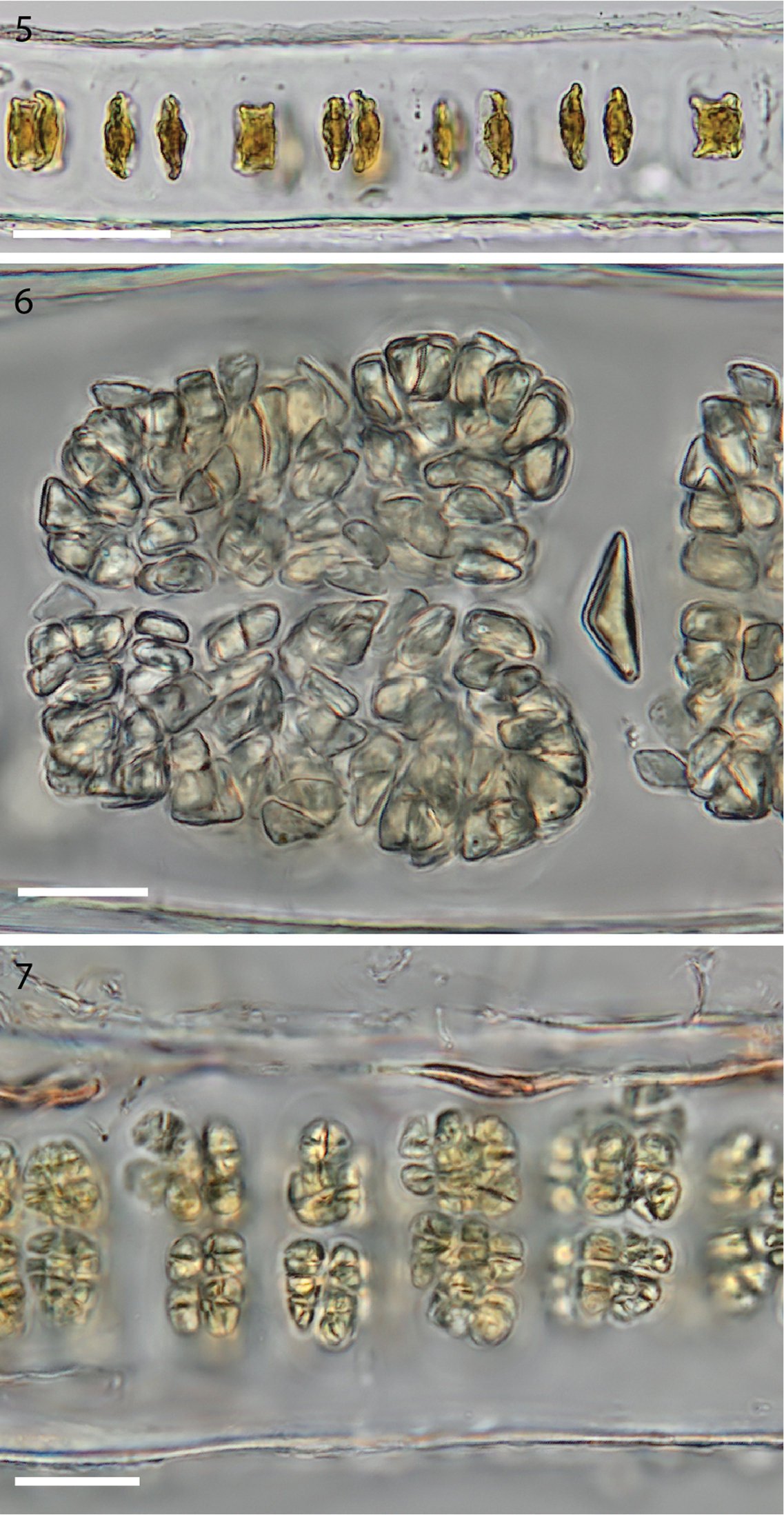
In the early stages of development spindle-shaped carpogonia form trichogynes on both sides of the blade, in marked contrast to the box-like shape of the neighbouring sterile cells (Figure 5). Blades increase in thickness to ca. 85–110 µm in zygotosporangial regions (Figure 6) and ca. 60–70 µm in mature spermatangial regions (Figure 7). The zygotosporangia when mature are deep red and the packets vary in size, becoming lozenge shaped at maturity with divisions up to a/8, b/8, c/8 (Figure 6). The spermatangial patches become golden as they develop and when mature are divided into packets ca. a/2, b/2, c/8 (Figure 7). Spermatia and zygotospores are usually released before reaching the maximum division formulae.
Typically Pyropia plicata is found on the upper intertidal shores of open coasts on rocky substrata. It has not been found growing epiphytically and is uncommon in sheltered areas. The deep pleats and central attachment of Pyropia plicata enable the retention of moisture between the folds in the blade. This morphology would appear to be advantageous in the upper intertidal habitats where it is found, as this species can be out of water for periods of up to eight hours between tidal cycles. The outer part of a clump of Pyropia plicata may be dried with a cellophane-like appearance yet within the folds, parts of the blade remain wet.
