(C) 2012 Margaret L. Stimpson. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Taxa in the Banksia spinulosa Sm. complex (Proteaceae) have populations with sympatric, parapatric and allopatric distributions and unclear or disputed boundaries. Our hypothesis is that under biological, phenetic and diagnosable species concepts that each of the currently named taxa within the Banksia spinulosa complex is a separate species. Based on specimens collected as part of this study, and data recorded from specimens in six Australian herbaria, complemented by phenetic analysis (semi–strong multidimensional scaling and UPGMA clustering) and a detailed morphological study, we investigated both morphological variation and geographic distribution in the Banksia spinulosa complex. All specimens used for this study are held at the N.C.W. Beadle Herbarium or the National Herbarium of New South Wales. In total 23 morphological characters (11 quantitative, five binary, and seven multistate characters) were analysed phenetically for 89 specimens. Ordination and cluster analysis resulted in individuals grouping strongly allowing recognition of distinct groups consistent with their recognition as separate species. Additional morphological analysis was completed on all specimens using leaf, floral, fruit and stem morphology, providing clear cut diagnosable groups and strong support for the recognition of Banksia spinulosa var. cunninghamii and Banksia spinulosa var. neoanglica as species.
Banksia spinulosa, Banksia cunninghamii, Banksia neoanglica, species limits, phenetics, new species, floral and inflorescence morphology
Banksia is a moderately sized genus currently of 212 taxa; viz. 78 species, 9 subspecies and 11 varieties (see
According to
Most herbaria follow
These competing taxonomic treatments have created confusion, examples of which can be found in species lists for some National Parks in New South Wales (unpublished visitor brochures), which include Banksia spinulosa var. neoanglica and Banksia cunninghamii subsp. A as separate entities. Some herbaria also concurrently use two names for the same entity (see the
The aims of this study were (1) to test and set the taxonomic status and circumscription of Banksia cunninghamii subsp. A; and (2) to search for novel diagnostic characters that could be used to distinguish individual taxa within the Banksia spinulosa complex (sensu
Although dried herbarium specimens were available for this study, it was considered necessary to collect fresh material to adequately investigate character homology though a detailed study of different developmental stages. Existing herbarium specimens were deficient in some developmental stages and often were not suitable for destructive sampling. We made collections from locations in New South Wales and Queensland encompassing the full geographic range of Banksia cunninghamii subsp. A. Vouchers have been lodged at NE and/or NSW (Table 1). Each site was visited twice; the first time in February to observe the development of the rachis, the second time in May to observe the flowering process. During both visits observations were made and vouchers prepared.
Vouchers used in phenetic and morphological analysis of the Banksia spinulosa complex.Numbers in the OTU code are M. L. Stimpson collection numbers. Bcu = Banksia cunninghamii subsp. cunninghamii; Bco = Banksia spinulosa var. collina; Bn = Banksia cunninghamii subsp. A; Bsp = Banksia spinulosa var. spinulosa; Bsp? = putative hybrid of Banksia spinulosa var. collina × Banksia spinulosa var. spinulosa. Abbreviations: NP = National Park; NSW = New South Wales; Qld = Queensland. Voucher codes are herbarium abbreviations following Thiers (continuously updated). All elements of the collections were available at NE and/or NSW during the study, replicates will be distributed.
| OTU Code | Location | Voucher |
|---|---|---|
| BcuHW30 | Hassans Walls, Hartley Vale, NSW | NE, NSW |
| BcuHW32 | Hassans Walls, Hartley Vale, NSW | NE, NSW |
| BcuHW41 | Hassans Walls, Hartley Vale, NSW | NE, NSW |
| BcuSR43a | Scenic Railway, Katoomba, NSW | NE, NSW |
| BcuSR43b | Scenic Railway, Katoomba, NSW | NE, NSW |
| BcuSR43c | Scenic Railway, Katoomba, NSW | NE, NSW |
| BcuHW114 | Hassans Walls, Hartley Vale, NSW | NE, NSW |
| BcuHW115 | Hassans Walls, Hartley Vale, NSW | BRI, NE, NSW |
| BcuEL117 | Evans Lookout, Blue Mountains, NSW | BRI, NE, NSW |
| BcuCF118 | Cataract Falls, Blue Mountains, NSW | NE, NSW |
| BcuML119 | McMahons Lookout, Blue Mountains, NSW | NE, NSW |
| BcuFF121 | Fitzroy Falls, E of Moss Vale, NSW | NE, NSW |
| BcuFF128 | Fitzroy Falls, E of Moss Vale, NSW | NE, NSW |
| BcuMW122 | Medway, W of Moss Vale, NSW | NE, NSW |
| BcuMW123 | Medway, W of Moss Vale, NSW | BRI, NE, NSW |
| BcuMW126 | Medway, W of Moss Vale, NSW | NE, NSW |
| BcoK25a | Kungala, NSW | NE |
| BcoK25c | Kungala, NSW | NE |
| BcoK60a | Kungala, NSW | NE, NSW |
| BcoK60b | Kungala, NSW | NE |
| BcoK60c | Kungala, NSW | NE, NSW |
| BcoKR61 | Kremnos, NSW | NE |
| BcoKR62 | Kremnos, NSW | NE, NSW |
| BcoTG88 | Tarragindi, Brisbane, Qld | NE, NSW |
| BcoDCK102 | Daves Creek track, Lamington NP, Qld | NE |
| BcoDCK85 | Daves Creek track, Lamington NP, Qld | NE, NSW |
| BcoDCK103 | Daves Creek track, Lamington NP, Qld | NE |
| BcoMY93 | Mullaway, NSW | NE |
| BnRN27a | Robinsons Knob Trail, New England NP, NSW | NE, NSW |
