Research Article |
|
Corresponding author: Daniel Testoni ( daniel.testoni@uns.edu.ar ) Academic editor: Marcin Nobis
© 2017 Daniel Testoni, H. Peter Linder.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Testoni D, Linder HP (2017) Synoptic taxonomy of Cortaderia Stapf (Danthonioideae, Poaceae). PhytoKeys 76: 39-69. https://doi.org/10.3897/phytokeys.76.10808
|
Abstract
Cortaderia (Poaceae; Danthonioideae) is a medium-sized genus of C3 tussock grasses, widespread in the temperate to tropic-alpine regions of South America. It is particularly important in the subalpine and alpine zones of the Andes. We revised the classification of the genus, and recognize 17 species grouped into five informal groups. We describe one new species, Cortaderia echinata H.P.Linder, from Peru. We provide a key to the groups and the species, complete nomenclature for each species including new lectotypes, and notes on the ecology, distribution and diagnostic morphological and anatomical characters.
Keywords
Leaf anatomy, key, nomenclature, South America, taxonomy
Introduction
Cortaderia Stapf (Danthonioideae, Poaceae) is best known for the pampas-grass, Cortaderia selloana (Schult. & Schult. f.) Asch. & Graebn., which is globally cultivated as a garden ornamental (
The genus was erected by
The taxonomy of the genus has never been reviewed in total, from a global perspective. The Cortaderia selloana group was revised by
There are several taxonomic problems in the genus. Species delimitations of the three species closely related to C. selloana present a major challenge, as already noted by
The reproductive biology of Cortaderia is complex, with apparently hermaphrodite, dioecious, gynodioecious and apomictic species.
The phylogeny of Cortaderia is as yet incompletely known. Phylogenetic analyses have been published by
This paper presents a critical review of the species limits in Cortaderia based on leaf anatomical features, investigation of field populations and the study of herbarium specimens. We also clarify the nomenclature and typification of all names in the genus, and provide a key to the species. A descriptive monograph of the whole subfamily is in preparation, and full descriptions will be published in that account, as well as the full lists of specimens examined.
Materials and methods
The morphological descriptions were compiled from the analysis of the available herbarium material at B,
| Species | Voucher | Country |
|---|---|---|
| araucana | Testoni, D., 656 ( |
Chile |
| araucana | Werdermann, E., 1360 (K) | Chile |
| bifida | Beck, S.G., 1816 ( |
Bolivia |
| bifida | Renvoize, S.A.; Cope, T.A.; Beck, S., 4202 (K) | Bolivia |
| bifida | Smith, D.N. & Canabilla, J., 7167 ( |
Peru |
| bifida | Testoni, D., 477 ( |
Ecuador |
| bolivensis | Beck, S.G., 21266 (K) | Bolivia |
| boliviensis | Beck, S.G., 11273 (K) | Bolivia |
| boliviensis | Renvoize, S.A., 5342 ( |
Bolivia |
| columbiana | Schultes, R.E., 7251 (K) | Venezuela |
| columbiana | Schultes, R.E., 7226 (K) | Colombia |
| columbiana | Schulz, J.P. & Rodri, L., 318 ( |
Venezuela |
| egmontiana | Green, S.W., 42385 (K) | Falkland/Malvinas |
| egmontiana | Moore, D.M., 1697 (K) | Argentina |
| egmontiana | Peterson, P.M., Soreng, R.J. & Refulio-Rodriguez, N., 17465 ( |
Argentina |
| egmontiana | Pisano, E. & Henriquez, M., 8802 ( |
Chile |
| egmontiana | Testoni, D., 634 ( |
Argentina |
| echinata | Peterson, P.M. & Soreng, R.J., 21587 (Z) | Peru |
| hapalotricha | Laegaard, S., 53805 (K) | Ecuador |
| hapalotricha | Renvoize, S.A. & Laegaard, S., 5023 (K) | Peru |
| hieronymi | Asplund, E. 11971 (K) | Peru |
| hieronymi | Garcia, Beck, S.G. & Michel 563 (K) | Bolivia |
| hieronymi | Testoni, D., 386 ( |
Argentina |
| hieronymi | Testoni, D., 496 ( |
Ecuador |
| modesta | Carauta, P., 927 ( |
Brazil |
| modesta | Chase, A., 8288 ( |
Brazil |
| modesta | Glaziou, A.F., 17913 (K) | Brazil |
| modesta | Luetzelburg, 6368 (M) | Brazil |
| nitida | Laegaard, S., 53121 (K) | Ecuador |
| nitida | Testoni, D. 516 ( |
Ecuador |
| nitida | Soderstrom, T.R., 1350 (K) | Colombia |
| nitida | Steyermark, J.& Dunsterville. G.C.K., 101134 ( |
unknown |
| roraimensis | Farney, C. 885 ( |
Brazil |
| roraimensis | Magire, B., Pires, J.M. & Magire, C.K., 60448 ( |
Venezuela |
| roraimensis | Steyermark, J., 103836 ( |
Venezuela |
| speciosa | Renvoize, S.A., 5341 (K) | Bolivia |
| selloana ssp. selloana | Linder, H.P., s.n. | South Africa |
| selloana ssp. selloana | Villamil, |
Uruguay |
| selloana ssp. jubata | Testoni, D., 435 ( |
Ecuador |
| sericantha | Laegaard, S., 55066 (K) | Ecuador |
| sericantha | Laegaard, S., 55728 (P) | Ecuador |
| sericantha | Ramsay, P.M.; Merrow-Smith, P.J., 967 (K) | Ecuador |
| sericantha | Testoni, D., 438 ( |
Ecuador |
| speciosa | Renvoize, S.A.; Flores, G.; Peca, C., 5272 (K) | Bolivia |
| speciosa | Testoni, D., 644 ( |
Chile |
| vaginata | Reitz, P.R., 2672 ( |
Brazil |
| vaginata | Smith, L.B., Reitz, P.R. & Klein, R., 7761 (B) | Brazil |
| vaginata | Zanin 1654 ( |
Brazil |
The assignation of holo- and lectotype status follows the analysis and recommendations of (
Taxonomic treatment
Cortaderia , nom. cons.
Cortaderia Stapf, Gard. Chron. ser. 3. 22: 378 (1897) nom. cons. Type species: C. selloana (Schult.) Asch. & Graebn. (Syn. Mitteleur. Fl. 2(1): 325. 1900) (Basionym Arundo selloana Schult.).
Moorea Lem., Ill. Hort. 2: Misc. 14 (1855) nom. rej., non Rolfe (1890). Type species: M. argentea (Nees) Lem. (C. selloana).
Lamprothyrsus Pilg., Bot. Jahrb. Syst. 37 (Beibl. 85): 58 (1906). Type species: L. hieronymi (Kuntze) Pilg. (Basionym Triraphis hieronymi Kuntze).
Description
Gynodioecious, dioecious, hermaphrodite or apomictic perennials, ranging from rounded vegetable hedgehogs less than 0.5 m tall to erect 4 m tall tussocks; innovations intravaginal; spreading stolons rare. Leaf sheaths variable: persisting intact, or fragmenting transversely, or decaying into a tangled mass of fibres, or occasionally persisting as burnt-off sheaths; glabrous or more rarely covered in a dense indumentum. Ligule of one or many rows of cilia, to 5 mm long. Leaf blades to 2 m long, tough, expanded, rolled or folded, occasionally pungent, usually persistent but occasionally disarticulating above the ligule, sometimes with an adaxial weft of hairs directly above the ligule; margins sometimes roughly scabrid and cutting. Inflorescences paniculate, sometimes compact but usually plumose, to 1 m long, many-spikeleted, pedicels and pulvini glabrous, scabrid or villous. Spikelets to 30 mm long, with 2–10 florets, disarticulating above the glumes, male spikelets usually less hairy than female spikelets and glabrous in the Selloana group; glumes glabrous, often papery or membranous, 4–30 mm long, usually 1-veined and rarely with no veins, upper and lower glumes similar. Lemmas (Fig.