Pyropia plicata shows no particular seasonal trends in its distribution, with reproductively mature specimens collected throughout the year. Collections of this species have been made from the northern tip of the North Island, through to areas on the south western and south eastern South Island, as well as on the Chatham Islands. It has not been found on the Three Kings Islands, Stewart Island, or any of the New Zealand subantarctic islands.
Distinctive features: Pyropia plicata can be distinguished from other New Zealand species of bladed Bangiales by a number of distinctive features. It is the only species of Pyropia present on mainland shores with a marked rosette-like growth form. Although the ribbon-like blades of Pyropia cinnamomea may become eroded with age, the basal position of the holdfast in this species differs from Pyropia plicata. In addition, these two species can be distinguished by colour, and also by the division formulae of zygotosporangia. On intertidal shores Pyropia plicata is characteristically found in the high intertidal but below the position occupied by Clymene coleana (W.A.Nelson) W.A.Nelson from which it can be easily distinguished. Clymene coleana has finely divided finger-like lobes rather than the continuous circular to oval deeply pleated blade of Pyropia plicata. Although both of these species have a predominantly grey colour in winter months, they bleach to different colours in bright light, with Clymene coleana becoming golden compared with the khaki colour of Pyropia plicata. In addition the zygotosporangia and spermatangia are arranged in separate areas of the blade in Clymene coleana rather than being intermixed in Clymene plicata.
New Zealand. North Island. North Auckland: Far North, east Tapotupotu Bay, 13 Nov 2001, R. Dunmore, WELT A030179 (34°26.1080'S, 172°43.0050'E); Muriwai Beach, Maori Bay (Maukatia), 04 Apr 2000, W. Nelson, T. Farr & G. Williams, WELT A024784 (36°50.30'S, 174°25.90'E). Bay of Plenty: Tauranga, Mount Maunganui main beach, 05 May 2000, G. Williams & T. Farr, WELT A024775 (37°38.00'S, 176°11.00'E); Maketu, Okurei Point East, 05 May 2000, T. Farr & G. Williams, WELT A024772 (37°44.95'S, 176°28.37'E). Wellington: Wellington City Harbour, Frank Kitts Lagoon reclamation, 12 Feb 2001, W. Nelson & T. Farr, WELT A030170 (41°17.20'S, 174°46.90'E); Lyall Bay, 5 Nov 2012, W. Nelson, WELT A032593 (41°21.0'S, 174°48.00'E; Southern Wairarapa, Ngawihi, 26 Oct 2000, W. Nelson, T. Farr & G. Williams, WELT A024816 (41°36.00'S, 175°14.00'E).
Chatham Islands. Reef at Owenga wharf, 10 Mar 2001, W. Nelson, J. Broom, W. Jones, T. Farr & M. Clayton, WELT A030169 (44°01.50'S, 176°22'W).
South Island. Marlborough: D’Urville Island, Bonne Point, 20 Sep 1999, W. Nelson & G. Williams, WELT A031087 (40°52.00'S, 173°55.00'E).
Kaikoura, Ocean View, 18 Oct 1997, W. Nelson, WELT A024408 (42°31'S, 173°30'E). Westland: West coast, Charleston, Constant Bay, 10 Mar 2000, W. Nelson & T. Farr, WELT A024727 (41°54.20'S, 171°26.00'E); Punakaiki, 12 Mar 2000, W. Nelson & T. Farr, WELT A024793 (42°06.70'S, 171°20.00'E). Canterbury: Banks Peninsula, Avon Heathcote estuary, 20 Mar 2000, J. Broom, WELT A023952 (43°33.00'S, 172°44.00'E); Christchurch, Sumner, Cave Rock, 20 Mar 2000, J. Broom, WELT A023953, also, 26 Jun 2005, J. Broom & S. Heesch, WELT A023946 (43°33.9370'S, 172°45.5190'E); Lyttelton Harbour, Corsair Bay, 17 Sep 2001, M. Parsons, W. Jones & K. Neill, WELT A030172 (43°37.00'S, 172°42.00'E). Otago: Purakanui, 19 Apr 2000, K. Neill, WELT A023949 (45°45.00'S, 170°38.00'E); Dunedin, Brighton, 22 Oct 1999, J. Broom & W. Nelson, WELT A023956 (45°57.05'S, 170°20.00'E). Southland: Catlins, Kaka Point, 28 Apr 2005, S. Heesch & J. Broom, WELT A031597 (46°23.010'S, 169°47.140'E); Fiordland, Edwardson Sound, Chalky Inlet, 23 Feb 2000, G. Williams, WELT A024786 (45°55.983'S, 166°38.067'E).
Holotype of Pyropia plicata sp. nov. (WELT A A032582). Scale bar = 5 cm.
Pyropia plicata exposed at low tide on upper intertidal rocks (ca 5 cm high).