| BnRN27b | Robinsons Knob Trail, New England NP, NSW | NE, NSW |
| BnRN27c | Robinsons Knob Trail, New England NP, NSW | NE, NSW |
| BnBP28a | Banksia Point, New England NP, NSW | NE |
| BnBP28b | Banksia Point, New England NP, NSW | NE |
| BnBP28c | Banksia Point, New England NP, NSW | NE, NSW |
| BnNE39a | Point Lookout road, New England NP, NSW | NE |
| BnNE39b | Point Lookout road, New England NP, NSW | NE, NSW |
| BnNE39c | Point Lookout road, New England NP, NSW | NE, NSW |
| BnDCK79 | Daves Creek track, Lamington NP, Qld | NE, NSW |
| BnDCK80 | Daves Creek track, Lamington NP, Qld | NE, NSW |
| BnDCK81 | Daves Creek track, Lamington NP, Qld | NE, NSW |
| BnDCK82 | Daves Creek track, Lamington NP, Qld | NE |
| BnBP96 | Banksia Point, New England NP, NSW | BRI, NE, NSW |
| BnTC97 | Tom’s Cabin, New England NP, NSW | BRI, NE, NSW |
| BnMM98 | Mount Mitchell, NSW | BRI, NE, NSW |
| BnMM99 | Mount Mitchell, NSW | BRI, NE, NSW |
| BnG100 | Girraween NP, Qld | BRI, NE, NSW |
| BnG101 | Girraween NP, Qld | BRI, NE, NSW |
| BnDCK104 | Daves Creek track, Lamington NP, Qld | NE |
| BnDCK105 | Daves Creek track, Lamington, NP, Qld | NE, NSW |
| BnBB106 | Boonoo Boonoo NP, Morgan’s Gully, NSW | BRI, NE, NSW |
| BnBB107 | Boonoo Boonoo NP, Morgan’s Gully, NSW | NE, NSW |
| BnBB108 | Boonoo Boonoo NP, Morgan’s Gully, NSW | NE, NSW |
| BnBB109 | Boonoo Boonoo NP, Cyprus Pine Camp, NSW | NE, NSW |
| BnGR110 | Gibraltar Range NP, Mulligans Hut, NSW | BRI, NE, NSW |
| BnGR111 | Gibraltar Range NP, Mulligans Hut, NSW | NE |
| BnGR112 | Gibraltar Range NP, Mulligans Hut, NSW | BRI, NE, NSW |
| BnGR113 | Gibraltar Range NP, Mulligans Hut, NSW | NE |
| BspDC42 | Darling Causeway, Blue Mountains, NSW | NE, NSW |
| BspHB44 | Hazelbrook, Blue Mountains, NSW | NE, NSW |
| BspHB45 | Hazelbrook, Blue Mountains, NSW | NE, NSW |
| BspJB46 | Jervis Bay, NSW | NE, NSW |
| BspJB59 | Jervis Bay, NSW | NE, NSW |
| BspML120 | McMahons Lookout, Blue Mountains, NSW | NE, NSW |
| BspFF127 | Fitzroy Falls, E of Moss Vale, NSW | NE, NSW |
| BspMW129 | Medway, W of Moss vale, NSW | NE, NSW |
| BspML130 | McMahons Lookout, Blue Mountains, NSW | NE, NSW |
| BspEL131 | Evans Lookout, Blue Mountains, NSW | NE |
| BspCF132 | Cataract Falls, Blue Mountains, NSW | NE, NSW |
| Bsp?BU36 | Bouddi NP, NSW | NE, NSW |
| Bsp?BU37 | Bouddi NP, NSW | NE |
| Bsp?BU38 | Bouddi NP, NSW | NSW |
| Bsp?CA52a | Calga, NSW | NSW |
| Bsp?CA52b | Calga, NSW | NE, NSW |
| Bsp?CA52c | Calga, NSW | NE, NSW |
| Bsp?CA53a | Calga, NSW | NE, NSW |
| Bsp?CA53b | Calga, NSW | NE, NSW |
| Bsp?CA53c | Calga, NSW | NE, NSW |
| Bsp?M54a | Morisset, NSW | NE, NSW |
| Bsp?M54b | Morisset, NSW | NE, NSW |
| Bsp?M54c | Morisset, NSW | BRI, NE, NSW |
| Bsp?RM66 | Morisset, NSW | NE, NSW |
| Bsp?YM67 | Morisset, NSW | NE, NSW |
| Bsp?JB124 | Jervis Bay, NSW | NE |
| Bsp?MM86 | Mount Mee, Qld | NE |
| Bsp?MM87 | Mount Mee, Qld | NE |
| Bsp?GM89 | Glasshouse Mountains, Qld | NE |
| Bsp?GM90 | Glasshouse Mountains, Qld | NE |
| Bsp?GM91 | Glasshouse Mountains, Qld | NE |
| Bsp?GM92 | Glasshouse Mountains, Qld | NE |
Micromorphology was examined using Leica MZ8 and MZ9 stereomicroscopes fitted with eyepiece graticules. Images were taken using a Wild M400 photomacroscope fitted with a Nikon DS-5M-L1 Digital Sight Camera System. Exploratory scanning electron microscopy of styles was undertaken using air and silca gel-dried samples mounted on double-sided carbon tabs on aluminium stubs. Specimens were coated with gold in a Neocoater sputter coater and examined at 15 kV using a Neoscope JCM-5000 bench-top SEM.
Phenetic analysis Selection of charactersThe character list was primarily constructed to include leaf, floral, stem and fruit morphology. Assessment of descriptions of the taxa in the Banksia spinulosa complex (
Characters used for phenetic analysis for the Banksia spinulosa species complex.
| No. | Character and states |
|---|---|
| Quantitative characters | |
| 1 | Length of complete conflorescence including peduncle ± 1 mm |
| 2 | Width of lamina at widest point excluding teeth ± 1 mm |
| 3 | Length of lamina including mucro ±1 mm |
| 4 | Length from base of lamina to first tooth excluding mucro ± 1 mm |
| 5 | Length of seed including wing ± 1 mm |
| 6 | Width of wing at widest point ± 1 mm |
| 7 | Length of seed excluding wing ± 1 mm |
| 17* | Number of floral pairs around circumference of conflorescence |
| 9 | Length of complete infructescence ± 1 mm |
| 10 | Circumference of complete infructescence ± 1 mm |
| 12 | Lamina interveinal thickness when dry ± 0.05 mm |
| Binary characters | |
| 11 | Lignotuber: 1 = absent 2 = present |
| 20* | Floral bract keel number: 1 = 1 2 = 2 |
| 21* | Distal bract margins: 1= plain 2 = recurved |
| 22* | Bract apiculum: 1= absent 2 = present |
| 23* | Bract apiculum: 1 = not incurved 2= incurved |
| Multistate characters | |
| 8 | Lamina apex: 1 = tridentate, 2 = bidentate, 3 = unidentate |
| 13 | Colour of lamina adaxial surface when dry~ |
| 14 | Colour of lamina abaxial surface when dry~ |
| 15 | Colour of lamina adaxial surface prior to drying~ |
| 16 | Colour of lamina abaxial surface prior to drying~ |
| 18* | Style colour pre anthesis~ |
| 19* | Style colour post anthesis~ |
~=RHS colours, see Table 3. * = new characters; i.e. not previously used in studies of
Banksia (cf.