Lemmas of selected species of Cortaderia. A C. selloana, Jürgens 40 (B) B C. araucana, Borchers s.n. (
Leaf anatomy
Leaf in transverse section sclerophyllous, leaves varying from expanded to setaceous, margins not thickened but with a sclerenchyma cap. Adaxial furrows vary from deep and cleft-like to absent; abaxial ribs sometimes present. Vascular bundles differentiated into two, rarely three, orders; primary vascular bundles 6–30, symmetrically distributed in the two leaf sections; either ad- or abaxially or centrally positioned, circular or elliptical, sometimes with sclerosed phloem; outer bundle sheath cells always distinct from the chlorenchyma and sometimes lignified, entire or interrupted by bundle sheath; adaxial sclerenchyma as narrow girders, as trapezoidal girders, as T-shaped girders or inversely anchor-shaped girders; abaxial sclerenchyma as small strands, as narrow girders, as wide girders, as trapezoidal girders, or as massive linked girders forming a continuous subepidermal layer; tertiary vascular bundles 1-several between the primary vascular bundles, adaxial sclerenchyma as small strands, as narrow girders, as trapezoid girders narrowing towards vascular bundles, as T-shaped girders or inversely anchor-shaped girders; abaxial sclerenchyma absent, as small strands, as narrow girders, as broad girders, as trapezoidal girders or as massive linked girders forming a continuous subepidermal layer. Mesophyll of small, angular isodiametric chlorenchyma cells with small air spaces; mesophyll islands of colourless cells usually absent, sometimes with colourless collenchyma cells connecting the adaxial and abaxial furrows and so partitioning the chlorenchyma. Abaxial subepidermal layer sometimes with collenchymatous or non-chlorophyllous cells in 1-several layers only along the margins, or flanking the midrib, and sometimes with this layer extending over the whole width of the leaf. Abaxial epidermal zonation present or absent; microhairs or macrohairs absent; silica bodies absent, or tall and narrow, or round and single. Adaxial epidermis sometimes with papillae, prickle-hairs, and microhairs.
Distribution and ecology
Widespread in South America, from Tierra del Fuego (Argentina) to Venezuela, from Brazil to Peru, from sea level to the Páramo.
Systematics
We arranged the species into five informal groups, which are coherent morphologically and anatomically.
Key to the species (anatomical characters in brackets)
| 1 | Lemma body continued up the awn, for at least the same length as the expanded portion of the lemma; plants forming massive tussocks to 4 m tall, inflorescences plumose (leaves with abaxial groves (Fig. |
Selloana group...2 |
| – | Lemma body not continued up the awn, lemmas consequently acute or obtuse or lobed, usually obviously awned; plants and inflorescences various (leaves rarely with abaxial grooves) | 5 |
| 2 | Glumes 9–17 mm long, ca. ½ length of basal lemmas; basal lemmas 14–25(–30) mm long; plants of southern (austral) Andean region | 2. C. araucana |
| – | Glumes 5–14 mm long, almost as long as or longer than the basal lemmas; basal lemmas 6–15 mm long; plants from southern Brazil, Uruguay, and Argentina northwards to Colombia | 3 |
| 3 | Lemma awn present above the insertion of the lateral setae (these often lost on herbarium material); spikelets 8–15 mm long; lemmas 7.0–12.5 mm long; glumes 6–8 mm long; plants from desert regions of the Andes | 3. C. speciosa |
| – | Lemma awn absent; spikelets 10–20 mm long; lemmas 6–15 mm long; glumes 5–14 mm long; widely distributed in South America | 4 |
| 4 | Gynodioecious plants, exceptionally populations exclusively pistillate; panicles pyramidal to fusiform, dense to lax, included or not in the foliage; southern Brazil, Uruguay and Argentina | 1a. C. selloana subsp. selloana |
| – | Only pistillate plants; panicles pyramidal, lax, much exserted above the foliage; northwest Argentina to Colombia | 1b. C. selloana subsp. jubata |
| 5 | Glumes without veins; lemmas with awns 14–35 mm long; sheaths always intact (primary vascular bundles with lignified sheaths and girders, tertiary vascular bundle sheaths and girders collenchyma) | Lamprothyrsus Group: 5. C. hieronymi |
| – | Glumes with 1 (rarely 2) vein; lemmas awnless or with awns up to 17 mm long; when longer than 13 mm the basal sheaths are lacerated, sheaths and girders of all vascular bundles similar | 6 |
| 6 | Lemmas acute, at most with vestigial lobes, mostly without awns; from southern and eastern South America (leaves with large bulliform cells – Fig. |
Egmontiana group...7 |
| – | Lemmas lobed, often with setae on the lobes, mostly with awns; from the Andes and the tepui (bulliform cells absent or poorly developed) | 9 |
| 7 | Inflorescence compact with the branches shorter than the spikelets; leaf blades disarticulating from a persistent sheath; southern South America (leaf anatomy with adaxial ribs, phloem-pole usually intact) | 5. C. egmontiana |
| – | Inflorescences plumose with the branches longer than the spikelets; leaf blades persistent on the sheath; eastern Brazil (leaf anatomy with hardly any ad- or abaxial grooves and with the phloem-pole split) | 8 |
| 8 | Glumes 8–12 mm long; lemma back villous; basal sheaths burnt off, ensheathing the tiller bases | 6. C. modesta |
| – | Glumes 4–6 mm long; lemma back glabrous; basal sheaths breaking up into fibres | 7. C. vaginata |
| 9 | Old leaf sheaths intact, or shattering transversally, rarely some lacerated (sometimes in C. boliviensis); (leaves, except in C. echinata, with a multilayered wide collenchyma below the adaxial epidermis and no sclerenchyma girder connecting the vascular bundle to the epidermis, Fig. |
Nitida group...10 |
| – | Old leaf sheaths lacerated (leaves never with a multilayered collenchyma below the abaxial epidermis, or when present then interrupted by a sclerenchyma girder connecting the vascular bundle to the epidermis) | Bifida group...14 |
| 10 | Plants caespitose, at least 0.5 m tall (leaf anatomy with adaxial ribs, and the adaxial surface papillate) | 11 |
| – | Plants usually forming vegetable hedgehogs (spiny cushions), rarely caespitose, up to 0.5 m tall | 12 |
| 11 | Tussocks up to 2.3 m tall; old sheaths remaining intact; inflorescence branches nitid to scaberulous, nodes villous; lemmas villous overall with callus indumentum longer than lemma hairs | C. nitida |
| – | Tussocks up to 1.5 m tall; old sheaths shattering transversely; inflorescence branches and nodes scabrid; lemma indumentum sometimes only basal with callus indumentum only as long as the lemma hairs | 9. C. boliviensis |
| 12 | Leaves densely pilose (leaves folded double, no adaxial ribs) | 10. C. sericantha |
| – | Leaves glabrous (leaves expanded, with adaxial ribs) | 13 |
| 13 | Plants caespitose; sheaths remaining intact; inflorescence branches villous; glumes less than 15 mm long; lemma setae, excluding lobes, to 1.5 mm long; from marshlands in Colombia (anatomy not known) | 11. C. pungens |
| – | Plants cushion-forming; sheaths splitting transversely; inflorescence branches scabrid; glumes more than 15 mm long; lemma setae, excluding lobes, at least 2 mm long; from epilithic habitats in Peru (leaves without abaxial collenchyma) | 12. C. echinata |
| 14 | Leaf upper surface, directly above the ligule, glabrous (leaves abaxially shallow grooves with collenchyma in the grooves (Fig. |
15 |
| – | Leaf upper surface, directly above the ligule, villous (leaves abaxially not grooved, with a weakly developed sclerenchyma layer below the abaxial epidermis) | 16 |
| 15 | Leaves not pungent, more than 20 cm long, when dry expanded, disarticulating from the sheath; inflorescences plumose, pedicels not obscured by spikelets; lemma awn 6–17 mm long | 13. C. bifida |
| – | Leaves pungent, to 20 cm long, when dry folded double, persistent on sheath; inflorescence contracted, pedicels obscured by spikelets; lemma awn 4–8 mm long (anatomy not known) | 14. C. planifolia |
| 16 | Glumes 10–22 mm long; lemma setae 1–3 mm long; from Andes | 15. C. hapalotricha |
| – | Glumes 5–13 mm long; lemma setae 0–2 mm long; from Andes or tepuis | 17 |
| 17 | Lemma indumentum 3–4 mm long; setae 3–9 mm; from Andes | 16. C. columbiana |
| – | Lemma indumentum 4–6 mm long; setae 0–2 mm; from tepuis | 17. C. roraimensis |
Leaf anatomy of Cortaderia, as evident from transverse sections. A–B C. selloana (Villamil 11738) C C. speciosa (Testoni 644) D C. vaginata (Zanín 1654). Comparison of bulliform cells in Egmontiana group: E C. egmontiana (Testoni 634) F C. modesta (Carauta 927) G C. vaginata (Zanín 1654). Structures referred to in the descriptions are labelled as follows: 1, multi-layered abaxial sub-epidermal collenchyma layer; 2, aerenchyma; 3, chlorenchyma; 4, primary vascular bundle; 5, midrib; 6, colourless cells; 7, empty cells; 8, bulliform cells.