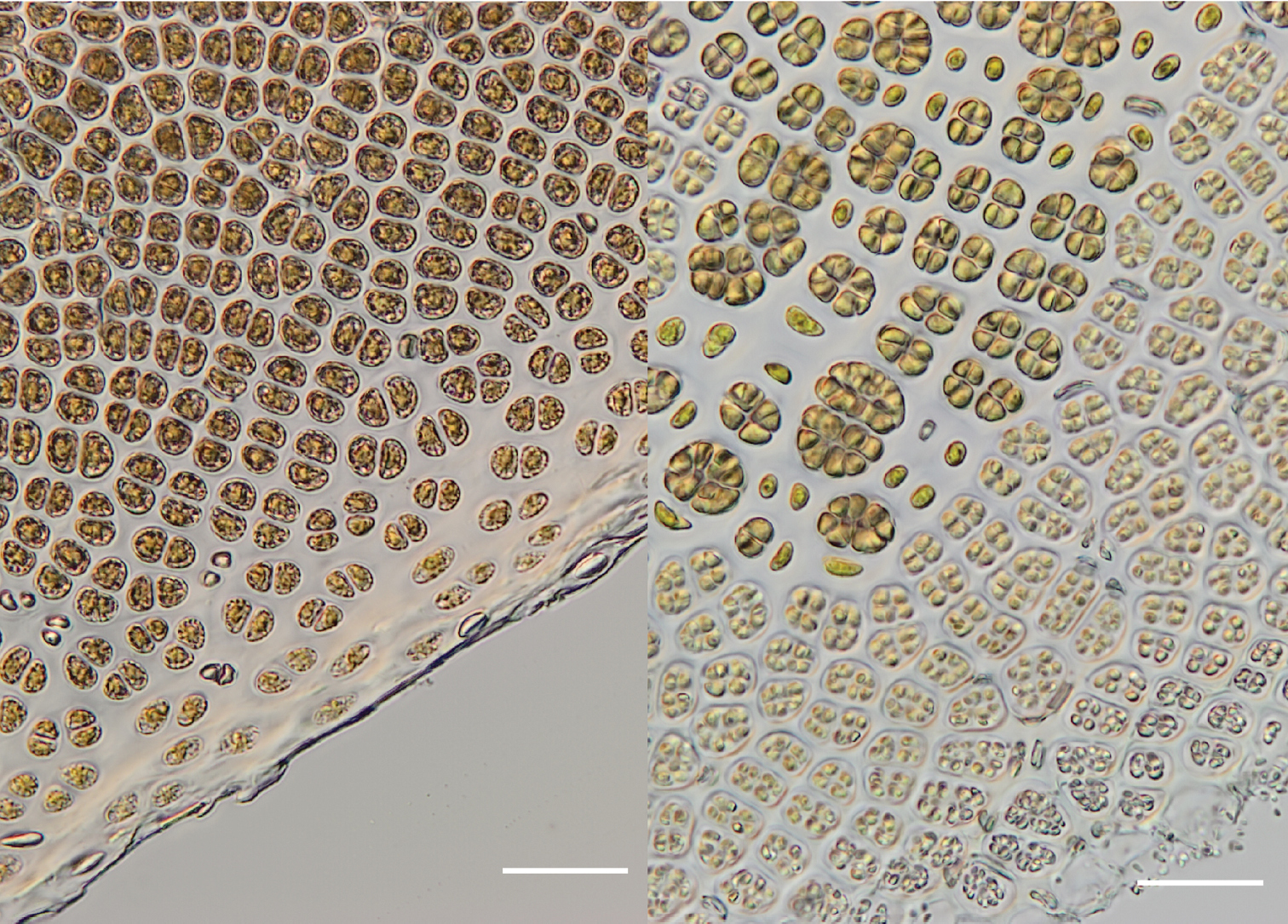
3 (left): Vegetative region of the blade showing the margin with small pale cells. (WELT A032593) 4 (right): Fertile region of the blade with packets of developing zygotosporangia (larger, darker-coloured clusters), single sterile cells (between zygotosporangia) and packets of spermatangia (smaller, paler-coloured clusters) releasing at the blade margin. (WELT A032593). Scale bar = 50 µm.
5 Cross section of monostromatic blade showing square sterile cells and spindle-shaped developing carpogonia. (WELT A032593) 6 Cross section view of mature zygotosporangia. (WELT A032593) 7 Cross section view of mature spermatangia. (WELT A032593). Scale bar 5: 50 µm, 6–7: 20 µm.
A major problem in Bangiales taxonomy has been the incorrect application of names, making studies of the ecology and comparative physiology of species exceedingly difficult. The need for molecular sequence data in Bangiales taxonomic studies has been emphasised by many authors over the past decade in order to clarify species concepts as well as the phylogenetic relationships amongst taxa (e.g.
As circumscribed by
I would like to thank Jenn Dalen for assistance with access to the herbarium and the registration of voucher material at Te Papa, and the many people who have assisted with field work and collections, particularly Judy Sutherland (Broom), Tracy Farr, and Kate Neill, as well as Margaret Clayton, Anne Conroy, Robyn Dunmore, Svenja Heesch, Wyn Jones, Glenys Knight, Peter de Lange, Murray Parsons, Louise Phillips, and Gina Williams. Erika Mackay is thanked for assistance with the figures. Funding for this research has been from the New Zealand Foundation for Research Science Technology, now MBIE Core Funding to NIWA (COBR1301).