Colours, however, were treated as multistate characters to maximise accuracy and repeatability, which allowed for some natural variation, thus avoiding spurious over-precision (see below). Royal Horticultural Society (RHS) colours were used to compare adaxial and abaxial leaf surfaces prior to, and after drying, as well as styles before and after anthesis. Each RHS colour was allocated a number from 1–26 (Table 3).
RHS colour codes used in phenetic analysis.
| Colours | RHS colours | Coded RHS colours |
|---|---|---|
| Green Group | 135a–d | 1 |
| Green Group | 137a–d | 2 |
| Yellow Green Group | 146a–d | 3 |
| Yellow Green Group | 147a–d | 4 |
| Greyed White Group | 156a– | 5 |
| Greyed White Group | 156b-d | 6 |
| Greyed White Group | 157a–d | 7 |
| Greyed Green group | 190a-c | 8 |
| Greyed Green Group | 190d | 9 |
| Greyed Yellow Group | 160a | 10 |
| Greyed Yellow Group | 162a–d | 11 |
| Yellow green group | 148d | 12 |
| Green White group | 157b | 13 |
| Red Purple Group | 59a–d | 14 |
| Red Purple Group | 61a–d | 15 |
| Black Group | 202a–d | 16 |
| Greyed Yellow group | 160b-160d | 17 |
| Greyed Green Group | 191a–d | 18 |
| Greyed Green Group | 195a–d | 19 |
| Greyed Green Group | 196a–d | 20 |
| Green Group | 139a–d | 21 |
| Greyed Green Group | 198a–d | 22 |
| Yellow Green Group | 148a–d | 23 |
| Yellow Green Group | 145a-d | 24 |
| Yellow Green Group | 152a-d | 25 |
| Greyed Green Group | 198a-d | 26 |
All leaf measurements were taken from leaves in the middle of a branchlet, selected from the whorl of branchlets subtending a resting terminal bud or conflorescence; leaves were measured after drying. Conflorescence characters such as number of floral pairs were counted live on the plant. Infructescences were measured vertically with a steel ruler and the circumference was measured with a sewing tape measure.
Infructescences were placed on a gas burner for 1–3 min then left on brown paper for two days in a dry place. Seeds were extracted using a pair of forceps and measured under a stereomicroscope using a calibrated eyepiece graticule.
DatasetA dataset (Appendix 1) was maintained in Microsoft Excel and exported to PATN v. 3 for Windows (
The conflorescences of all taxa in the Banksia spinulosa species complex consist of an elongate woody rachis that has three types of bracts. Below the base of the rachis on the short peduncle are the involucral bracts. The second type of bract is the common bract each of which subtends a flower pair on the conflorescence axis. The third type of bract, a smaller floral bract, subtends each flower in a pair (
The perianth segments or tepals in Banksia each consist of a limb and a claw (