Leaf anatomy of Cortaderia, as evident from transverse sections. A C. egmontiana (Moore 2677) B C. modesta (Glaziou 17913) C C. hieronymi (Garcia 563) D C. nitida (Laegaard 53121) E C. boliviensis (Beck 11273); F C. sericantha (Ramsay 967); G C. echinata (Peterson 21587) H C. bifida (Renvoize 4202) I C. hapalotricha (Laegaard 53305) J C. roraimensis (Maguire 60448). Structures referred to in the descriptions are labelled as follows: 1, multi-layered abaxial sub-epidermal collenchyma layer; 2, adaxial islands of collenchyma in the abaxial grooves; 3, chlorenchyma; 4, primary vascular bundle; 5, midrib.
Notes on species
Selloana group
In this group three morphologically and anatomically similar species, with both gynodioecious and apomictic breeding systems, are included. They are easily distinguishable from other species in the genus: they form big tussocks 1.5 to 3 m in diameter and up to 4 m in height, and the leaf edges are strongly cutting. The panicles are large and plumose, very showy, and much larger than in most of the other species. The spikelets have 1-veined glumes, the lemmas are long-acuminate, with or without evident awns, unlobed, 3-veined and with long hairs only in female plants (hermaphrodites glabrous).
The leaf, in transversal section (Fig.
Cortaderia selloana
Arundo dioeca Spreng., Syst. Veg. (ed. 16) 1: 361. 1825 [1824], nom. illeg. (non Lour. 1790); Cortaderia dioeca (Spreng.) Speg., Anales Mus. Nac. Buenos Aires 7: 194. 1902; Arundo selloana Schult. & Schult. f., Mant. 3(1): 605. 1827; Gynerium argenteum Nees, Agrost. Bras. 462. 1829, nom. illeg.; Moorea argentea (Nees) Lemaire, Ill. Hort. 2: 14. 1855; Cortaderia argentea (Nees) Stapf, Gard. Chron. ser. 3, 22: 396. 1897, nom. illeg.
Type. Uruguay. Montevideo, I-1836, F. Sellow 570 (lectotype, designated here: B 10 0185657! (http://ww2.bgbm.org/Herbarium/specimen.cfm?Barcode=B100185657); isolectotypes:
Etymology
selloana: In honour of Friedrich Sellow (1789–1831), German botanist, a major collector of Brazilian flora.
Nomenclatural comments
Arundo dioeca Spreng. is a later homonym of Arundo dioica Lour. (1790) from Indochina, and is consequently illegitimate. Arundo selloana Schult. & Schult.f. is a new name for A. dioica Spreng.; A. dioeca Spreng. is cited in the protologue, and the diagnoses are identical. Furthermore, both description cite a Sellow collection, without number, from Montevideo. It is most likely that the type is Sellow 570 from Montevideo, which is in B, and is designated here as lectotype. Curiously,
Cortaderia selloana subsp. selloana
Common names
pampas grass, cortadera, cola de zorro, carrizo de las pampas. The origins of the popular name “pampas grass” are somewhat obscure, and do not reflect the ecology of the species (
Taxonomy
Cortaderia selloana ssp. selloana can be diagnosed by the glumes about as tall as the basal lemma, and lemma without a distinct awn. The plants are generally larger than those of C. araucana and C. speciosa, and the panicles are larger (0.5 to 1 m long), more lax, and coloured white, pink or yellowish. The similar size of basal lemmas and glumes (6–15 mm) further separates it from C. araucana (glumes 9–17 mm long, ca. ½ length of basal lemmas), whereas the larger glumes separate it from C. speciosa (glumes 6–8 mm, ca. ¾ length of basal lemmas). The large size may also lead to confusion with C. nitida, but it is easily separated by the larger and laxer panicles, 3-veined, awnless lemmas that are glabrous on hermaphrodite plants; and female plants with tiny staminodes. For the distinction from ssp. jubata see below.
Cortaderia selloana ssp. selloana was originally described as dioecious, but
Cortaderia selloana subsp. jubata
Gynerium jubatum Lemoine, Rev. Hort. 50: 449. 1878; Cortaderia jubata (Lemoine) Stapf, Bot. Mag. 124: t. 7607. 1898.
Type: Ecuador, “sent by Lemoine of Nancy and collected at Chimborazo by the botanical collector Roezl”, sine data, B. Roezl s.n. (lectotype designated by Connor & Edgar, Taxon 23: 598 (1974): K 000307978!).
Etymology
jubata (Lat.): Having mane, crest, in allusion to the panicle.
Common names
pink pampas grass, jubata grass, cortadera
Taxonomy
This subspecies is generally similar to ssp. selloana, and includes all the morphologically homogenous apomictic populations of the Yungas region. It can be separated from ssp. selloana by the inflorescences which extend far beyond the foliage, and the pink, 75–90 cm long, very lax, pyramidal and nodding panicles. In Ecuador it is sympatric with C. nitida, from which it can be separated by its larger size and its spectacular pink panicles. They can also easily be distinguished by the leaves: in subsp. jubata they are flat and folded V-shaped, while in C. nitida leaves are inrolled from both margins.
Cortaderia araucana
Moorea araucana (Stapf) Stapf, Gard. Chron. ser. 3, 34: 400. 1903.
Type: Chile, llanos de Valdivia, 20-XII-1852, W. Lechler 613 (lectotype designated by Connor & Edgar, Taxon 23: 598 (1974): K 000308157!; isolectotypes: P photo!, W photo!,
Cortaderia quila var. patagonica Speg., Anales Mus. Nac. Buenos Aires 7: 194. 1902.
Type: Argentina, Chubut, “non rara in rupestribus secus Carren-leofú, aest. 1899-900”, N. Illín s.n. (lectotype, here designated:
Cortaderia longicauda Hack., Repert. Spec. Nov. Regni Veg. 10 (243–247): 169. 1911.
Type: Chile, Valdivia, “Potrero Coihue, I-1861”, R. A. Philippi s.n. (lectotype designated as holotype by Connor & Edgar, Taxon 23: 598 (1974): W-1916-0039626 (http://jacq.nhm-wien.ac.at/djatoka/jacq-viewer/viewer.html?rft_id=w_19160039626&identifiers=w_19160039626); isolectotype:
Cortaderia araucana var. fuenzalidae Acevedo, Bol. Mus. Nac. Hist. Nat. Santiago de Chile 27(4): 239. 1959.
Type: Chile, Curico, Potrero Grande, 5-XI-1943, M. Espinosa s.n. (lectotype, here designated:
Cortaderia araucana var. skottsbergii Acevedo, Bol. Mus. Nac. Hist. Nat. Santiago de Chile 27(4): 240. 1959.
Type: Chile, provincia Chiloé, región del Corcovado, sine data, C. Reiche s.n. (lectotype, here designated:
Etymology
-ana, indicating connection. From the Araucania region of Chile.
Common names
cortadera
Taxonomy
In the Selloana group, C. araucana is readily diagnosed by the basal lemmas longer than 12.5 mm and much longer than the glumes. The spikelets are 20–35 mm long and the lemma of the basal floret 14–25 (30) mm long (including awn of 5–11 mm long). The species is found in the southern (austral) Andean region.
Cortaderia araucana includes extensive morphological variation, and both gynodioecious and apomictic populations. This variability led Acevedo Vargas (1959) to recognize three varieties, which are no longer maintained. In northern Patagonia C. araucana and C. selloana are sympatric, but the plants of C. araucana are somewhat smaller, with less lax panicles and flowering in the austral spring (late November and early December), whereas C. selloana flowers in the austral summer (January and February). Further, the spikelets are different: the glumes are shorter than the basal floret, the lemma may terminate in an awn that arises between two lower lateral setae. The leaf anatomy of both species is similar.
Cortaderia speciosa
Gynerium speciosum Nees & Meyen, Nov. Act. Nat. Cur. 19 suppl. 1: 153. 1843; Gynerium argenteum var. strictum E. Desv., Fl. Chile. 6: 328. 1854; Moorea speciosa (Nees & Meyen) Stapf, Gard. Chron. Ser. 3, 34: 400. 1903.
Type: Chile, ad flumen Copiapo dictum circa Nantoco in provincia Copiapó reipublicae Chilensis, III-1831, F. J. F. Meyen s.n. (lectotype designated by Connor & Edgar, Taxon 23: 603 (1974): B 10 0217503! (http://ww2.bgbm.org/Herbarium/specimen.cfm?Barcode=B100217503); isolectotype: K! frag. ex B).
Gynerium quila Nees & Meyen, Nov. Act. Nat. Cur.19 suppl. 1: 153. 1843; Cortaderia quila (Nees & Meyen) Stapf, Gard. Chron. Ser. 3: 22: 396. 1897; Moorea quila (Nees & Meyen) Stapf, Gard. Chron. Ser 3, 34: 400. 1903.
Type: Chile, ad Copiapó fluvium circa Nantoco, sine data, F. J. F. Meyen s.n. (syntype: B!); Perú, ad lacum Titicacam et ad pedem vulcani Arequipensis. Femina planta. Mascula ignota est., 1000 m, Maio, F. J. F. Meyen s.n. (syntype: B 10 0217504 (http://ww2.bgbm.org/Herbarium/specimen.cfm?Barcode=B100217504; isosyntype:
Gynerium quila var. pygmaeum Meyen, Nov. Act. Nat. Cur. 19 Suppl. 1: 153. 1843.
Type: Perú, “ad lacum Titicacam. ♀”, IV-1841, F. J. F. Meyen s.n. (lectotype, designated here: B 10 0217506! (http://ww2.bgbm.org/Herbarium/specimen.cfm?Barcode=B100217506).
Gynerium argenteum var. parviflorum E. Desv., Fl. Chile. 6: 328. 1854.
Type: Chile, Mal Paso, cordillera de Guanta, a la orilla de los arroyos, 2490 m., en donde forma copas apretadas de un metro y más, sine data, C. Gay s.n. (lectotype, designated here: P 00506920!).
Gynerium atacamense Phil., Linnaea 33: 289.1865. Cortaderia atacamensis (Phil.) Pilg., Bot. Jahrb. 37: 374. 1906.
Type: Chile, prope San Pedro de Atacama, I-1854, R. A. Philippi s.n. (lectotype, designated as holotype by Connor & Edgar, Taxon 23: 597 (1974):
Cortaderia rudiuscula Stapf, Gard. Chron. Ser. 3, 22: 396. 1897. Moorea rudiuscula (Stapf) Stapf, Gard. Chron. Ser. 3, 34: 400. 1903.
Type: Chile, Santa Rosa de los Andes, V-1882, J. Ball s.n. (lectotype, designated by Connor & Edgar, Taxon 23: 601 (1974): K!; isolectotype:
Etymology
speciosus (Latin), beautiful, showy.
Nomenclatural comments
The binomials Gynerium speciosum, G. neesii and G. pygmaeum – mentioned as new species by Meyen (1834), from Copiapo (Chile) and Lake Titicaca (Peru), respectively – are synonyms of Cortaderia speciosa, but are invalid (nomina nuda) as no descriptions were published. Their identity can be determined, because the specimens in B! were annotated with the Meyen names. Gynerium speciosum was validated by Nees in 1943. Tropicos (Downloaded 14 December 2016) lists the species as described by Nees in 1841 (
Common names
cortadera
Taxonomy
In the Selloana group, C. speciosa can be diagnosed by the short basal lemmas, which are less than 13 mm long. The spikelets are 8–15 mm long and the basal lemma 7.0–12.5 mm long (including awn, 1–4 mm). It differs from other species in the group by its very compact, bright brown panicles with ascending, short and stiff branches. The species is readily distinguished by the small floret sizes. The leaf anatomy is also somewhat different from the other species of the group (Fig.
This species is completely apomictic, and several morphological subgroups can be recognized. As these are all apomicts, it is presumed that they derive from the same ancestral sexual population. The material previously separated as C. rudiuscula has longer (9–12 mm) and more slender lemmas, than the material previously separated as C. speciosa (lemmas ca. 8 mm), but there is no clear separation between these two forms.
Lamprothyrsus group
This group is very distinct within Cortaderia. Morphologically, it differs by the long, filiform awns, 14–35 mm long; glumes without veins; and by the sheaths which are always intact. Furthermore, the leaf anatomy differs by the primary vascular bundles with lignified sheaths and girders, tertiary vascular bundle sheaths and girders collenchyma (Fig.
Cortaderia hieronymi
Triraphis hieronymi Kuntze, Revis. Gen. Pl. 3(3): 373. 1898; Danthonia hieronymi (Kuntze) Hack., Anales Mus. Nac. Buenos Aires ser. 3, 6: 484. 1906; Lamprothyrsus hieronymi (Kuntze) Pilg., Bot. Jahrb. Syst. 37 (Beibl. 85): 58. 1906.
Type: Argentina, Córdoba, “prope urbem”, 6 Nov. 1881, G. H. E. W. Hieronymus s.n. (lectotype, designated as holotype by Conert, Syst. Anat. Arundineae 128 (1961): B!; isolectotype: K!).
Triraphis hieronymi var. jujuyensis Kuntze, Revis. Gen. Pl. 3(3): 374, 1898; Danthonia hieronymi var. jujuyensis Kuntze, Anales Mus. Nac. Buenos Aires ser. 3, 6: 486 (1906); Lamprothyrsus hieronymi var. jujuyensis (Kuntze) Pilg., Bot. Jahrb. Syst. 37 (Beibl. 85): 59, 1906.
Type: Argentina, Jujuy, sine data, O. Kuntze s.n. (lectotype, designated as holotype by Conert, Syst. Anat. Arundineae 130 (1961): B!).
Lamprothyrsus hieronymi var. pyramidatus Pilg., Bot. Jahrb. Syst. 37 (Beibl. 85): 59. 1906.
Type: Bolivia, ad Toldos prope oppium Bermejo, 2000m, 8 Dec. 1903, K. A. G. Fiebrig 2372 (lectotype, designated as holotype by Conert, Syst. Anat. Arundineae 128 (1961): B 10 0249138! (http://ww2.bgbm.org/Herbarium/specimen.cfm?Barcode=B100249138); isolectotypes: K,
Lamprothyrsus hieronymi var. nervosus Pilg., Bot. Jahrb. Syst. 37 (Beibl. 85: 59. 1906.
Type: Argentina, Cordoba, Sierra Achala, 11 Nov. 1878, G. H. E. W. Hieronymus 43 (lectotype, designated as holotype by Conert, Syst. Anat. Arundineae 129 (1961): B 01 0272938! (http://ww2.bgbm.org/Herbarium/specimen.cfm?Barcode=B100272938); isolectotype: W).
Lamprothyrsus hieronymi var. tinctus Pilg., Bot. Jahrb. Syst. 37 Beibl. 85: 59. 1906.
Type: Bolivia, Bermejo, 1400m, 16 Nov. 1903, K. Fiebrig 2099 (lectotype, designated as holotype by Conert, Syst. Anat. Arundineae 129 (1961): B 10 0249137! (http://ww2.bgbm.org/Herbarium/specimen.cfm?Barcode=B100249137); isolectotypes: K, L!,
Lamprothyrsus peruvianus Hitchc., Proc. Biol. Soc. Washington 36: 195. 1923; Cortaderia peruviana (Hitchc.) N.P.Barker & H.P.Linder, Ann. Missouri Bot. Gard. 97(3): 342. 2010.
Type: Peru, Yanahuanca, 16–22 Jun 1922, J. F. Macbride & W. Featherstone 1205 (lectotype, designated as holotype in F: F-V0040645F, photo F-50163 (http://emuweb.fieldmuseum.org/web/pages/common/imagedisplay.php?irn=39615&reftable=efmnh&refirn=257048); isolectotypes:
Lamprothyrsus venturi Conert, Syst. Anat. Arundineae 130. 1961.
Type: Argentina, prov. Tucuman, Famailla, Villa Nougues, 21-10-1923., S. Venturi 2534 (lectotype, designated as holotype by Conert, Syst. Anat. Arundineae 131 (1961): K; isotype:
Etymology
In honour of George Hans Emmo Wolfgang Hieronymus (1846–1921), German botanist, sometimes resident of Argentina.
Common names
Seringuilla, sivinga (Tucuman).
Taxonomy
This species contains substantial variation in the robustness of the plants.
Only apomictic populations are known, but a few fertile staminate specimens with long hairs on the lemmas were found (
In the central and northern Argentina to Ecuador C. hieronymi is sympatric with the two subspecies of C. selloana, but it is easily separated by its smaller panicles, spikelets with glumes without veins, and 5-veined, 3-awned lemmas. In Peru and Ecuador it is sympatric with C. bifida, with which it is often confused: in both species the old leaf sheaths are lacerated and the spikelets have long awns, but the spikelets of C. hieronymi are bigger, and the lemmas with longer and robust central awns.
Egmontiana group
This group includes three quite distinctive species. Cortaderia vaginata and C. modesta have an unusual (for Cortaderia) leaf anatomy lacking ribs, and with deeply split phloem poles (Fig.
Cortaderia egmontiana
Arundo egmontiana Roem. & Schult., Syst. Veg., ed. 15 b [Roemer & Schultes] 2: 511. 1817. Phragmites egmontiana (Roem. & Schult.) Trin. ex Steud., Nomen. Bot. (ed. 2) 2: 324. 1840.