The conflorescences in the Banksia spinulosa complex have the appearance of being a particular colour, i.e. black, red, yellow orange, or purple. It is the styles that are most boldly coloured with red, black, green, yellow or purple pigment, not the limb and claw (
All taxa within the Banksia spinulosa complex have leaves that are scleromorphic in texture, discolourous, and linear in shape. The indumentum on the abaxial leaf surface is felted and the midvein is raised on the abaxial surface of all leaves in all taxa within the complex. Continuous variation was found in the colour of adaxial and abaxial leaf surfaces both within and between populations of all taxa within the Banksia spinulosa complex.
LignotubersThe term lignotuber refers to a woody swelling which may take the form of an extensive subterranean lignotuber, basal lignotuber, or an above ground lignotuber (
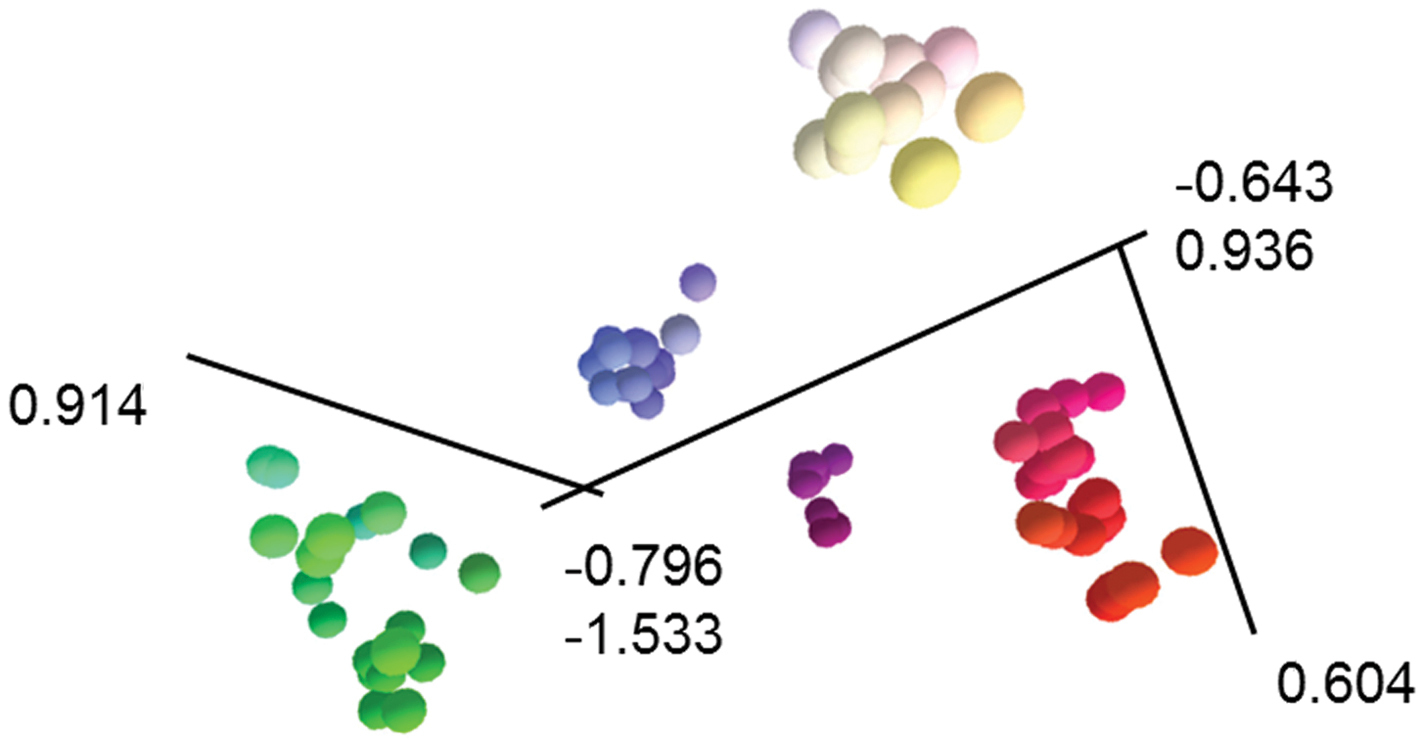
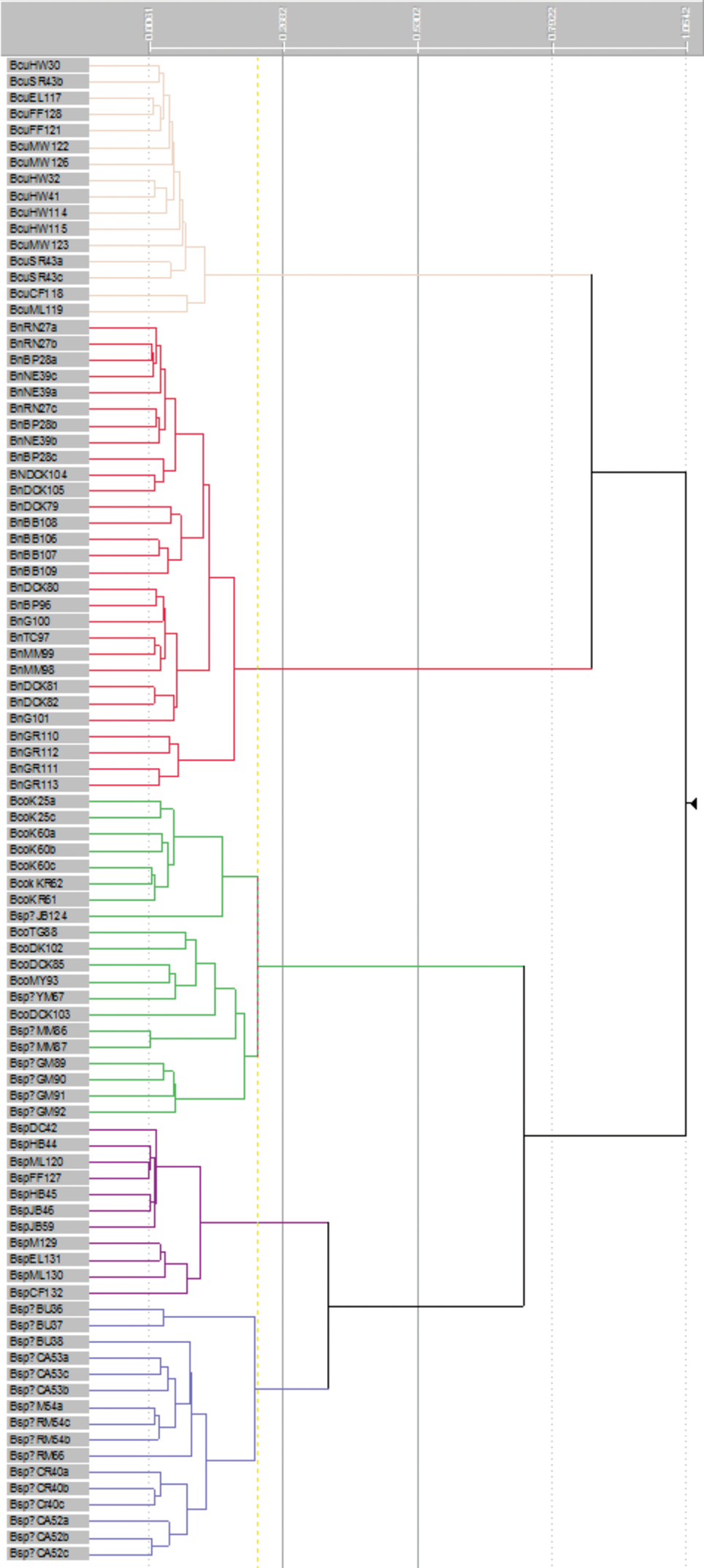
Ordination (Figure 1) and clustering (Figure 2) of the data matrix found five distinct groups of OTUs in the Banksia spinulosa complex: corresponding to a priori names Banksia spinulosa var. collina sens. lat., Banksia spinulosa var. collina × Banksia spinulosa var. spinulosa from near the New South Wales locations of Morisset, Bouddi and Calga, Banksia spinulosa var. spinulosa, Banksia cunninghamii subsp. cunninghamii, B. cunninghamii subsp. A. The phenogram displays the same five groups of OTUs (Figure 2). Even when we reran the analyses excluding all the binary characters (Characters 11, 20–23; ordination and phenogram not presented), the same five groups of OTUs were obtained, which, along with the very low stress value (Figure 1) indicate that the results are robust. Twelve of the 23 characters, including quantitative, binary and multistate characters had correlated more than 70% with the ordination (Table 4) indicating sound choice of characters, a broad base of evidence for the patterns obtained, and confidence in the results obtained.
3D ordination from semi-strong multidimensional scaling of the Banksia spinulosa complex. From to leftto right, Banksia spinulosa var. collina sens. lat., Banksia spinulosa from Morisset, Bouddi and Calga, Banksia spinulosa var. spinulosa, Banksia cunninghamii subsp. cunninghamii, Banksia cunninghamii subsp. A. Ordination stress = 0.795. Size and colour of OTUs represents perspective. Ordination orientated to highlight separation of groups of OTUs. See Table 2 for characters and Appendix 1 for data.
Flexible UPGMA phenogram of OTUs in the Banksia spinulosa complex. Major groups from top to bottom: Banksia cunninghamii subsp. cunninghamii, Banksia cunninghamii subsp. A, B. spinulosa var. collina sens. lat., Banksia spinulosa var. spinulosa, Banksia spinulosa from Morisset, Bouddi and Calga. See Table 2 for characters and Appendix 1 for data.