Type: Falkland / Malvinas Islands, Port Egmont, R. J. Schuttleworth s.n. (type:
Arundo pilosa d’Urv., Mém. Soc. Linn. Paris 4: 603. 1826; Cortaderia pilosa (d’Urv.) Hack. ex Dusén, Bol. Acad. Nac. Ci. 16: 253. 1900; Gynerium pilosum (d’Urv.) Macloskie in Scott, Rep. Princeton Univ. Exped. Patagonia, Botany 8, part 1: 213. 1904; Phragmites pilosa (d’Urv.) Macloskie & Dusén in Scott, Rep. Princeton Univ. Exped. Patagonia, Botany 8, suppl. bot.: 50. 1915. Ampelodesmos australis Brongn. in Duperrey, Voy. Monde 2(2): 31. 1829, nom. illeg.
Type: Falkland / Malvinas Islands, 1825, J. S. C. D. D’Urville s.n. (central inflorescence designated as lectotype by Connor & Edgar, Taxon 23: 600 (1974): P 00740221! (http://mediaphoto.mnhn.fr/media/1443644100310dGB3ZqFqm8JGPDaz; isolectotype: B!).
Calamagrostis patula Steud., Syn. Pl. Glumac. 1(6): 422. 1854.
Type: Chile, Huiti, sine data, W. Lechler 760 (lectotype, selected here: P-00740220 (http://mediaphoto.mnhn.fr/media/1443644088798jsLY8AS29Euj4oHx); isolectotypes:
Poa phragmites Phil., Anales Univ. Chile 43: 576. 1873.
Type: Chile, volcan de Osorno, 1872, C. Juliet s.n. (holotype:
Gynerium nanum Phil., Anales Univ. Chile 94: 155. 1896.
Type: Falkland / Malvinas Islands, Dec. 1884, C. Martin s.n. (lectotype, designated as holotype by Connor & Edgar, Taxon 23: 600 (1974):
Calamagrostis scirpiformis Phil., Anales Univ. Chile 94: 20. 1896.
Type: Chile, ad lacum Llanquihue, I-1866, F. Philippi s.n. (lectotype, designated here:
Cortaderia minima Conert, Syst. Anat. Arundineae 119. 1961; Cortaderia pilosa var. minima (Conert) Nicora, Darwiniana 18(1–2): 80. 1973.
Type: Chile, Andes, Villarrica, “in feuchten Schluchten nahe der Waldgrenze”, 1897, F. W. Neger s.n. (lectotype, designated as holotype by Conert, Syst. Anat. Arundineae 119 (1961): M; isolectotypes: W5945! B! fragm. ex M).
Etymology
egmontiana: called after Port Egmont in the Falklands / Malvinas Islands.
Nomenclatural comments
Brongniart (1829) described Ampelodesmos australis, and explicitly included Arundo pilosa D’Urville as a synonym, noting that this species is better placed in Ampelodesmos.
Taxonomy
The species can be readily diagnosed by the combination of compact inflorescences, almost glabrous leaves, and either no, or poorly developed, awns and setae on the lemmas. The habit and dense inflorescences are as in C. sericantha, but C. egmontiana differs by the absence of setae, and by the almost completely glabrous leaves. The lemma and spikelet morphology (reduced or absent awns and setae) suggests an affinity to the eastern Brazilian species C. vaginata and C. modesta. From these two species C. egmontiana can be separated by the compact inflorescences and the tendency of the leaf blades to disarticulate from the sheaths. It is the only Cortaderia species in southern South American temperate zone. The leaf anatomy (Figs
There is remarkable intraspecific variation in the spikelet and floret sizes, and
Cortaderia modesta
Gynerium modestum Döll, Fl. Bras. [Martius] 2(3): 240. 1880.
Type: Brasil, near Rio de Janeiro, Serra dos Órgãos, au Frade (2 ou 3 mois après l’incendie de la forêt), 11-X-1869, A. F. M Glaziou 4352 (lectotype, designated by Connor & Edgar, Taxon 23: 600 (1974): W 10406!; isolectotypes K!,
Gynerium ramosum Hack., Arq. Mus. Nac. Rio de Janeiro 13: 73. 1903. Gynerium modestum f. ramosa (Hack.) Hack., Ark. Bot. 9(5): 4. 1909.
Type: Brasil, Campo 2100 m, 18 Dec. 1895, P. K. H. Dusén s.n. (lectotype, designated here: W!).
Etymology
modesta (Latin) = moderate, presumably referring to the culms of average height.
Nomenclatural comments
The locality information given by
Common names
cabeça de negro, capim-de-anta.
Taxonomy
Some specimens show a poorly developed axillary inflorescence developed at the penultimate node of the flowering culm. The almost awnless lemmas, with the paleas as long as the lemmas, and the very dense callus hairs compared to the short lemma back hairs, are almost unique in the genus. Its closest relative might be C. vaginata from Santa Catarina, further south along the Brazilian Atlantic coast. It is readily distinguished from C. vaginata by the persistent leaf sheaths and the awnless lemmas. According to herbarium labels the plant forms massive tussocks with persistent red, burnt sheaths.
Cortaderia vaginata
Type
Brasil, Santa Catarina, Bom Retiro, Campo dos Padres, 16 Dec. 1948, R. Reitz 2398 (lectotype, designated as holotype by Connor & Edgar, Taxon 23: 603 (1974):
Etymology
vagina (Latin) = sheath. Possibly referring to the conspicuous leaf-sheaths, a feature that is common to most of the genus.
Common names
Penacho, Capim-Penacho.
Taxonomy
According to
The leaf anatomy is identical to that of C. modesta, except that all sections appear to have large empty cells in the middle of the leaf (Fig.
Nitida group
The Nitida group can be characterized by the leaf sheaths which generally remain intact, and the leaves which, in transverse section, show a massive abaxial sub-epidermal collenchyma layer. Cortaderia nitida, C. pungens and C. boliviensis are very similar, whereas C. sericantha is quite distinct by the villous, folded leaves with no adaxial ribs. The distinction of C. pungens is not clear, and needs fieldwork. The new C. echinata is also included in here although anatomically it fits into the next group. Leaf anatomically, C. nitida and C. boliviensis are very similar, with papillate adaxial surfaces, deep adaxial grooves, and a well developed abaxial collenchyma layer. The leaf anatomy of C. pungens is not known.
Cortaderia nitida
Arundo nitida Kunth in Humb. et Bonpl., Nov. Gen. Sp. [H.B.K.] 1: 149. 1816; Gynerium nitidum (Kunth) Pilg., Bot. Jahrb. Syst. 27: 31. 1899.
Type: Colombia, inter Guachucal et Tuqueres, sine data, A. J. A. Bonpland s.n. (lectotype, designated as holotype by Connor & Edgar, Taxon 23: 600 (1974): B; isolectotypes:
Cortaderia sodiroana Hack., Oesterr. Bot. Z. 52: 238. 1902.
Type: Ecuador, in reg. silvat. suband., 1872, L. Sodiro s.n. (lectotype, designated by Connor & Edgar, Taxon 23: 600 (1974): W 25246!; isolectotype:
Etymology
niteo (Latin) = shine. It may refer to the persistently intact, more or less white, leaf sheaths.
Common names
“Sigse de Páramo”.
Taxonomy
Cortaderia nitida is a distinctive grass. It is the tallest and most robust species of this group. The lamina margins are inrolled. The basal sheaths gradually become shorter with age, but do not become lacerated, the leaf blades are scabrid in the upper half but not the lower, and the inflorescence branches which are scaberulous while the pulvini often have a few long hairs (the latter seems to be unique in the genus). The callus usually has very long spreading hairs (more than 2 mm, almost equivalent to the lemma hairs), and the setae are less than 2 mm long. The other tall Cortaderia, C. bifida, has central awns that are longer than 8 mm, and very well developed setae. The lemma shape is similar to C. columbiana, but the inflorescence branches are scaberulous in C. nitida, and villous in C. columbiana.
This species also approaches the Selloana group by it large size, big plumose inflorescences, and especially by the lemma shape. It is easy to confuse the lemmas of the two groups, but in Nitida group the lemmas are 5–7 veined, hairy in both sexes, while in Selloana group the lemmas are 3-veined, hairy in female plants and glabrous in hermaphrodite plants. The plastid sequence data also places this species as sister to the Selloana group, but this is not corroborated by the ITS-based phylogeny.
The leaf anatomy (Fig.
Cortaderia boliviensis
Cortaderia bifida var. grandiflora Henrard, Meded. Rijks-Herb. 40: 67. 1921.
Type: Bolivia, Departamento Cochabamba: “Charactergrass der Andenwiesen über Tablas, feuchte Stellen, 3400 m, Mai 1911, T. C. J. Herzog 2194 (holotype: L; isotypes: S,
Etymology
-ense (Latin), denoting origin. From Bolivia.
Taxonomy
This species is very similar to C. nitida, with which it shares the (usually) non-lacerated, entire leaf sheaths and the shape of the lemmas, as well as largely similar leaf anatomy. However, neither chloroplast nor nuclear genome indicates such a relationship for C. boliviensis (
The leaf anatomy (Fig.
Cortaderia sericantha
Danthonia sericantha Steud., Syn. Pl. Glumac. 1(3): 246. 1854.
Type: Ecuador, Quito “On boggy plains on the eastern Cordillera at 13000 feet above sea level”, sine data, W. Jameson 93 (lectotype designated by Connor & Edgar, Taxon 23: 602 (1974): K!; isolectotypes: K! - frag
Danthonia jubata Sodiro, Revista Colegio Nac. Vicente Rocafuerte 12: 91. 1930.
Type: Ecuador, Pinchincha, sine data, A. S. J. Mille s.n. (
Etymology
serios (Greek) = silken + Anthos (Greek) = flower. Presumably this refers to the silky-haired leaves, a diagnostic trait for this species.
Taxonomy
This species is very distinctive in Cortaderia by its very villous leaves, which are rolled rather than flat, and quite pungent; the compact inflorescences with short inflorescence branches; the glumes with three veins and which are much longer than the packet of florets; and the tuft of hair at the base of the spikelets. The inflorescences are similar to those of C. egmontiana, but the villous leaves immediate distinguish our species from C. egmontiana. The intact leaf sheaths, pungent leaf tips, and compact growth form related this species to C. pungens and C. echinata. The remarkably large glumes, much overtopping the packet of florets, are shared with C. echinata.
The leaf anatomy (Fig.
Cortaderia pungens
Danthonia confusa L.B.Sm., Phytologia 22(2): 89. 1971, non D. pungens Cheeseman, 1906.
Type: Colombia, Dept. Santander, Páramo de Santurban, near Vetas, 17 Jan. 1927, E. P. Killip & A. C. Smith 17467 (lectotype, designated as holotype by Connor & Edgar, Taxon 23: 600 (1974):
Etymology
pungens (Latin): piercing, terminating in a sharp point. This describes the leaf tips.
Taxonomy
This species is often placed with C. hapalotricha, from which it differs by (a) shorter growth-form (less than 1 m tall); (b) the intact leaf bases; (c) the rolled, pungent leaves; and (d) deeply lobed lemmas. The two species have much in common (leaf anatomy, spikelet and inflorescence structure). It is possible that they are ecotypes of each other, and the problem needs critical field work. We keep them separate on the very different growth-form. The intact leaf bases and pungent leaves suggest a relationship to C. sericantha and C. echinata, but the species is readily separated from these two by the much shorter glumes.
The leaf anatomy was not studied.
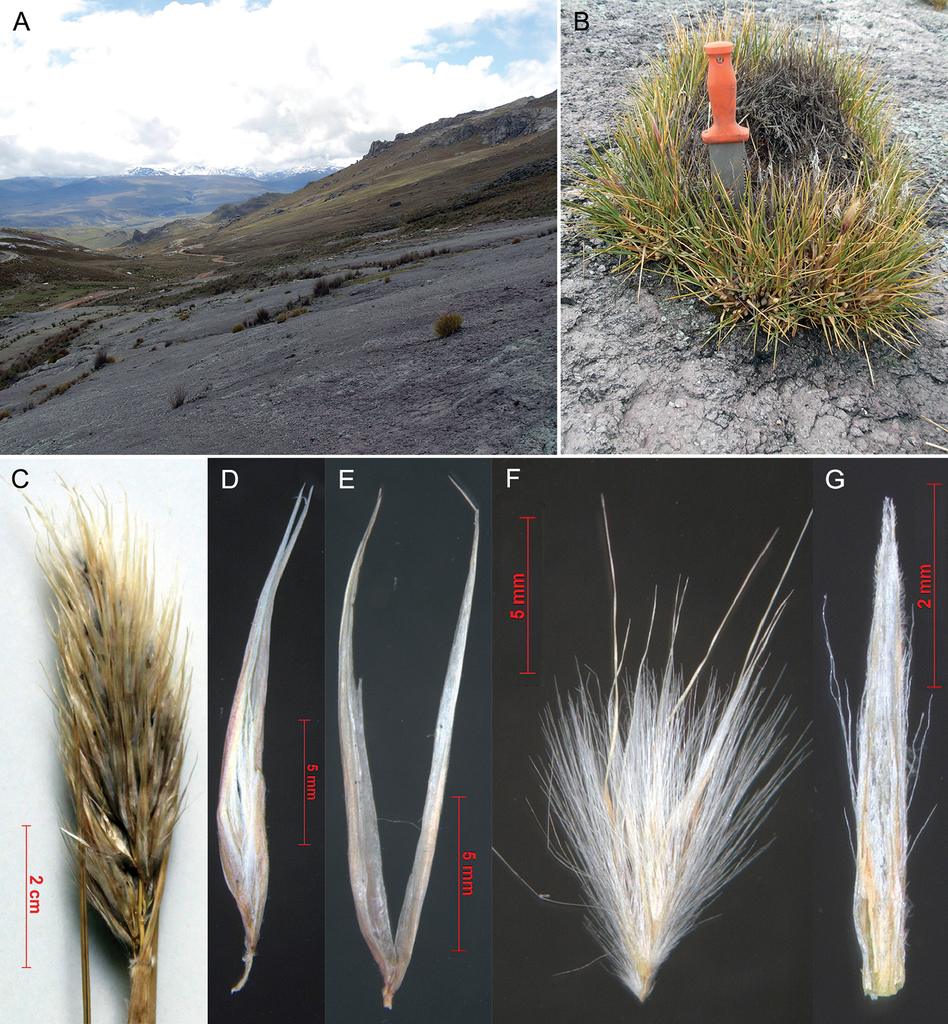
Cortaderia echinata , sp. nov.
Type
Peru, vicinity of Cerro Ayrahnanca pass ca. 1 km E of Lugo Ututo on road between Cataparaco and Utcuyau, 4223 m. Rocky slopes, 11 Mar 2008, P. M. Peterson, R. J. Soreng, M. I. la Torre & J. V. Rojus Fox 21587 (holotype: Z!, isotype:
Diagnosis
Similar to C. pungens by the small compact habit and pungent leaves, but differing by the shattering leaves and the longer spikelets.
Description
Plants forming tough, perennial cushions (vegetable hedgehogs) to 30 cm in diameter and to 30 cm tall. Basal sheaths white, shiny, persistent, when old splitting transversely into segments, puberulous between the veins. Ligule a dense ring of hairs 2–3 mm long, sheath mouth glabrous. Leaf blades 80–150 × 2–3 mm; C-shaped at base and margins incurved towards apex, forming a rolled, viciously pungent tip; disarticulating from the persistent sheath at the ligule. Inflorescence paniculate, contracted, ovate, 60–100 × 15–25 mm, with 100–300 spikelets; branches and pedicels shorter than and obscured by the spikelets, scaberulous. Female-fertile spikelet 16–22 mm long; with ca. 3 florets. Glumes 16–22 × 0.6–0.8 mm; twice as long as the packet of florets; 1 veined, acute, glabrous, straw to almost white, upper and lower glumes similar. Callus ca. 0.75 mm long; indumentum 2–2.5 mm long, overtopping the base of the lemma hairs length; rhachilla 0.75 mm long. Second lemma ca. 4 mm long, 5 veined, indumentum scattered on lower half of lemma back, about as long as the lemma lobes, 5–6 mm long; lemma-lobes acute, 3–4.5 mm long, setae 2–3 mm long, distinctly shorter than lemma lobes, included in the glumes; awn simple, 8.5–10 mm, longer than setae. Palea linear, 5 × 0.5 mm, obscurely bilobed, keels sinuose; scabrid, with hair-tufts along mid-margins. Lodicules obtriangular and with bristles.
Leaf anatomy
Leaf in transverse section expanded, sclerophyllous; margins gently tapering, sclerenchyma caps well-developed; adaxial furrows located between all vascular bundles, the same over primary and tertiary vascular bundles, about half depth of leaf, forming narrow clefts, ribs flat-topped; abaxial ribs and furrows present. Vascular bundles closer to abaxial surface, 3 primary vascular bundles in half a leaf section, with 1–2 tertiary vascular bundles between the primary vascular bundles. primary vascular bundles elliptical; phloem without lignified cells; metaxylem vessels narrower than outer bundle sheath cells; outer bundle sheath clearly distinct from chlorenchyma, cells larger and colourless, with adaxial and abaxial interruptions; inner bundle sheath walls thickened anticlinally, cells smaller than outer bundle sheath cells; adaxial sclerenchyma as inversely anchor-shaped girders; abaxial sclerenchyma as trapezoidal girders. tertiary vascular bundles outer bundle sheath cells distinct from and larger than chlorenchyma cells, walls thickened anticlinally or all round; with abaxial interruption only; adaxial bundle sheath extension present with cells smaller than outer bundle sheath cells; adaxial sclerenchyma inversely anchor-shaped girders; abaxial sclerenchyma as trapezoidal girders; phloem without lignified cells or with only the inner bundle sheath lgnified. Mesophyll of small, angular isodiametric chlorenchyma cells with small air spaces. Abaxial epidermal cells all larger than adaxial ones; outer wall twice as thick as inner wall; walls equal to mesophyll walls. Subepidermal layer of sclerified fibres only in marginal regions of leaves, absent from the middle of the leaf (directly next to leaf margins), 2-3 cells thick; with large clear parenchymatous cells below abaxial furrow present, connected via collenchyma cells to the adaxial furrow to the epidermis and so partitioning the chlorenchyma. Bulliform cells absent; abaxial epidermal zonation present (Fig.