Principal component correlation (PCC) attributes and ordination vectors for ordination of the Banksia spinulosa complex. See Table 2 for Character numbers.
| Character | X | Y | Z | Correlation (r2) |
|---|---|---|---|---|
| 21 | -0.115 | 0.983 | 0.146 | 0.978 |
| 20 | 0.35 | 0.67 | 0.655 | 0.953 |
| 14 | -0.254 | 0.695 | 0.672 | 0.938 |
| 23 | -0.604 | 0.473 | -0.641 | 0.938 |
| 18 | -0.464 | 0.482 | -0.743 | 0.929 |
| 13 | 0.872 | 0.195 | -0.45 | 0.917 |
| 22 | -0.787 | -0.327 | 0.523 | 0.893 |
| 11 | -0.534 | -0.77 | -0.35 | 0.847 |
| 8 | 0.767 | 0.269 | -0.583 | 0.84 |
| 9 | -0.678 | 0.6 | -0.425 | 0.826 |
| 10 | 0.146 | 0.72 | 0.679 | 0.766 |
| 19 | -0.104 | 0.415 | -0.904 | 0.732 |
| 3 | -0.477 | 0.868 | 0.141 | 0.581 |
| 6 | 0.348 | 0.868 | -0.355 | 0.537 |
| 2 | -0.339 | 0.662 | -0.669 | 0.454 |
| 1 | -0.47 | 0.702 | -0.535 | 0.413 |
| 16 | 0.174 | -0.25 | -0.952 | 0.391 |
| 15 | 0.45 | 0.036 | -0.892 | 0.372 |
| 12 | -0.326 | -0.601 | 0.729 | 0.308 |
| 7 | 0.433 | 0.858 | -0.277 | 0.279 |
| 5 | 0.005 | 0.635 | -0.772 | 0.193 |
| 4 | 0.714 | 0.694 | 0.092 | 0.146 |
| 17 | -0.614 | 0.567 | -0.549 | 0.123 |
The cluster of OTUs of Banksia spinulosa from Morisset, Bouddi and Calga (Table 1) is characterised by red styles, at Morisset and Bouddi and black styles at Calga, multi-stemmed habit and occurs between the Hawkesbury River and Hunter Valley. Herbarium specimens from these locations have been determined by A.S. George and other botanists as “Banksia spinulosa var. collina × Banksia spinulosa var. spinulosa”.
Slight outliers in the Banksia spinulosa var. collina cluster represent some discontinuous morphological variation, which we also plan to investigate.
Taxonomic inferenceGiven the consistent clear cut groups in the ordination and cluster analysis across a broad geographic and morphological range of OTUs (Table 1), we propose the following taxonomic arrangement, which we use hereafter in this paper: recognising Banksia cunninghamii subsp. cunninghamii as Banksia cunninghamii sensu stricto; recognising Banksia spinulosa var. collina as Banksia collina sensu lato; recognising Banksia spinulosa var. spinulosa as Banksia spinulosa sensu stricto; formalising Banksia cunninghamii subsp. A at species rank under the name Banksia neoanglica . Although the OTUs of Banksia spinulosa from the Morisset and Bouddi populations could be considered to constitute a distinct species on the evidence we present here, we refrain from recognising these populations as a distinct taxon until we have more thoroughly tested the hypothesis that they are part of an extensive hybrid swarm and searched for any additional populations that might provide evidence for integradation between Banksia collina and Banksia spinulosa.
Morphological analysis Growth forms within the Banksia spinulosa species complexBanksia cunninghamii sensu stricto is a single-stemmed tree to 7 m tall, and is non-lignotuberous. Banksia spinulosa sensu stricto forms a multi-stemmed, rounded shrub to 3 m high. The lignotuber is subterranean. Banksia collina sensu lato is a multi-stemmed upright shrub to 3 m tall, with a subterranean lignotuber (
Banksia neoanglica has a variety of growth forms ranging from small rounded multi-stemmed shrubs to single-stemmed trees. The growth forms of Banksia neoanglica appear to be related to the degree of exposure of plants to fire. At sites where there have been no fires for more than 15 years, such as at Binna Burra, Lamington National Park, Queensland and some parts of Gibraltar Range National Park, New South Wales (Pers. Comm. Justin Kreis Ranger Glen Innes National Park), Banksia neoanglica is a single-stemmed tree and exhibits all the traits of an obligate seeder such as a greater infructescence load and spontaneous opening of the follicles. In the tree form, Banksia neoanglica has a slight swelling at the base of the trunk just below the soil or there are epicormic buds which often develop into branches, well above ground level, similar to those of some eucalypts (
Styles: The structure of the conflorescence, including perianth and styles is similar for all taxa in the Banksia spinulosa complex. Size, shape and colour of the individual parts of the conflorescence, however, differ considerably across the species. Style colour in the Banksia spinulosa complex varies depending on the proportions of chlorophyll (green), carotenoid (yellow to orange), anthoxanthin (yellow) and anthocyanin (red to purple) pigments that develop in them (
The style apex in B. cunninghamii sensu stricto seems to have substantially more anthocyanin pigment than either Banksia spinulosa sensu stricto or Banksia neoanglica. In Banksia cunninghamii sensu stricto we observed that the style length is usually longer than either Banksia spinulosa sensu stricto or Banksia neoanglica and is a similar length to Banksia collina sensu lato. The black pigmentation of the styles of Banksia cunninghamii sensu stricto starts to develop one third of the way along the style above the ovary. In Banksia spinulosa sensu stricto and Banksia neoanglica the dark pigmentation in the style develops one half to two thirds of its length above the ovary. In all populations in the Banksia spinulosa complex with the exception of Banksia collina sensu lato we observed what appeared to be yellow-styled conflorescences. Upon closer inspection they are green styled and appear to have less chlorophyll in both the styles and leaves than is found in Banksia collina sensu lato which is also green-styled. Green styled variants are found in less than 2% of any one population except in Banksia collina. Polymorphism is a common trait in Proteaceae where, for example, 40% of all species of Protea exhibit variation in the bract, style and perianth colour (
Perianth colour: The colours of the perianth in the Banksia spinulosa complex vary according to their developmental stage and their exposure to sunlight. The perianth colours can vary within and between populations in all four of the species in the Banksia spinulosa complex. The factor that seems to have the most influence on the perianth colour in the early stages of development is exposure to sun, often mediated by the position of an conflorescence on the outside or inside branches of the plant or by shading from other plants. In Banksia spinulosa sensu stricto, Banksia collina and Banksia neoanglica, the conflorescences that are exposed to full sun tend to have orange or yellow perianths. Those that are exposed to a limited amount of sun tend to be green. The perianth colour of Banksia cunninghamii sensu stricto is diagnostic for the species. At maturity the perianth always has a distinct pink hue and this colouring continues through to anthesis. The pink hue does not vary between and within populations of Banksia cunninghamii sensu stricto, nor does exposure to full sun orfull shade effect the colour of the perianth at maturity.