Etymology
echinus (Latin) = hedge-hog or sea-urchin. The plant is spiny like a hedgehog.
Distribution and ecology
South America, Peru.
Altitude
4220–4230 m.
Habitat
Rock ledges (bedrock slabs); moisture regime: in soil pockets on rock. Forming cushions on almost flat rock slabs, in pockets of soil.
Cortaderia echinata (all from Peterson 21587). A habitat on bare rock slabs B habit, forming a vegetable hedgehog C inflorescence D spikelet, somewhat squashed (all very compact in the inflorescence) E glumes F floret package, with three florets, note long lemma indumentum G palea with sparse indumentum on the lateral palea flaps. A and B were photographed by Paul Peterson and Robert Soreng.
Conservation status
Known only from the type collection.
Phenology
Flowering month March or April.
Taxonomy
The small compact hedgehog form with pungent leaves is similar to C. pungens, from which it differs by the shattering leaves and the longer spikelets (glumes 15–25 mm long). The shattering leaf-sheaths link the species to C. boliviensis, but it differs by the very different growth form. The compact inflorescences are reminiscent of C. egmontiana, but the pungent leaves provide a simple diagnostic difference.
The leaf anatomy is reminiscent of that of C. bifida, but the outer bundle sheath is not lignified, and form an extension adaxially on the vascular bundles, connecting them to the lignified anchor-shaped girders.
Bifida group
The leaf sheaths of this group are highly lacerated and form a tangled mat around the base of the plant. Anatomically there is nothing unusual about these species. The distinction between C. columbiana and C. roraimensis needs critical investigation.
Cortaderia bifida
Cortaderia bifida Pilg., Bot. Jahrb. Syst. 37: 374. 1906.
Type: Peru, “zwischen den Tambo Yuncacoya und Ramospata (Weg von Sandia nach Chunchusmayo), 2000–2400m”, 27 Jul. 1902, A. Weberbauer 1328 (lectotype, designated as holotype by Connor & Edgar, Taxon 23: 597 (1974): B-100217561! (http://ww2.bgbm.org/Herbarium/specimen.cfm?Barcode=B100217561); isolectotype:
Cortaderia aristata Pilg. Bot. Jahrb. Syst. 37: 375. 1906.
Type: Peru, Prov. Huamalies, Dep. Huanuco, “Berge südwestlich von Monzon, 3400–3500m”, 11 Jul. 1903., A. Weberbauer 3349 (lectotype, designated as holotype by Connor & Edgar, Taxon 23: 597 (1974): B-100217562! (http://ww2.bgbm.org/Herbarium/specimen.cfm?Barcode=B100217562); isolectotypes: K!,
Cortaderia trianae Stapf ex Conert, Syst. Anat. Arundineae 100. 1961.
Type: New Granada, February 1892, J. Triana 289 (lectotype, designated as holotype by Conert, Syst. Anat. Arundineae 100 (1961): K!).
Etymology
bis (Latin) = twice + fidu, divide, this presumably refers to the lemma setae.
Taxonomy
This species can be diagnosed by the combination of the lacerated sheath bases, the long awns and especially the long setae. The shape of the lemmas with lobes and setae are shared with C. hapalotricha, and the curly fibrous leaf remains are similar to C. roraimensis. It is separated from C. roraimensis by the hairy lemmas and by the much longer awns and setae. It differs from C. hapalotricha by the glabrous adaxial surface above the ligule and the scaberulous inflorescence branches. From the other tall species, C. nitida, it can be separated by the longer awns (more than 8 mm long). The long awns and setae result in the inflorescences looking similar to those of C. peruviana, but the bases of the plants are quite different. Consequently, it can be difficult to determine collections which consist only of inflorescences.
The leaf anatomy (Fig.
Cortaderia planifolia
Type
Colombia, Dept. Valle del Cauca, Cordillera Occidental, extremo N, vertiente NW, entre Alto del Buey y Quebrada de los Ramos, 12 Oct. 1944, J. Cuatrecasas 18059 (lectotype, designated as holotype by Connor & Edgar, Taxon 23: 601 (1974):
Etymology
planus (Latin) = flat + folium (Latin) = leaf. Leaf-blades flat.
Taxonomy
Cortaderia planifolia has many similarities to C. pungens, but is separated by the flat or folded, but not rolled, leaves; somewhat taller tussocks (05-1 m, compared to 0.2-0.5m); adaxial leaf surface above the ligule glabrous; glumes 8-15 mm long, compared to 12-16 mm; lemmas 4-8 mm, compared to 3-4 mm long; lemma awn less than 8 mm long, compared to more than 9 mm in C. pungens. These numerous small differences suggest that these are two species.
It has also been grouped with C. hapalotricha, from which it differs by the smaller size, the flat leaves glabrous above the ligule, the shorter lemma awn and setae.
Leaf anatomy not investigated.
Cortaderia hapalotricha
Danthonia hapalotricha Pilg., Bot. Jahrb. Syst. 25: 715. 1898.
Type: Colombia, Páramo between Usme and Pasca, Cudinamarca, June 1868, M. A. Stübel 111C (lectotype, designated as holotype by Connor & Edgar, Taxon 23: 598 (1974): B, frag.
Cortaderia scabriflora Swallen, Contr. U.S. Natl. Herb. 29: 252. 1948.
Type: Ecuador, near Toreador, between Molleturo and Quinoas, Province of Azuay, along lake shore, 15 June 1943, J. A. Steyermark 53188 (lectotype, designated as holotype by Connor & Edgar, Taxon 23: 602 (1974):
Etymology
hapalos (Greek) = soft + thrix (Greek) = hair. It presumably refers to the densely pubescent rhachilla.
Nomenclatural comments
The type specimen of Cortaderia scabriflora is intermediate between C. hapalotricha, C. pungens and C. planifolia. It has the lemma structure of C. pungens, the folded leaves typical of C. planifolia, the pungent leaves typical of both, but the size of C. hapalotricha. Overall, it approaches C. hapalotricha.
Taxonomy
Leaf anatomically (Fig.
Cortaderia columbiana
Gynerium columbianum Pilg., Bot. Jahrb. Syst. 27: 31. 1899.
Type: Colombia, Merida, sine data, J. W. K. Moritz 1558 & 1559 (lectotype, designated by Connor & Edgar, Taxon 23: 597 (1974): B 10 0217508! (http://ww2.bgbm.org/Herbarium/specimen.cfm?Barcode=B100217508); isotype:
Cortaderia parviflora Swallen, Contr. U.S. Natl. Herb. 29: 253. 1948.
Type: Venezuela, between La Trampa and Casadero, State of Merida, 28 April 1944, J. A. Steyermark 56182 (lectotype, designated as holotype by Connor & Edgar, Taxon 23: 600 (1974):
Etymology
-ana, indicating connection. From Republic of Colombia.
Taxonomy
Connor & Edgar (1974) imply a similarity to C. hapalotricha, but note that the panicle is longer, more laxly flowered, and dull brown, and that this separates the two species. Cortaderia columbiana is superficially similar to C. hapalotricha, and also has short felty hair on upper leaf surface above the ligule, but is different by the shorter setae. Leaf anatomically they can be separated by the presence of a continuous lignified sub-epidermal layer on the abaxial side. It is also very similar to C. roraimensis by the lemma shape, in particular with the very short setae. However, the plant bases differ: in C. roraimensis the leaf bases are lacerated and curly, a feature less well developed in C. columbiana. Possibly the best way to separate the two species might be by the much more villous leaf margins, and often the villous adaxial leaf surface of C. columbiana. Geographically, the two species are also adjacent.
The leaf anatomy is like that of C. hapalotricha, but differs by a continuous sclerenchyma layer below the abaxial epidermis.
Cortaderia roraimensis
Arundo roraimensis N.E.Br., Trans. Linn. Soc. London, Bot. ser. 2, 6: 74. 1901.
Type: British Guiana, summit Mt. Roraima, autumn 1898, F. V. McConnel & J. J. Quelch 673 (lectotype, designated as holotype by Connor & Edgar, Taxon 23: 601 (1974): K!).
Etymology
-ensis (Latin), denoting place of origin. From Mt Roraima, Guyana.
Taxonomy
This is the only Cortaderia species from the tepuis. It is very similar to C. columbiana. It shares with C. columbiana and C. bifida a base of dense clustered lacerated sheaths. From the similar C. columbiana it is separated by the almost absent indumentum on the leaf margin directly above the simple ligule. From C. bifida it is distinct by the lobed lemma, where the lobes are not extended into slender setae.
The leaf anatomy (Fig.
Acknowledgements
We thank Paul Peterson and Rob Soreng for the material of the new species, for encouragement and hosting HPL at the Smithsonian Insitution, and sharing their extensive knowledge of the South American grasses; Zensi Hopf Schalch for preparing many anatomical sections; Belén Montes for training in anatomical techniques; the Swiss National Science foundation SNF for funding the research on the Danthonioideae (Grant 31003A-107927 to HPL); Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET); and the numerous herbaria for loans and for working space during visits; Jeff Saarela and Marcin Nobis for a very constructive review.
References
- Acevedo de Vargas R (1959) Las especies de Gramineas del genero Cortaderia en Chile. Boletin del Museo Nacional de Historia Natural 27: 204–246.
- Astegiano ME, Anton AM, Connor HE (1995) Sinopsis del genero Cortaderia (Poaceae) en Argentina. Darwiniana 33: 43–51.
- Barker NP, Linder HP, Morton CM, Lyle M (2003) The paraphyly of Cortaderia (Danthonioideae; Poaceae): evidence from morphology and chloroplast and nuclear DNA sequence data. Annals of the Missouri Botanical Garden 90: 1–24. https://doi.org/10.2307/3298522
- Bernardello LM (1979) Sobre del género Lamprothyrsus (Poaceae) en Argentina. Kurtziana 12-13: 119–132.
- Conert J (1961) Die Systematik und Anatomie der Arundineae. J. Cramer, Weinheim, 208 pp.
- Connor HE (1965) Breeding systems in New Zealand grasses V. Naturalised species of Cortaderia. New Zealand Journal of Botany 3: 17–23. https://doi.org/10.1080/0028825X.1965.10428709
- Connor HE (1973) Breeding systems in Cortaderia (Gramineae). Evolution 27: 663–678. https://doi.org/10.2307/2407199
- Connor HE, Charlesworth D (1989) Genetics of male-sterility in gynodioecious Cortaderia (Gramineae). Heredity 63: 373–382. https://doi.org/10.1038/hdy.1989.111
- Connor HE, Dawson MI (1993) Evolution of reproduction in Lamprothyrsus (Arundineae, Gramineae). Annals of the Missouri Botanical Garden 80: 512–517. https://doi.org/10.2307/2399797
- Connor HE, Edgar E (1974) Names and types in Cortaderia Stapf (Gramineae). Taxon 23: 595–605. https://doi.org/10.2307/1218786
- Davidse G (2004) Cortaderia. In: Steyermark JA, Berry PE, Yatskievych K, Holst BK (Eds) Flora of the Venezuelan Guayana, vol 8. Missouri Botanical Garden Press, St Louis, 78–79.
- Dusén P (1909) Beiträge zur Flora des Itatiaia. Arkiv für Botanik 9: 1–50.
- Ellis RP (1976) A procedure for standardizing comparative leaf anatomy in the Poaceae. I. The leaf-blade as viewed in transverse section. Bothalia 12: 65–109. https://doi.org/10.4102/abc.v12i1.1382
- Ellis RP (1979) A procedure for standardizing comparative leaf anatomy in the Poaceae. II. The epidermis as seen in surface view. Bothalia 12: 641–671. https://doi.org/10.4102/abc.v12i4.1441
- Gadgil RL, Barton PG, Allen PJ, Sandberg AM (1990) Growth of the pampas grass Cortaderia spp in New Zealand Pinus radiata forests. New Zealand Journal of Forestry Science 20: 176–183.
- Grounds R (1979) Ornamental Grasses. Pelham Books, London, 216 pp.
- Harradine AR (1991) The impact of pampas grasses as weeds in southern Australa. Plant Protection Quarterly 6: 111–115.
- Hitchcock AS (1927) The grasses of Ecuador, Peru, and Bolivia. Contrib US Natl Herb 24 part 8: 291–556.
- Laegaard S (1997) Gramineae (part 1). In: Harling G, Andersson L (Eds) Flora of Ecuador. Council for Nordic Publications in Botany, Copenhagen, 3–56.
- Lambrinos JG (2001) The expansion history of a sexual and asexual species of Cortaderia in California, USA. Journal of Ecology 89: 88–98. https://doi.org/10.1046/j.1365-2745.2001.00524.x
- Linder HP, Baeza PCM, Barker NP, Galley C, Humphreys AM, Lloyd KM, Orlovich DA, Pirie MD, Simon BK, Walsh NG, Verboom GA (2010) A generic classification of the Danthonioideae (Poaceae). Annals of the Missouri Botanical Garden 97: 306–364. https://doi.org/10.3417/2009006
- Lyle M (1996) Change in name and status of a Pampas grass (Cortaderia, Poaceae: Arundinoideae) from Bolivia. Novon 6: 72–77. https://doi.org/10.2307/3392215
- McNeill J (2014) Holotype specimens and type citations: General issues. Taxon 65: 1112–1113. https://doi.org/10.12705/635.7
- Moore DM (1983) Flora of Tierra del Fuego. Anthony Nelson, London, 396 pp.
- Nees ab Esenbeck CG (1841) Florae Africae Australioris. Illustrationis monographicae. I. Gramineae. Prausnitzianis, Glogau.
- Nicora EG (1978) Flora Patagonica, parte III, Gramineae, ed. M.N. Correa. Buenos Aires.
- Okada M, Ahmad R, Jasieniuk M (2007) Microsatellite variation points to local landscape plantings as sources of invasive pampas grass (Cortaderia selloana) in California. Molecular Ecology 16: 4956–4971. https://doi.org/10.1111/j.1365-294X.2007.03568.x
- Pilger R (1906) Lamprothyrsus, eine neue Gattung der Gräser und ihre Verwandten. Botanische Jahrbücher für Systematik, Beiblatt 85: 58–67.
- Pirie MD, Humphreys AM, Barker NP, Linder HP (2009) Reticulation, data combination, and inferring evolutionary history: an example from Danthonioideae (Poaceae). Systematic Biology 58: 612–628. https://doi.org/10.1093/sysbio/syp068
- Pirie MD, Humphreys AM, Galley C, Barker NP, Verboom GA, Orlovich D, Draffin SJ, Lloyd K, Baeza CM, Negritto M, Ruiz E, Cota JHS, Reimer E, Linder HP (2008) A novel supermatrix approach improves resolution of phylogenetic relationships in a comprehensive sample of danthonioid grasses. Molecular Phylogenetics and Evolution 48: 1106–1119. https://doi.org/10.1016/j.ympev.2008.05.030
- Renvoize SA (1998) Gramineas de Bolivia. The Royal Botanic Gardens, Kew, 644 pp.
- Robinson ER (1984) Naturalized species of Cortaderia (Poaceae) in southern Africa. South African Journal of Botany 3: 343–346. https://doi.org/10.1016/S0022-4618(16)30023-7
- Stapf O (1897) The botanical history of the Uva, Pampas Grass and their allies. The Gardener’s Chronicle, 396 pp.
- Swallen JR (1948) New grasses from Honduras, Colombia, Venezuela, Ecuador, Bolivia, and Brazil. Contributions from the United States National Herbarium 29: 251–275.
- Swallen JR (1956) New grasses from Santa Catarina. Sellowia 7: 7–12.
- Testoni D (2016) Studies in the genus Cortaderia (Poaceae) Ill. Cortaderia peruviana, a new synonym of C. hieronymi. Boletin De La Sociedad Argentina De Botanica 51: 359–365.
- Testoni D, Villamil CB (2014) Estudios en el género Cortaderia (Poceae). I. Sistematica y nomenclatura de la sect. Cortaderia. Darwiniana, nueva serie2: 260–276. https://doi.org/10.14522/darwiniana/2014.22.591
- Thiers B (continuously updated) Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium.
- Tolivia D, Tolivia J (1987) Fasga: a new phytochromatic for simultaneous and differential staining of plant tissue. Journal of Microscopy 148: 113–117. https://doi.org/10.1111/j.1365-2818.1987.tb02859.x
- Tovar O (1993) Las Gramineas (Poaceae) del Peru. Monografias del Real Jardin Botanico, Consejo Superior de Investigaciones Cientificas, Madrid.