Common bracts: Common bracts have been mentioned in previous studies (
Common bractson young conflorescences in the Banksia spinulosa species complex: A Banksia neoanglica (BanksiaL. Stimpson 98) B Banksia cunninghamii sensu stricto (M.L. Stimpson 122) C Banksia collina sensu lato (M.L. Stimpson 25A) D Banksia spinulosa sensu stricto. (M.L. Stimpson 120). Scale bar = 1 mm.
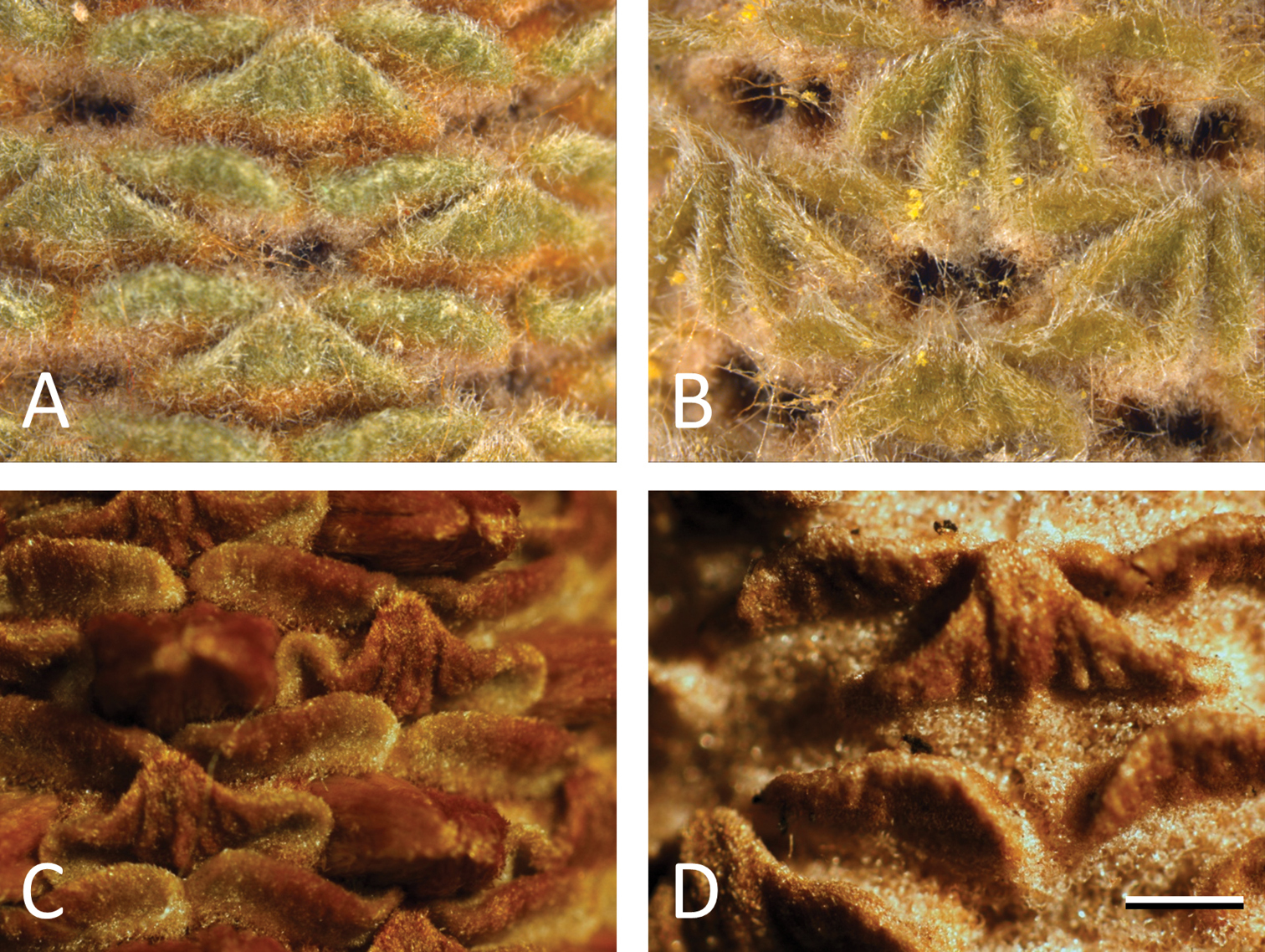
Involucral bracts: Involucral bracts appear to be taxonomically informative at the species level in the study group. The involucral bracts of Banksia cunninghamii sensu stricto are caudate with an abaxial ‘spine’ (Figure 4A). The involucral bracts in Banksia spinulosa sensu stricto (Figure 4B) are longer and more scleromorphic, with little or no hair and no external spine. In Banksia neoanglica (Figure 4C) these bracts are more hirsute without an external spine and in Banksia collina sensu lato (Figure 4D) the involucral bracts are shorter, have no external spine and limited hair. There are differences in the distal and proximal portions of the involucral bracts (Figure 4A–D) in each species that warrant further examination.
Involucral bracts on young conflorescences in the Banksia spinulosa complex. A Banksia cunninghamii sensu stricto (M.L. Stimpson 122) B Banksia spinulosa sensu stricto (M.L. Stimpson 125) C Banksia neoanglica (M.L. Stimpson 81) D Banksia collina sensu lato (M.L. Stimpson 25A). Scale bar = 2.5 mm.
The diversity of species concepts in the biological literature is an asset, not a liability when considering the Banksia spinulosa complex and are an integral part of biological theory. We have taken into account the co-varying morphological discontinuities, the phenetic species concept, geographical and ecological isolation and the biological species concept of reproductive isolation. The use of differing concepts has been useful in suggesting multiple lines of evidence for testing taxonomic boundaries in the Banksia spinulosa complex (cf.
Comparison of some attributesof Banksia neoanglica, Banksia spinulosa sensu stricto and Banksia cunninghamii.
| Character | Banksia neoanglica | Banksia spinulosa | Banksia cunninghamii |
|---|---|---|---|
| Lignotuber | present | present | absent |
| Leaf length | 43–75 mm | 50–72 mm | 53–88 mm |
| Leaf width | 3–4.5 mm | 1.5–2.5 mm | 3–4.5 mm |
| Leaf margins | not recurved | tightly recurved | not recurved |
| Length of inflorescence | 84–119 mm | 96–144 mm | 99–152 mm |
| Common bract keels | single thickened keel | single keel apex | two thin keels |
| Common bract apex | apex rounded | apiculate | apex rounded |
| Number of floral pairs | 12–14(–16) pairs | 13–16 pairs | 12–14 pairs |
| Perianth colour | orange, or yellow | orange or yellow | pink |
| Style colour prior to anthesis | red/maroon | red/maroon | red/maroon |
| Style colour after anthesis | purple/black | purple/black | purple/black |
| Circumference of infructescence | 141–160 mm | 153–159 mm | 113–125 mm |
| Length of infructescence | 85–120 mm | 96–144 mm | 113–140 mm |
The geographic distribution of Banksia neoanglica falls within the biogeographic region known as the “Macpherson–Macleay Overlap” of
Banksia cunninghamii sensu stricto and Banksia neoanglica have often been misidentified because Banksia cunninghamii sensu stricto, on occasions, has a brown indumentum; Banksia neoanglica sometimesalso exhibitsbrowning on the abaxial leaf surface. This character has been used in the past as an aid to distinguishing Banksia cunninghamii sensu stricto and the two other ‘varieties’ recognised at that time (
Disjunct populations in central and northern Queensland currently assigned to Banksia spinulosa var. spinulosa warrant inclusion in a more broadly framed analysis, as do the northern and southern populations of Banksia collina sensu lato and Victorian populations of Banksia cunninghamii sensu stricto. There are also other populations of Banksia that clearly belong with the Banksia spinulosa group but are as yet unstudied. Further work is needed to enable suitable placement of these populations. Analysis using molecular data, together with expanded use of the novel characters presented here, would likely resolve these long-outstanding taxonomic issues.
Taxonomic treatmenthttp://species-id.net/wiki/Banksia_neoanglica
AUSTRALIA: New South Wales: Northern Tablelands, 900 m along Waterfall Way towards Ebor from turn-off to New England National Park, 22 May 2011, M.L. Stimpson 180, J.J. Bruhl & I.R. Telford; neotype: NSW; isoneotype: AD, BRI, CANB, CNS, K, MEL, NE, MO, PERTH. Figure 5.
Photograph of the neotype of Banksia spinulosa var. neoanglica A.S.George (M.L. Stimpson 180, J.J. Bruhl & I.R. Telford, NE 98613).
Banksia spinulosa Sm. var. cunninghamii (Sieber ex Rchb.) A.S.George, Nuytsia 3: 396 (1981) pro parte, excluding type.
Banksia cunninghamii Sieber ex Rchb. subsp. A: G.J. Harden in G.J. Harden (ed.), Flora of New South Wales 1: 71 (1991); G.J Harden, D.W. Harden & D.C. Godden (2000) Proteaceae of New South Wales: 170 (2000); G.J. Harden in G.J. Harden (ed.), Flora of New South Wales 2, edn 2: 86 (2002).
The protologue of Banksia spinulosa var. neoanglica quotes the type:
“1 km N of turnoff to New England National Park, Ebor–Armidale road, N.S.W., 6 April 1986, S.C. Clemesha; holo: NSW; iso: CANB, BRI, MEL, PERTH”.
No specimens so labelled have been located in NSW, BRI, CANB or MEL herbaria after repeated searches. Alex George (pers. comm. 2010–2011) could find no specimens in PERTH and he believes it likely that specimens were never distributed. Accordingly, we have nominated a neotype, collected from the same population as the type.
Shrubs with 2–8(–10) stems to 2.5 m from a lignotuber or trees to 7 m tall. Juvenile leaves: petiole 2–3.8 mm long; lamina narrowly obovate, 30–66 mm long, 5–11 mm wide, strongly dentate along full leaf margin, apex bidentate. Adult leaves: petiole 1.8–3.5 mm long; lamina linear, 43–75 mm long, 3–4.5 mm wide, occasionally toothed towards the usually unidentate, occasionally bidentate apex; adaxial surface glabrous, with colour after drying RHS greyed green group 195a-d; abaxial surface felted, colour after drying RHS greyed white group 156a–d. Involucral bracts subulate, thickened at base, 3–15mm long, grey-brown pubescent. Conflorescence 84–119 mm long, 70–85 mm diameter at anthesis; floral pairs 12–14(–16) around the circumference of the conflorescence axis. Common bract with a single thickened keel on the abaxial surface that extends from the apex of the bract down to the visible part of the base of the bract, distal margins slightly concave, apex rounded, indumentum villous, lower third of bract uniformly brown and upper two thirds uniformly green (Fig. 3A). Perianth 18–23 mm long, pubescent, yellow–orange at maturity but may be green, orange or yellow during developmental stages; limb c. 3.5 mm long; anthers c. 1 mm long. Style 25–38 mm long, apically hooked, colour grading from red to maroon to black just prior to anthesis. Infructescence 85–120 mm long, 35–45mm diam. Seed 15–19 mm long, including wing. Figure 6.
Banksia neoanglica at neotype locality. A Habitat B Conflorescences on shrub C Conflorescence from the neotype collection(M.L. Stimpson 180, J.J. Bruhl & I.R. Telford) showing basipetal development; upper flowers with pollen on pollen presentors D Conflorescence and infructescence with black styles at preanthesis. E–G Apex of conflorescences at successive stages of development exhibiting variation in perianth and style colour. Scale bars = 1 cm.
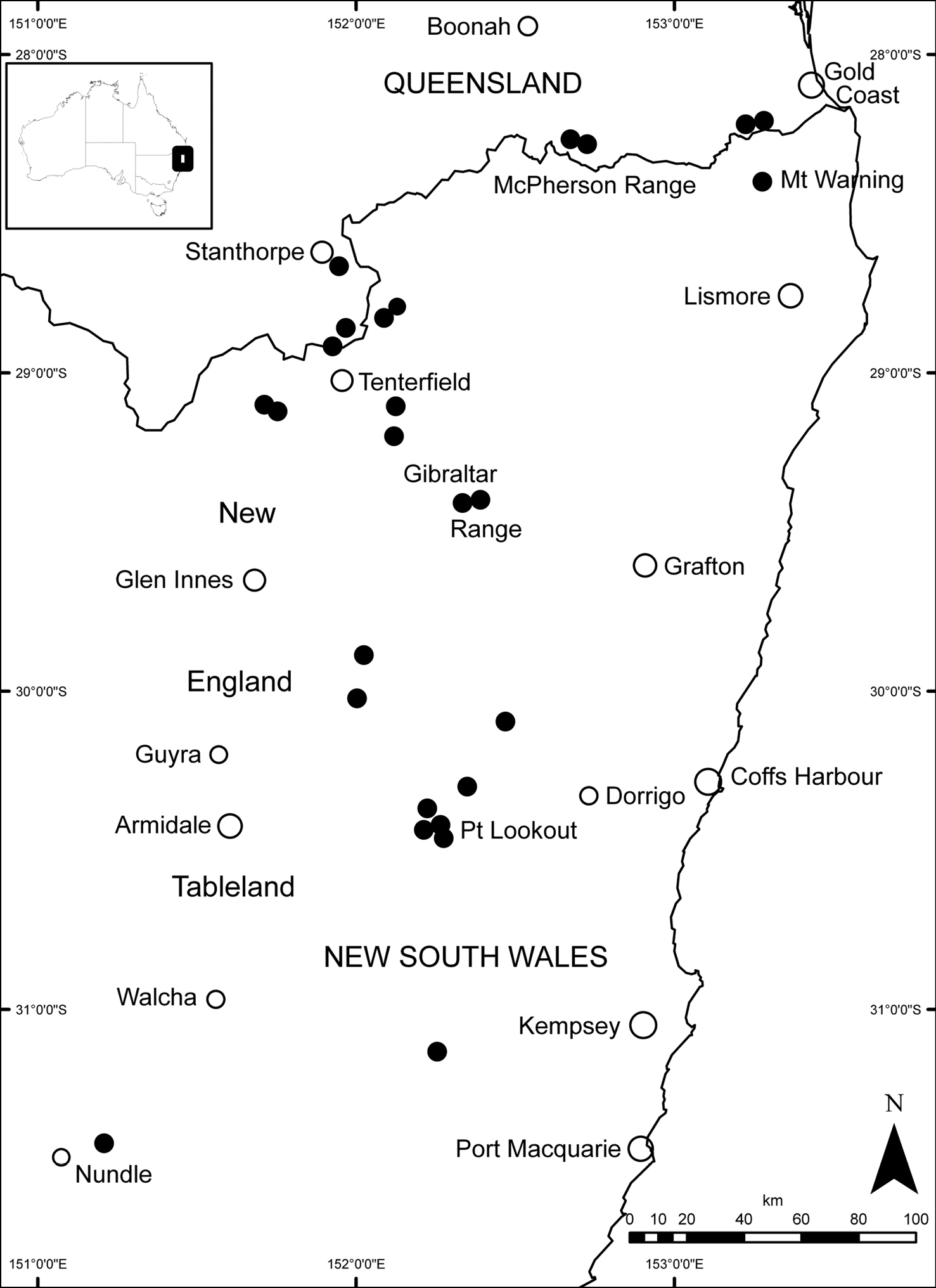
Banksia neoanglica occurs on the McPherson Range, just north of the Queensland–New South Wales border, Mt Warning and the eastern edge of the New England Tableland southwards to near Hanging Rock, New South Wales. Figure 7.
Distribution of Banksia neoanglica(solid black circles). Towns and cities indicated by open circles.
Grows in sandy soil on granite and acid volcanics, rarely on basalt, in Eucalyptus open forest (Figure 6), woodland and heath at altitudes of 850–1480 m. The species is sympatric with Banksia integrifolia subsp. monticola throughout its range, with Banksia marginata sensu lato on the Gibraltar Range and with Banksia conferta in the Daves Creek area.
The growth forms that Banksia neoanglica assume appear to be dependent upon the exposure to fire (
The species is widespread, often locally common, and is not considered at risk. It is conserved in several reserves: Lamington, Springbrook and Girraween National Parks in Queensland, and Boonoo Boonoo, Gibraltar Range and New England National Parks and Torrington State Conservation Area in New South Wales.
AUSTRALIA. Queensland: Moreton District: McPherson Range, Lamington National Park, Daves Creek track, M.L. Stimpson 79 (BRI, NE, NSW); Darling Downs District: Girraween National Park, track to Mt Norman, 21 Jan. 2009, I.R. Telford 13278 & J.J. Bruhl (NE). New South Wales: North Coast: Mount Warning, 3 Oct. 1939, F.A. Rodway s.n. (NSW); Northern Tablelands: 19 km E of Deepwater on Miles Shaw Rd, Butterleaf State Forest, J.T. Hunter 3750 & P.J. Clarke (NE); ); 0. 4 km N of Torrington, 19 Nov 1972, J.B. Williams s.n. (NE); Pheasant Mountain, 32 km NE of Guyra, 24 Apr. 1972, H.J. Wissmann s.n. (NE); Mount Chaelundi, E side just below crest, J.T. Hunter 157 & V.H. Hunter (NE); New England National Park, Banksia Point, M.L. Stimpson 28 (BRI, NE, NSW); NE of Bakers Downfall Hill, Nundle State Forest, J.R. Hosking 1877 (CANB, MEL, NE, NSW).
Resting buds start to expand in late January and conflorescences are fully developed by late March with flowering continuing until early July. These times are dependent on climatic conditions.
Extensive experiments conducted between May 1986 and July 1987 found that the New England population of Banksia neoanglica studiedwas autogamous (
MLS thanks Leanne and David Rowbotham, Bob and Maureen Anderson for considerable assistance in the location and collection of some coastal populations of Banksia, and Mark and Wendy Alexander for permission to collect on their property. We acknowledge permission from National Parks authorities in New South Wales and Queensland to collect in areas under their administration. Thanks also go to R.D.B. (Wal) Whalley, Ray South, Justin Kries and Alex George for constructive suggestions, and to the directors/curators of herbaria BRI, CANB, CNS, MEL NE, and NSW for specimen data and, where relevant, searching for the original type collection. We acknowledge access to facilities and collections at NE and NSW. Open access to this paper was supported by the Encyclopedia of Life (EOL) Open Access Support Project (EOASP).
Dataset of Banksia spinulosa complex used for phenetic analysis. See Table 1 for OTU codes and Table 2 for character list. (doi: 10.3897/phytokeys.14.3415.app) File format: MS Word (DOC).
Explanation note: The dataset (organised in MS Excel) presented here as a MS Word document, includes 23 characters and 92 individuals (operational taxonomic units = OTUs).